Iridotomía para desacelerar la evolución de la pérdida del campo visual en el glaucoma de ángulo cerrado
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012270.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 junio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JL, BR, and GG designed and wrote the protocol.
JL and BR screened studies for inclusion.
JL and BR extracted data from studies.
JL, BR, and GG drafted the final review and will be responsible for updates.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Cochrane Eyes and Vision US Project, supported by cooperative agreement 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
Epidemiology and Biostatistics of Aging Training Program, T32 AG000247, National Institute on Aging, National Institutes of Health, USA.
-
JL is a doctoral candidate supported by the Epidemiology and Biostatistics of Aging Training Program.
-
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
JL: none known.
BR: none known.
GG has received travel funding and both educational and unrestricted research funding from pharmaceutical and equipment manufacturers that are involved in the treatment of glaucoma; however none of the funding is otherwise related to (or competing with) the subject of this review.
Acknowledgements
We thank Kristina Lindsley, Tianjing Li, Barbara Hawkins, Sonal Singh, Andrew Law, Anupa Shah, Xuan Hui, Jennifer Evans and other members of Cochrane Eyes and Vision (CEV) for their comments and suggestions during title registration and writing the review. We thank our peer reviewers for providing comments to the review manuscript. We are also grateful to Amanda Bicket who provided feedback on the background of our review. Our protocol was adapted from a previous (withdrawn) protocol by Aravind Reddy and Sandra M Johnson for a Cochrane Review that was could not be completed.
We thank Lori Rosman, Information Specialist for CEV for devising and executing the electronic search strategies. We thank the methodologists and staff at CEV for assistance with full‐text screening of non‐English articles.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Jan 09 | Iridotomy to slow progression of visual field loss in angle‐closure glaucoma | Review | Benjamin Rouse, Jimmy T Le, Gus Gazzard | |
| 2018 Jun 13 | Iridotomy to slow progression of visual field loss in angle‐closure glaucoma | Review | Jimmy T Le, Benjamin Rouse, Gus Gazzard | |
| 2016 Jun 29 | Iridotomy to slow progression of angle‐closure glaucoma | Protocol | Jimmy T Le, Benjamin Rouse, Gus Gazzard | |
Differences between protocol and review
We added methods for assessing the certainty of the evidence and presenting outcomes in a 'Summary of findings' table in accordance with revised Cochrane standards and GRADE. We revised our background to be more concise and clarified that comparator (observation) refers to no iridotomy. For our secondary outcomes, we also considered data for longer‐term follow‐up closest to one year if trials did not report outcomes at one year.
Methods not implemented
We did not conduct a meta‐analysis as planned because data are not available for all outcomes and the full reports of the trials are still under preparation. Accordingly, we did not perform assessment of reporting biases, subgroup analyses and sensitivity analyses.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
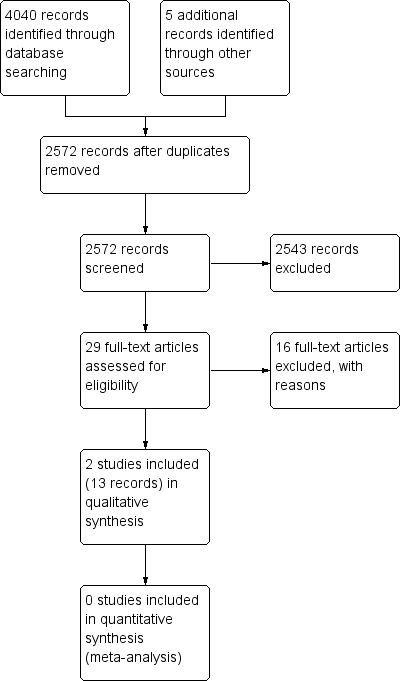
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
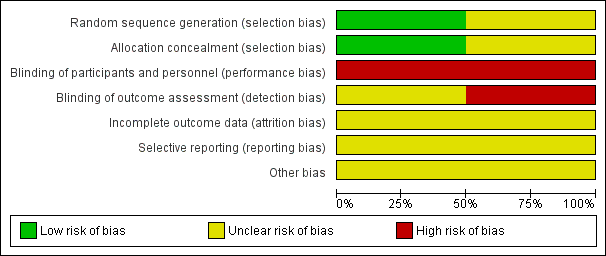
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Iridotomy vs No treatment, Outcome 1 Angle Width.

Comparison 1 Iridotomy vs No treatment, Outcome 2 Adverse events.
| Iridotomy compared to no iridotomy for patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Patient or population: patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of eyes | Certainty of the evidence | Comments | |
| Risk with no iridotomy | Risk with Iridotomy | |||||
| Proportion of progressive visual field loss at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Intraocular pressure: mean IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Gonioscopic findings: mean angle width at 1 year | The mean angle width was 11.3° in the no iridotomy group | The mean angle width in the iridotomy group was 12.7° higher | MD 12.7 | 1550 | ⊕⊕⊕⊝ | Participants in the study were primary angle‐closure suspects. Data were only available at 18 months. |
| Need for additional surgery: proportion of participants who received additional surgery to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Medications: mean number of medications used to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Quality of life measures | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Adverse events ‒ IOP spike (rise greater than or equal to 8 mmHg) at 1 hour | 4 per 1000 | 98 per 1000 | RR 24.00 | 1468 | ⊕⊕⊝⊝ | Participants in the study were primary angle‐closure suspects. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias, as the study is at unclear risk of bias for incomplete outcome data, selective outcome reporting, and other sources of bias due to the lack of availability of a full trial report. 2 Downgraded by one level for imprecision, as the confidence interval of the risk ratio between the groups is wide. | ||||||
| Primary angle‐closure suspect (PACS) | Primary angle closure (PAC) | Primary angle‐closure glaucoma (PACG) | |
| Iridotrabecular contact greater than or equal to 180° | X | X | X |
| Elevated intraocular pressure OR peripheral anterior synechiae | X | X | |
| Optic nerve damage | X |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angle Width Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 IOP spike (rise greater than or equal to 8 mmHg) at 1 hour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

