Suplementos nutricionales para pacientes en tratamiento para la leishmaniasis visceral activa
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012261.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades infecciosas
- Clasificada:
-
- Actualizada
No new studies identified with search
No studies were identified for inclusion in the last search (12 Sep, 2017)Evaluada: 8 May 2019
- Actualizada
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
| Tasks | Authors |
| Protocol development | EC, JLA, MH, CB, CJ, TLC, AB, JA |
| Guarantor | EC |
| Contact person | EC |
| Piloted the selection stage | EC, JLA, JA |
| Screened titles and abstracts and assessed full texts | EC, JLA, MH, CJ, SSgB, JA |
| Assessed conferences | EC, CJ, MH, SSgB, TM, JA |
| Requested information to researchers and companies | EC, MH, TM |
| Designed the data extraction form | EC, JLA |
| Wrote the background | EC, JA |
| Wrote the methodological sections of the review | EC, JLA, MH, CB, TLC |
| Wrote the results, discussion and conclusions sections | EC, JLA, JA |
| Prepared the flow‐chart | JLA |
| Prepared ‘Summary of findings' tables | JLA |
| Made an intellectual contribution and provided the clinical perspective | EC, JLA, MH, CB, CJ, SSgB, TM, TLC, AB, JA |
| Edited the review | EC, JLA, MH, CB, CJ, SSgB, TM, TLC, AB, JA |
| Assessed MECIR standards | JLA |
| Approved final version of the protocol prior to submission | EC, JLA, MH, CB, CJ, SSgB, TM, TLC, AB, JA |
Abbreviations: Agustin Benito (AB); Carmen Bouza (CB); Carolina Jimenez (CJ); Estefanía Custodio (EC); Jesús López Alcalde (JLA); Jorge Alvar (JA); Mercè Herrero (MH); Stefan Storcksdieck genannt Bonsmann (SSgB); Teresa López‐Cuadrado (TLC); Theodora Mouratidou (TM).
Sources of support
Internal sources
-
Centro Nacional de Medicina Tropical, Instituto de Salud Carlos III, Spain.
-
Red de Investigación Cooperativa en Enfermedades Tropicales (RICET), Spain.
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
Agustin Benito has no known conflicts of interest.
Carmen Bouza has no known conflicts of interest.
Carolina Jimenez has no known conflicts of interest.
Estefanía Custodio has no known conflicts of interest.
Jesus Lopez Alcalde has no known conflicts of interest.
Jorge Alvar has no known conflicts of interest.
Merce Herrero has no known conflicts of interest.
Stefan Storcksdieck genannt Bonsmann has no known conflicts of interest.
Teresa López‐Cuadrado has no known conflicts of interest.
Theodora Mouratidou has no known conflicts of interest.
Acknowledgements
We acknowledge Raimundo Alcázar for bibliographical support, and Fabiana Alves for her contribution to the technical discussions.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in here do not necessarily reflect UK government policy.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 26 | Nutritional supplements for patients being treated for active visceral leishmaniasis | Review | Estefanía Custodio, Jesús López‐Alcalde, Mercè Herrero, Carmen Bouza, Carolina Jimenez, Stefan Storcksdieck genannt Bonsmann, Theodora Mouratidou, Teresa López‐Cuadrado, Agustin Benito, Jorge Alvar | |
| 2016 Jun 29 | Nutritional supplements for patients being treated for active visceral leishmaniasis | Protocol | Estefanía Custodio, Mercè Herrero, Carmen Bouza, Jesús López‐Alcalde, Agustin Benito, Jorge Alvar | |
Differences between protocol and review
Review information
-
Jesús López Alcalde is now an author, with equal contribution to Estefanía Custodio.
-
New authors joined the team: CJ, TLC, SSgB, and TM.
Search methods
In the review protocol, we did not plan to contact micronutrient companies.
Assessment of risk of bias in the included studies
In the protocol, we had planned to assess risk of bias in non‐randomized trials according to the Cochrane tool for non‐randomized studies (ACROBAT‐ NRSI; (Sterne 2014)). However, this tool is now called ROBINS‐I.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
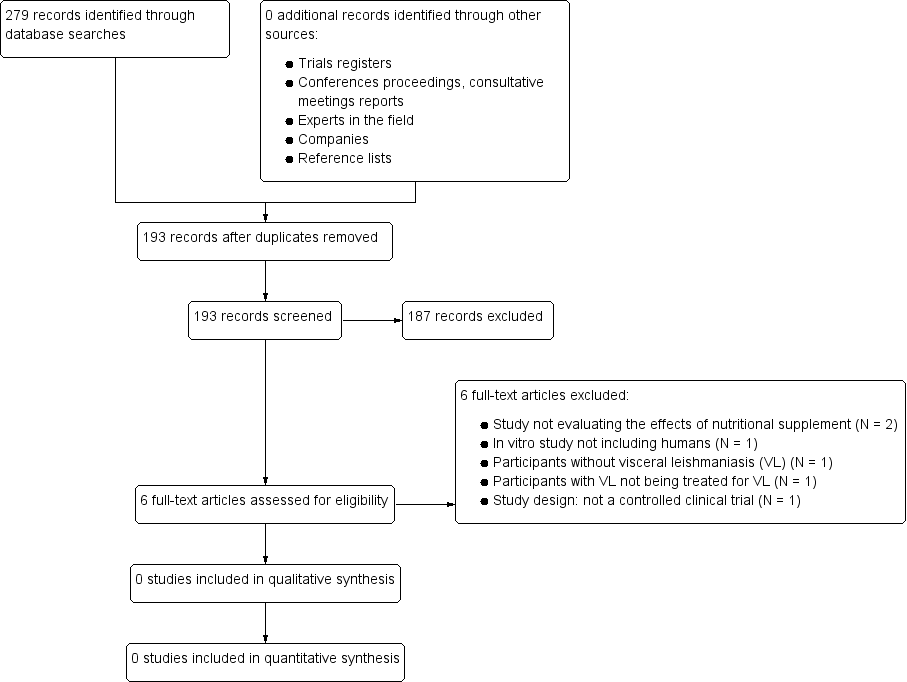
Study flow diagram
| What are the effects of nutritional supplements in patients being treated for active visceral leishmaniasis (VL)? Evidence: we found no eligible studies for this systematic review. | ||
| Elements | Proposal | Comments |
| Population | People being treated for VL |
|
| Intervention | Oral nutritional supplement |
|
| Comparison | Placebo | |
| Outcomes | Relevant outcomes for key stakeholders defined, measured, collected, and reported in an objective, reliable, accurate, and actionable way |
|
| Study type | RCT | Cluster‐RCTs (RCTs that randomize groups (clusters) rather than individuals) have several advantages compared to individual‐RCTs (López‐Alcalde 2015). For example, they may be less costly and time‐consuming, as they simplify the logistics of implementation (Smith 2008); they control for confounding (Safdar 2008), and minimize treatment contaminationb between intervention and control participants (Hayes 2000); they are better for measuring the overall group effect of an intervention, and for judging effectiveness (Hayes 2000), that is, the extent to which a specific intervention, when used under ordinary circumstances, does what it is intended to do (Cochrane 2017). Moreover, they have broader generalizability. For example, cluster‐RCTs minimize the Hawthorne effect, which is the effect on the people being studied (usually positive or beneficial), of being under study (Porta 2008). |
| Abbreviations: COS: core outcome set; HIV: human immunodeficiency virus; RCT: randomized controlled trial; VL: visceral leishmaniasis. | ||
| Nutritional supplements versus no nutritional supplements for people who are being treated for active VL | ||||||
| Patient or population: people who are being treated for active VL | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with no nutritional supplements | Risk with nutritional supplements | |||||
| Primary cure | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| Definitive cure | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| Treatment completion | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| Self‐reported recovery from illness or resolution of symptoms | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| Weight gain, increased skinfold thickness, or other measures of lean or total mass, or growth in children | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| Adverse outcomes | No trials met the inclusion criteria. Thus, there is no data for this outcome. | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Abbreviations: CI: confidence interval; VL: visceral leishmaniasis; RCT: randomized controlled trial; RR: risk ratio; OR: odds ratio. | ||||||

