Tratamiento con hormona del crecimiento para pacientes con talasemia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012284.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MKT, NML and CFN conceived the review title and developed the search strategy
CFN, SLT, AR and NML participated in screening, selection, data extraction, risk of bias assessment and data entering via Covidence.
NML developed the SOF table using GDT GRADEPRO.
CFN wrote the first draft of the review. All authors participated in revising the draft review and approved the final version.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
JH received a honorarium for giving a talk in an event sponsored by a pharmaceutical company related to growth hormone.
CFN, NML, SLT, AR, PM and MKT have no conflict of interest to declare.
Acknowledgements
We thank the Cochrane Cystic Fibrosis and Genetic Disorders Review Group, especially the Managing Editors, Tracey Remmington and Nikki Jahnke in the development of this review. We also thank the review group editors and referees for their comments on our draft review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 May 28 | Growth hormone therapy for people with thalassaemia | Review | Chin Fang Ngim, Nai Ming Lai, Janet YH Hong, Shir Ley Tan, Amutha Ramadas, Premala Muthukumarasamy, Meow-Keong Thong | |
| 2017 Sep 18 | Growth hormone therapy for people with thalassaemia | Review | Chin Fang Ngim, Nai Ming Lai, Janet YH Hong, Shir Ley Tan, Amutha Ramadas, Premala Muthukumarasamy, Meow‐Keong Thong | |
| 2016 Jul 15 | Growth hormone therapy for people with thalassaemia | Protocol | Chin Fang Ngim, Nai Ming Lai, Janet Y Hong, Shir Ley Tan, Amutha Ramadas, Premala Muthukumarasamy, Meow‐Keong Thong | |
Differences between protocol and review
A secondary outcome of 'Serum insulin‐like growth hormone (IGF‐1)' was added in agreement with the input by a peer reviewer that this outcome has additional value in assessing response to GH therapy.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Female; Humans; Male;
PICO

Study flow diagram.

Comparison 1 Growth hormone versus control, Outcome 1 Oral glucose tolerance test sum (mg/dL).
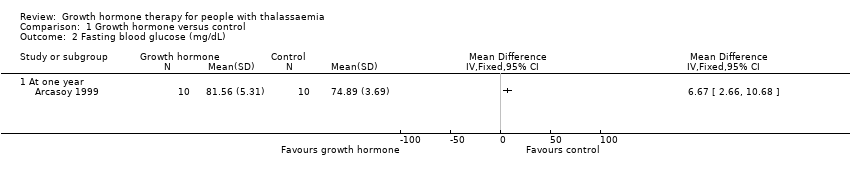
Comparison 1 Growth hormone versus control, Outcome 2 Fasting blood glucose (mg/dL).

Comparison 1 Growth hormone versus control, Outcome 3 Height SD score.

Comparison 1 Growth hormone versus control, Outcome 4 Change from baseline in height SD score.
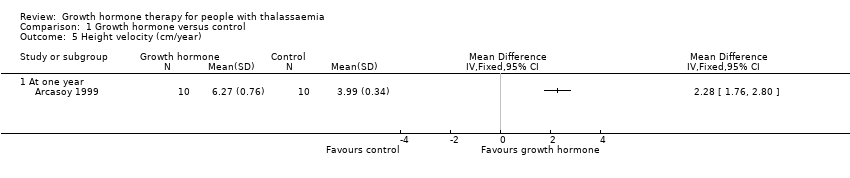
Comparison 1 Growth hormone versus control, Outcome 5 Height velocity (cm/year).

Comparison 1 Growth hormone versus control, Outcome 6 Height velocity SD score.
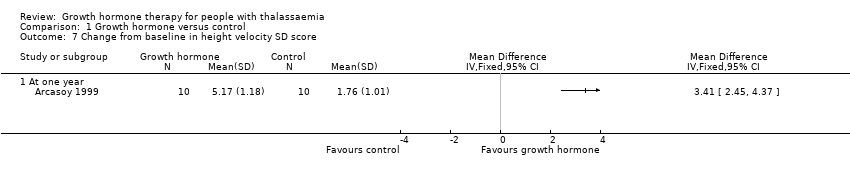
Comparison 1 Growth hormone versus control, Outcome 7 Change from baseline in height velocity SD score.

Comparison 1 Growth hormone versus control, Outcome 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL).
| Growth hormone for people with thalassaemia | ||||||
| Patient or population: people with thalassaemia (any age) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with growth hormone | |||||
| Final height and change in height | The included trial did not assess either of these outcomes. | |||||
| Adverse effects Oral glucose tolerance test (mg/dL) (at one year) | The mean oral glucose tolerance test was 336.56 mg/dL. | MD 0.03 lower (17.45 lower to 17.39 higher). | ‐ | 20 | ⊕⊕⊕⊝ | Fasting blood glucose levels in the growth hormone group were significantly higher than in the control group but both were still within the normal range. |
| Height SDS | The mean height SDS was ‐2.85. | MD 0.09 lower | ‐ | 20 | ⊕⊕⊕⊝ | |
| Change in height SDS (difference between baseline and final visit at one year) | The change in mean height SDS was ‐0.05. | MD 0.26 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Height velocity (cm/year) | The mean height velocity was 3.99 cm/year. | MD 2.28 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Height velocity SDS | The mean height velocity SDS was ‐1.56. | MD 3.31 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| Change in height velocity SDS (difference between baseline and final visit at one year) | The change in mean height velocity SDS was 1.76. | MD 3.41 higher | ‐ | 20 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Data contributed by a single trial with small sample and 95% CI is wide. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral glucose tolerance test sum (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting blood glucose (mg/dL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change from baseline in height SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Height velocity (cm/year) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change from baseline in height velocity SD score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Serum insulin‐like growth hormone (IGF‐1) (ng/mL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

