Intervencije usmjerene liječnicima o propisivanju antibiotika za akutne respiratorne infekcije u primarnoj njezi: pregled sustavnih pregleda
Abstract
Background
Antibiotic resistance is a worldwide health threat. Interventions that reduce antibiotic prescribing by clinicians are expected to reduce antibiotic resistance. Disparate interventions to change antibiotic prescribing behaviour for acute respiratory infections (ARIs) have been trialled and meta‐analysed, but not yet synthesised in an overview. This overview synthesises evidence from systematic reviews, rather than individual trials.
Objectives
To systematically review the existing evidence from systematic reviews on the effects of interventions aimed at influencing clinician antibiotic prescribing behaviour for ARIs in primary care.
Methods
We searched the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), MEDLINE, Embase, CINAHL, PsycINFO, and Science Citation Index to June 2016. We also searched the reference lists of all included reviews. We ran a pre‐publication search in May 2017 and placed additional studies in 'awaiting classification'.
We included both Cochrane and non‐Cochrane reviews of randomised controlled trials evaluating the effect of any clinician‐focussed intervention on antibiotic prescribing behaviour in primary care. Two overview authors independently extracted data and assessed the methodological quality of included reviews using the ROBIS tool, with disagreements reached by consensus or by discussion with a third overview author. We used the GRADE system to assess the quality of evidence in included reviews. The results are presented as a narrative overview.
Main results
We included eight reviews in this overview: five Cochrane Reviews (33 included trials) and three non‐Cochrane reviews (11 included trials). Three reviews (all Cochrane Reviews) scored low risk across all the ROBIS domains in Phase 2 and low risk of bias overall. The remaining five reviews scored high risk on Domain 4 of Phase 2 because the 'Risk of bias' assessment had not been specifically considered and discussed in the review Results and Conclusions. The trials included in the reviews varied in both size and risk of bias. Interventions were compared to usual care.
Moderate‐quality evidence indicated that C‐reactive protein (CRP) point‐of‐care testing (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.66 to 0.92, 3284 participants, 6 trials), shared decision making (odds ratio (OR) 0.44, 95% CI 0.26 to 0.75, 3274 participants, 3 trials; RR 0.64, 95% CI 0.49 to 0.84, 4623 participants, 2 trials; risk difference ‐18.44, 95% CI ‐27.24 to ‐9.65, 481,807 participants, 4 trials), and procalcitonin‐guided management (adjusted OR 0.10, 95% CI 0.07 to 0.14, 1008 participants, 2 trials) probably reduce antibiotic prescribing in general practice. We found moderate‐quality evidence that procalcitonin‐guided management probably reduces antibiotic prescribing in emergency departments (adjusted OR 0.34, 95% CI 0.28 to 0.43, 2605 participants, 7 trials). The overall effect of these interventions was small (few achieving greater than 50% reduction in antibiotic prescribing, most about a quarter or less), but likely to be clinically important.
Compared to usual care, shared decision making probably makes little or no difference to reconsultation for the same illness (RR 0.87, 95% CI 0.74 to 1.03, 1860 participants, 4 trials, moderate‐quality evidence), and may make little or no difference to patient satisfaction (RR 0.86, 95% CI 0.57 to 1.30, 1110 participants, 2 trials, low‐quality evidence). Similarly, CRP testing probably has little or no effect on patient satisfaction (RR 0.79, 95% CI 0.57 to 1.08, 689 participants, 2 trials, moderate‐quality evidence) or reconsultation (RR 1.08, 95% CI 0.93 to 1.27, 5132 participants, 4 trials, moderate‐quality evidence). Procalcitonin‐guided management probably results in little or no difference in treatment failure in general practice compared to normal care (adjusted OR 0.95, 95% CI 0.73 to 1.24, 1008 participants, 2 trials, moderate‐quality evidence), however it probably reduces treatment failure in the emergency department compared to usual care (adjusted OR 0.76, 95% CI 0.61 to 0.95, 2605 participants, 7 trials, moderate‐quality evidence).
The quality of evidence for interventions focused on clinician educational materials and decision support in reducing antibiotic prescribing in general practice was either low or very low (no pooled result reported) and trial results were highly heterogeneous, therefore we were unable draw conclusions about the effects of these interventions. The use of rapid viral diagnostics in emergency departments may have little or no effect on antibiotic prescribing (RR 0.86, 95% CI 0.61 to 1.22, 891 participants, 3 trials, low‐quality evidence) and may result in little to no difference in reconsultation (RR 0.86, 95% CI 0.59 to 1.25, 200 participants, 1 trial, low‐quality evidence).
None of the trials in the included reviews reported on management costs for the treatment of an ARI or any associated complications.
Authors' conclusions
We found evidence that CRP testing, shared decision making, and procalcitonin‐guided management reduce antibiotic prescribing for patients with ARIs in primary care. These interventions may therefore reduce overall antibiotic consumption and consequently antibiotic resistance. There do not appear to be negative effects of these interventions on the outcomes of patient satisfaction and reconsultation, although there was limited measurement of these outcomes in the trials. This should be rectified in future trials.
We could gather no information about the costs of management, and this along with the paucity of measurements meant that it was difficult to weigh the benefits and costs of implementing these interventions in practice.
Most of this research was undertaken in high‐income countries, and it may not generalise to other settings. The quality of evidence for the interventions of educational materials and tools for patients and clinicians was either low or very low, which prevented us from drawing any conclusions. High‐quality trials are needed to further investigate these interventions.
Laički sažetak
Strategije koje pomažu liječnicima promijeniti način na koji oni propisuju antibiotike za pacijente s akutnim upalama dišnog sustava
Istraživačko pitanje
Ovaj Cochrane sustavni pregled literature je imao za cilj sažeti sve dokaze iz sustavnih pregleda o strategijama usmjerenim na liječnike kojima je cilj smanjiti broj propisanih recepata za antibiotike koje daju bolesnicima s akutnim upalama dišnog sustava (akutne respiratorne infekcije) kao štu su upale uha, nosa, grla ili prsa.
Dosadašnje spoznaje
Važno je da se antibiotici koriste za bolesti gdje mogu utjecati na smanjenjenje simptoma i oporavak pacijenata te da su dostupni za ozbiljne infekcije koje mogu dovesti do invaliditeta ili smrti. Antibiotici mogu dovetsi do vrlo male ili nikakve razlike u bolesnika koji imaju upalu uha, nosa, grla ili pluća uzrokovane virusom (npr. prehlada, gripa ili upala grla). Liječnici ponekad previše propisuju antibiotike bolesnicima s navedenim simptomima. Stoga su razvijene strategije za promjenu liječničkih navika propisivanja antibiotika kako bi se smanjio broj antibiotika koji se daju bolesnicima s tim simptomima. Postoji nekoliko vrsta strategija, a važno je analizirati sva istraživanja koja su ispitala koliko dobro te strategije djeluju.
Značajke istraživanja
Pronašli smo pet Cochrane sustavnih pregleda i tri koja nisu iz Cochranea. Pregledi su varirali u broju uključenih istraživanja i broju sudionika u koji su bili uključeni u istraživanje. Kvaliteta tih sustavnih pregleda i istraživanja koja su u njih bila uključena razlikovala se.
Ključni rezultati
Pronašli smo umjerenu kvalitetu dokaza da tri vrste strategija vjerojatno pomažu smanjiti propisivanje antibiotika u primarnoj praksi. Strategije koje potiču korištenje zajedničkog odlučivanja među liječnicima i njihovim pacijentima, jeste testiranje C‐reaktivnog proteina i prokalcitonina (testovi koji mjere količinu proteina u krvi koja se može povećati u slučaju infekcije) te na osnovu toga smanjiti propisivanje antibiotika u općoj praksi. Upotreba određivanja prokalcitona vjerojatno smanjuje propisivanje antibiotika na hitnim prijemima. Čini se da te strategije mijenjaju propisivanje antibiotika, a pacijenti su bili zadovoljni savjetovanjem te uvjereni da se ne moraju vratiti svom liječniku za istu bolest. Nije bilo podataka o troškovima tih strategija pa je bilo teško procijeniti koristi i troškove.
Kvaliteta dokaza o strategijama koje imaju za cilj educirati liječnike o propisivanju antibiotika i pružaju pomoć liječnicima da im pomognu u promjeni propisivanja, a za upotrebu brzih virusnih dijagnostika u hitnim službama bila je niska ili vrlo niska, što znači da nisu mogli donijeti čvrste zaključke o učincima tih strategija.
U zaključku smo utvrdili da neke strategije usmjerene na liječnike vjerojatno mogu pomoći u smanjenju primjene antibiotika u primarnoj praksi. Daljnje studije su potrebne za druge vrste strategija gdje postoji manje informacija o tome mogu li promijeniti propisivanje antibiotika.
Authors' conclusions
Background
Description of the condition
Antibiotic resistance is a major threat to human health worldwide (WHO 2015). Two million people are directly affected by antibiotic‐resistant infections, of whom 23,000 die, annually in the USA (CDC 2013), with similar numbers in Europe (Lancet 2009). Infections caused by drug‐resistant bacteria put patients at increased risk of worse clinical outcomes and death, and consume more healthcare resources (WHO 2015). The economic cost has been estimated at USD 55 billion per year in the USA, although the real cost may be much higher (Smith 2013). Unless addressed, this situation will worsen, with 10 million deaths estimated globally every year by 2050, and economic costs of USD 100 trillion from a reduction in overall economic production (O'Neill 2014).
Antibiotic resistance is an inevitable consequence of antibiotic use because antibiotics kill only bacteria that are sensitive and not pre‐existing antibiotic‐resistant bacteria (Spellberg 2013). Globally, human consumption of antibiotics increased by 36% between 2000 and 2010 (Van Boeckel 2014). This is reflected in European increases in antibiotic prescriptions (Adriaenssens 2011). In the UK, 949.9 tonnes of antibiotics were used in 2013, with 56% being for human, rather than animal, use (PHE 2013). In Australia, 47% of the population are prescribed at least one antibiotic every year (ACSQHC 2016). For the individual patient, recent antibiotic use is the single most important risk factor for antibiotic‐resistant infection (Chung 2007; Malhotra‐Kumar 2007), with longer and multiple courses of antibiotics associated with even higher rates of resistance (Costelloe 2010). No new classes of antibiotics have been developed in the last two decades, and urgent investment in the discovery and development of new antimicrobial drugs has been proposed (Huttner 2013; O'Neill 2014).
However, resistance is reversible: in individuals, stopping the use of antibiotics results in the exponential decay of resistance in the bacteria of their microbiome (Costelloe 2010). This supports the case for strategies that promote more prudent use of antibiotics (O'Neill 2014). There are various approaches to this, including: promotion of narrow‐ over broad‐spectrum antibiotics; prescribing the shortest clinically effective course; and achieving a total reduction in antibiotics prescribed.
Most antibiotics are prescribed in primary care, and most commonly for acute respiratory infections (ARIs) (Goossens 2005; Gulliford 2014; Shapiro 2014). Antibiotics are highly effective for some ARIs (including community‐acquired bacterial pneumonia and acute exacerbation of chronic obstructive pulmonary disease (COPD)). However, the vast majority of ARIs are seen in primary care and in most cases are spontaneously resolved without antibiotics. These ARIs derive only marginal clinical benefits from antibiotics, which have to be balanced against the increased risks of harms associated with mild adverse events, and antibiotic resistance. Treated with antibiotics, one‐third fewer children with acute otitis media had pain at days 2 to 3 (number needed to treat for an additional beneficial outcome (NNTB) = 20) (Venekamp 2015), and the duration of sore throat and acute bronchitis (cough) was reduced by 12 to 16 hours (NNTB to prevent one sore throat = 21) (Smith 2014; Spinks 2013). Meanwhile, the risk of vomiting, diarrhoea, or rash increased (number needed to treat for an additional harmful outcome (NNTH) = 9 for acute otitis media, and NNTH = 24 for acute bronchitis).
The management of ARIs in primary care is therefore a key target for influencing the antibiotic prescribing behaviour of clinicians. This is most often done by encouraging reduced prescribing of antibiotics for ARI. The use of delayed prescriptions by clinicians can also change both clinician and patient behaviour by changing the type of prescription written and decreasing the likelihood that an antibiotic prescription is used.
Description of the interventions
Many interventions that target clinicians also frequently target patients or the public, acknowledging the influence of patient expectations and concerns on prescribing. However, in many countries an antibiotic cannot be prescribed without the prescribing clinician’s consent. In addition, the type of prescription written, whether it is for immediate or delayed use, is also the clinician’s decision and should be considered as an additional, distinct type of prescribing behaviour. This overview focussed on two prescribing behaviours, that is whether an antibiotic is:
-
prescribed;
-
prescribed for immediate or delayed use.
We focussed on interventions aimed at influencing primary care clinicians’ antibiotic prescribing behaviour for patients with ARIs. We have included all ARIs, acknowledging that antibiotic prescribing for some conditions (such as bacterial pneumonia and mastoiditis) is entirely appropriate for all cases, while for others (such as acute otitis media, sore throat, acute bronchitis, and acute sinusitis) antibiotic prescribing may be useful for only a proportion. The interventions could have included:
-
educational materials for clinicians: printed, electronic, or audio‐visual materials that target the healthcare professional;
-
educational meetings: healthcare professionals attending conferences, lectures, training courses, or workshops;
-
educational outreach visits: healthcare professionals receiving information from a trained professional in their practice setting;
-
audit and feedback: any summary of clinical performance of health care over a specified time period provided to the healthcare professional;
-
reminders: verbal, written, or electronic information intended to prompt a healthcare professional to recall information, to include (computer) decision support systems;
-
financial interventions: targeting the healthcare professional to include financial incentives (e.g. fee‐for‐service) and financial penalties (e.g. direct or indirect financial penalty for inappropriate behaviour);
-
point‐of‐care tests (POCTs): equipment for use by healthcare professionals in their practice setting, to be used at the time and place of patient care, to provide rapid diagnostic information to help reduce the uncertainty associated with clinical diagnosis;
-
communication strategies: any resource targeted at the healthcare professional that encourages discussion with a patient about management options including:
-
clinician‐delivered patient educational interventions;
-
improved communication interventions (for clinician‐patient interaction);
-
shared decision making (as defined by Coxeter 2015, i.e. the process of enabling a health professional and patient to make a joint decision about management based on the best available evidence and the patient’s values and preferences);
-
-
mass media campaigns: targeted at the healthcare professional at the population level employing varied use of communication;
-
delayed prescription strategy: any resource targeted at the healthcare professional that encourages giving a prescription for a patient to collect or use later than the initial consultation if symptoms do not improve;
-
any other intervention targeted at the clinician and aimed at changing antibiotic prescribing behaviour.
How the intervention might work
Strategies targeting clinician behaviour are complex interventions, meaning there is no single proposed mechanism of action. Multiple factors influence clinician antibiotic prescribing behaviour: knowledge of guidelines, previous clinical experience, diagnostic uncertainty, workload, and perceived patient expectations for antibiotics (Tonkin‐Crine 2011).
Interventions may provide education (including professional continuing education, provision of guidelines, decision support, educational outreach visits, audit and feedback, and patient information leaflets) to fill knowledge gaps and misperceptions. Interventions may seek to tackle diagnostic uncertainty by providing more information to the clinician and thus increasing self efficacy in managing the patient (e.g. through use of POCTs). Interventions may also seek to encourage enhanced communication between clinician and patient to discuss the benefits and harms of antibiotic treatment, thus decreasing concerns about negatively affecting patient satisfaction (e.g. shared decision making, enhanced communication skills training). Different combinations of these might be expected to achieve a greater effect if they operate through different mechanisms (Arnold 2005).
Much of the research undertaken hitherto has examined the effect of interventions, with less emphasis on process evaluation, which examines how interventions work (or do not work) (Moore 2015). Where process evaluations have been carried out, interventions that support general practitioners to use C‐reactive protein (CRP) POCTs or communication skills training, or both, appear to be effective because they increased the perceived importance of reducing antibiotic prescribing and decreased concerns regarding the safety of reducing antibiotic prescribing (Yardley 2013).
Why it is important to do this overview
Antibiotic prescribing is a major driver for the development of antibiotic‐resistant infections. Antibiotics are commonly prescribed in the management of ARIs in primary care, despite good evidence that they are only weakly effective in the vast majority.
There are many interventions aimed at influencing antibiotic prescribing for ARIs. However, the multiple systematic reviews evaluating their effectiveness have not been synthesised. This overview aimed to synthesise evidence from systematic reviews (rather than individual trials) and assess the effectiveness of these interventions to enable policymakers as well as clinicians to design processes for future management of antibiotic resistance in primary care, and researchers to focus on any gaps in the current evidence.
Objectives
To systematically review the existing evidence from systematic reviews on the effects of interventions aimed at influencing clinician antibiotic prescribing behaviour for ARIs in primary care.
Methods
Criteria for considering reviews for inclusion
Types of reviews
We included all published systematic reviews (Cochrane and non‐Cochrane) of randomised controlled trials (RCTs) (including parallel‐group, cluster, and factorial) testing interventions aimed at changing antibiotic prescribing in primary care for ARIs. We included reviews that included primary studies of non‐RCT designs, but only where RCT data were reported separately, where individual study data could be obtained. As stated in our protocol, we excluded reviews when there was complete overlap with an existing included review (overview authors decided which review to include, with Cochrane Reviews given priority over non‐Cochrane reviews, as they reported more detail) and where reviews were rated as having a high risk of bias.
Types of participants
We included reviews that studied interventions targeted at the antibiotic prescribing behaviour of clinicians for the treatment of ARIs in primary care. We included all ARIs, acknowledging that antibiotic prescribing for some conditions (such as bacterial pneumonia and mastoiditis) is entirely appropriate for all cases, while for others (such as acute otitis media, sore throat, acute bronchitis, and acute sinusitis) antibiotics prescribing may be useful for only a proportion of patients. Clinicians included anyone qualified to prescribe antibiotics. We included reviews that included trials from a variety of primary and ambulatory care settings. We also included reviews with trials that recruited participants from hospital inpatient settings, as well as primary or ambulatory care settings, providing data from the latter were reported separately. We defined primary care as any point‐of‐care in which patients are managed at the first point of patient contact, and included general practice, out‐of‐hours services, and emergency departments. We excluded reviews solely in hospital inpatient settings and residential settings such as nursing homes, as these were not classed as primary care settings. Patients could be any age, presenting with an ARI, which was defined as any sudden‐onset respiratory tract infection.
Types of interventions
We included any intervention designed to change the antibiotic prescribing behaviour of healthcare professionals for the management of ARIs in primary care. We included the following interventions: educational materials for clinicians, educational meetings, educational outreach visits, audit and feedback, reminders, financial interventions, point‐of‐care tests, communication strategies, mass media campaigns, delayed prescribing, or any other relevant intervention. Interventions could target healthcare professionals as a single population or as one of several groups. Interventions could be compared to usual care or an alternative intervention.
Types of outcome measures
Primary outcomes
-
Change in antibiotic prescriptions for ARI (total number prescribed or proportion of patients prescribed antibiotics, to include a delayed prescription, measured as absolute change or relative percentage change).
Secondary outcomes
-
Prescribing outcomes:
-
proportion of patients with an ARI given an antibiotic prescription for immediate use;
-
proportion of patients with an ARI given a delayed antibiotic prescription.
-
-
Patient outcomes:
-
proportion of patients with an ARI colonised or infected with antibiotic‐resistant bacteria;
-
adverse events;
-
symptom duration or severity;
-
health‐related quality of life;
-
patient satisfaction;
-
any measure of management failure, e.g. reconsultation for the same illness, hospital or emergency department attendance.
-
-
Healthcare resource costs:
-
management costs for any medication for the treatment of an ARI or associated complications.
-
Search methods for identification of reviews
Electronic searches
We searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE). We searched five additional databases in order to identify any other relevant systematic reviews. We incorporated search terms to target antibiotics, primary care settings, and ARIs but did not include intervention‐specific search terms. Information Specialist Nia Roberts developed search strategies for all databases, which are presented in Appendix 1. We applied no language restrictions to the searches. We searched the following databases:
-
Cochrane Database of Systematic Reviews (Issue 6 of 12, June 2016) and the Database of Abstracts of Reviews of Effects (Issue 2 of 4, April 2015) in the Cochrane Library (searched 9 June 2016);
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 9 June 2016);
-
Embase OvidSP (1974 to 9 June 2016);
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) EBSCO (1982 to 9 June 2016);
-
PsycINFO (from 1967 to June Week 2 2016);
-
Science Citation Index (Web of Science Core Collection) (1945 to 9 June 2016).
We also ran a pre‐publication, updated search on 19 May 2017, screened the results, and placed relevant studies in 'Characteristics of studies awaiting classification' in Appendix 2. We will incorporate these in the next version of this review as appropriate.
Searching other resources
In addition to database searches, two overview authors (STC and OvH) searched the reference lists of all included reviews.
Data collection and analysis
Selection of reviews
Two overview authors (STC and OvH) independently assessed the titles and abstracts of reviews identified by the search strategy. We excluded studies that were clearly not relevant. Both overview authors independently screened the full texts of potentially eligible reviews by applying the selection criteria. We agreed upon inclusion of reviews by consensus and, if necessary, by discussion with a third overview author (AMcC).
Data extraction and management
Two overview authors (STC and PST) independently extracted data from the full texts using a standardised data extraction form. The form included the following information:
-
general information (citation, author details, review ID);
-
aims and rationale;
-
extent of search (databases searched, restrictions);
-
eligibility criteria (types of studies included, whether RCTs reported separately);
-
participants within reviews (number of patients, age, primary care setting, indication for treatment);
-
interventions (type, target population);
-
comparator(s);
-
outcomes assessed; and
-
conclusions, recommendations, and limitations of the review.
We extracted data on details of the intervention(s) and comparison, effect of the intervention relative to control, number of trials and participants (patients), and quality of the evidence (using the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011).
We resolved discrepancies by consensus or by discussion with a third overview author (MPH). Where individual trials appeared in more than one included review, we noted the overlap and considered how trials had been interpreted by the author of each review. Where there was complete overlap in trials included within two or more reviews, two overview authors (STC and PST) discussed which review should contribute to the overview, based on the outcomes reported and risk of bias (Table 1).
| Intervention | Review | RCTs contributed and sample size (n) | ROBIS assessment | ||||
| Phase 2 domains | Phase 3 | ||||||
| 1. Study eligibility criteria | 2. Identification and selection of studies | 3. Data collection and study appraisal | 4. Synthesis and findings | 5. Risk of bias of review | |||
| POCT: C‐reactive protein | Andreeva 2014 (179) Cals 2009 (431) Cals 2010 (258) Diederichsen 2000 (812) Little 2013 (4264) Melbye 1995 (239) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Cals 2009 (431) Cals 2010 (258) Cals 2011 (330) Cals 2013* (379) Diederichsen 2000 (812) Gonzales 2011 (131) Melbye 1995 (229) | Low risk | Low risk | Low risk | High risk | Low risk | ||
| POCT: rapid viral detection test | Bonner 2003 (391) Doan 2009 (200) Poehling 2006 (300) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| POCT: procalcitonin | Briel 2008 (458) Burkhardt 2010 (550) Christ‐Crain 2004 (243) Christ‐Crain 2006 (302) Kristoffersen 2009 (210) Long 2009 (127) Long 2011 (156) Schuetz 2009 (1359) Stolz 2007 (208) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Educational materials for clinician or decision support, or both | Christakis 2001 (NR) Wilson 2002 (NR) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Interventions to support shared decision making | Altiner 2007 (2164) Briel 2006 (552) Butler 2012 (479,502) Cals 2009 (431) Cals 2013* (379) Francis 2009 (558) Légaré 2011 (151) Légaré 2012 (359) Little 2013 (4264) Welschen 2004 (1723) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Patient information leaflets to be used by clinician | Francis 2009 (558) Macfarlane 1997 (1014) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Multifaceted interventions | Finkelstein 2001 (NR) Flottorp 2002 (NR) McIsaac 1998 (NR) McIsaac 2002 (NR) Mainous 2000 (NR) | Low risk | Low risk | Low risk | High risk | Low risk | |
*Cals 2013 is a follow‐up study of the trial reported in Cals 2009.
NR: not reported
POCT: point‐of‐care‐test
RCT: randomised controlled trial
Assessment of methodological quality of included reviews
Quality of included reviews
Two overview authors (STC and PST) independently assessed the methodological quality of each review using the ROBIS tool (Whiting 2016). ROBIS is an up‐to‐date tool that provides a thorough way of assessing risk of bias in reviews with a comprehensive set of items on which to judge reviews. The ROBIS tool has three phases, as follows.
Phase 1: Assessing relevance (optional)
This was not required, as the reviews had already been assessed for relevance to the research question.
Phase 2: Identifying concerns with the review process
This consists of four domains against which a review is assessed:
-
study eligibility criteria;
-
identification and selection of studies;
-
data collection; and
-
study appraisal and synthesis and findings.
Each domain has five or six questions that are answered as ‘Yes’, ‘Probably Yes’, ‘Probably No’, ‘No’, and ‘No Information’. We rated domains as ‘Low Risk’ if all questions were ‘Yes’ or ‘Probably Yes’; ‘High Risk’ if they were ‘No’ or ‘Probably No’; and judged the remainder as ‘Unclear’ (Whiting 2016).
Phase 3: Judging risk of bias
This summarises the concerns identified in Phase 2 and assesses whether conclusions are supported by evidence by considering three points:
-
interpretation of findings addresses all concerns identified in Phase 2;
-
relevance of identified studies to the research question is considered;
-
avoids emphasising results on basis of statistical significance.
We excluded reviews that were assessed as being at high risk of bias based on Phase 2 and Phase 3 of the ROBIS assessment. Some reviews scored high risk on only a few items. We considered whether this influenced the overall result, and included reviews where high risk of bias due to omissions in original review reporting could be addressed in the overview.
We resolved differences in each overview author’s assessment of quality by discussion. If we were unable to reach agreement, we recorded this, and a third overview author (MPH) adjudicated.
Quality of evidence in included reviews
Risk of bias of individual trials
The review authors reported risk of bias of trials in reviews. The overview authors extracted and summarised the data.
GRADE assessments for each comparison/outcome
Two overview authors (STC and PST) examined the included reviews for information on the quality of the trials within each review, and where described, extracted it for each outcome. Where this information was not provided, we used the GRADE tool to make a retrospective assessment (Guyatt 2008; Higgins 2011). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence of the trials contributing data for each outcome.
Data synthesis
We produced a narrative summary of all the results reported in the included systematic reviews and presented a summary of data using an ’Overview of reviews’ table, which provides details of reviews based on all relevant outcomes.
We planned to perform subgroup analysis using Review Manager 5 software, data permitting, for the following (RevMan 2014);
-
adults (aged ≥ 18 years) versus children;
-
placebo versus no intervention; and
-
combined interventions versus single intervention.
Data were not available to undertake the planned subgroup analysis or sensitivity analysis.
Results
We searched the databases in June 2016. Figure 1 shows the reviews identified at each stage of the search process. Our database searches identified 910 records, of which 314 were duplicates. We identified four additional papers from searching the references of included reviews. Of the 600 records, we excluded 577 after screening titles and abstracts. We assessed the full texts of the remaining 23 reviews, and excluded another 12. This left 11 reviews for potential inclusion. We excluded an additional three reviews following the ROBIS assessment (Table 2; Appendix 3).
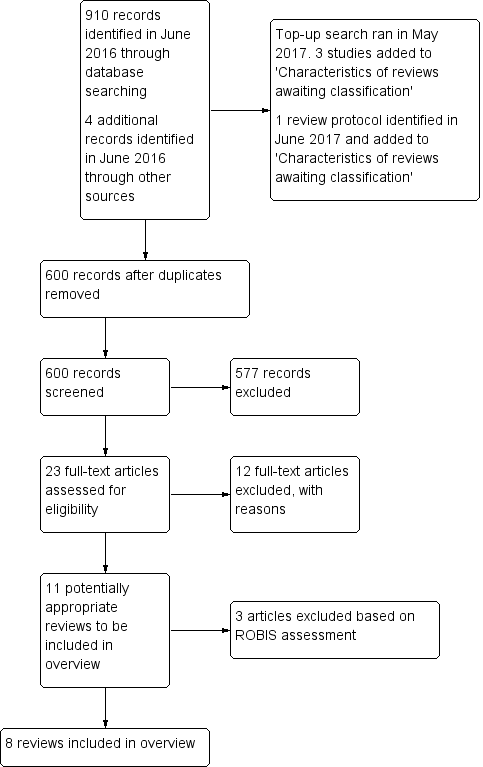
Study flow diagram.
| Reason for exclusion | Reviews excluded (n = 15) |
| Does not include RCTs including parallel‐group, cluster, or factorial RCTs | |
| Does not include studies that include patients presenting to primary care with acute respiratory infection | |
| Does not include interventions aimed at health professional with the primary goal of reducing antibiotic prescribing | |
| Does not investigate the effect of the intervention on antibiotic prescribing compared to usual care or control | Andrews 2012; Arroll 2003; Rausch 2009; Schuetz 2011; Spurling 2013 |
| Duplication of included review | Schuetz 2013 (duplicate of included Cochrane review Schuetz 2012) |
| Data were not reported at an individual‐study basis. | |
| No novel coverage in addition to included Cochrane Review | Cooke 2015; Engel 2012 (both fully overlap with Aabenhus 2014 in terms of included trials) |
| Rated as high risk in ROBIS quality assessment |
RCT: randomised controlled trial
We re‐ran the search in May 2017, identifying a total of 96 new references. We selected a further three reviews for in‐depth assessment (Hu 2016; McDonagh 2016; O'Sullivan 2016). We also identified a systematic review protocol published in June 2017 that would likely meet our inclusion criteria once completed (Martinez‐Gonzalez 2017). We added these four potential new reviews of interest to a list of ‘Characteristics of studies awaiting classification’, and we will incorporate them into overview findings during the next update (Appendix 2).
Description of included reviews
A summary of the included reviews can be found in Table 3. A list of reviews, interventions, and trials that contributed to the overview is presented in Table 1.
| Review | Date assessed as up‐to‐date | Included RCTs (n)* | Population* | Intervention | Comparison intervention | Primary outcome* | Limitations |
| Around January 2014 | 6 | Patients presenting with ARI in general practice | Point‐of‐care tests including C‐reactive protein, procalcitonin, and white blood cell count | Usual care | Number of patients given antibiotic prescription at index consultation and at 28 days' follow‐up | Small number of included studies | |
| December 2002 | 5 | Patients presenting with RTI in primary care | Professional interventions in the Cochrane Effective Practice and Organisation of Care Group. To include: education materials for clinician, educational meetings, local consensus processes, educational outreach visits, local opinion leaders, patient‐mediated interventions, audit and feedback, reminders, marketing, mass media, financial interventions | Usual care or other intervention | Decision to prescribe an antibiotic or not | None reported. | |
| February 2009 | 2 | Children presenting with URTI in primary care | Computerised evidence‐based decision support, development of clinical practice guidelines, and educational materials for clinician | Usual care | Antibiotic prescribing and reduced proportion of prescribed antibiotic courses | Potential for publication bias in search. Cost‐effectiveness not evaluated. Insufficient information to judge risk of bias in some areas | |
| December 2014 | 9 (reported in 10 papers) | Patients presenting with ARI in primary care | Shared decision making | Usual care or other intervention | Prescription of antibiotics | Intraclass correlation was imputed for 2 studies. | |
| April 2014 | 2 | Patients presenting with ARI in general practice | Patient information leaflets (PIL) for use by clinicians in consultations | No PIL | Antibiotic prescribing | Studies were at high risk of bias as blinding to the intervention was not possible. Patients included and outcomes assessed were diverse. No heterogeneity analysis was conducted. | |
| Around July 2014 | 3 | Children presenting with ARI in the emergency department | Rapid viral diagnosis by testing | No test or test result not made known to clinician | Antibiotic prescribing rate | None reported. | |
| June 2013 | 6 (reported in 7 papers) | Patients presenting with ARI in general practice | C‐reactive protein point‐of‐care test | No test or usual care, or both | Antibiotic prescribing at index consultation | Meta‐analysis is based on aggregate data rather than individual‐participant data, so difficult to explore sources of heterogeneity. | |
| 2011 | 9 | Adults presenting with ARI in primary care | Strategies to initiate or discontinue antibiotic therapy based on procalcitonin cut‐off ranges | No use of procalcitonin | Initiation of antibiotics | Variation in patient populations/settings and treatment failure was defined differently for each context. |
*relevant to this overview
ARI: acute respiratory infection
RCT: randomised controlled trial
RTI: respiratory tract infection
URTI: upper respiratory tract infection
Two reviews focussed specifically on interventions used in emergency departments, whilst the remainder focussed on general practice or family practice depending on the countries included. Nearly all trials were undertaken in high‐income countries, in particular Europe and North America, with the remainder in China. Both trials from China focussed on the use of procalcitonin‐guided therapy in the emergency department. Most reviews and their included trials were conducted in the last 10 years; the oldest review was dated 2005, and the oldest trial 1995. The majority of trials were carried out between 2003 and 2010.
Some trials were reported in more than one review (Table 1). Trials by Cals and colleagues, Cals 2009, Cals 2010, Cals 2013, appeared in two or more reviews (Aabenhus 2014; Coxeter 2015; Huang 2013). Little 2013 also appeared in Aabenhus 2014 and Coxeter 2015. Cals 2009 and Little 2013 both tested the effectiveness of two types of intervention on antibiotic prescribing: CRP testing and communication skills training for the clinician. As such, results about the effectiveness of different trial arms appear in reviews on CRP and on shared decision making. Due to the overlap in trials between Aabenhus 2014 and Huang 2013, only the three additional trials reported in Huang 2013 are discussed in addition to the results of Aabenhus 2014. The trial by Francis 2009 appears both in de Bont 2015 and Coxeter 2015, which consider the use of patient information leaflets by clinicians and shared decision making. The interactive aspect of the intervention by Francis 2009 is discussed, and its novelty in comparison to other patient information leaflets which are not used interactively is noted.
Point‐of‐care tests
Four of the eight included reviews assessed the use of a POCT as an intervention to change clinician antibiotic prescribing. Two assessed CRP testing, most often used as a near patient test which provided a result within minutes. One assessed rapid viral diagnostic testing in the emergency department (ED), where results were available during a patient's stay in the ED (within hours). Another assessed procalcitonin‐guided management, where results were also available within hours.
Aabenhus 2014 searched six databases up to January 2014 and included six trials, all of which tested the effectiveness of CRP point‐of‐care testing on antibiotic prescribing for ARIs. The trials included 3284 participants; five trials included only adults (older than 17 years), and one trial included both adults and children (Diederichsen 2000). All trials were carried out in European general practice. The trials included three RCTs and three cluster‐RCTs.
Huang 2013 searched two databases and included 13 studies, seven RCTs and six observational studies, to explore the effectiveness of CRP testing in the management of respiratory tract infections (RTIs) in general practice. We extracted only data from randomised trials for this overview: four were RCTs and three were cluster‐RCTs. All trials were carried out in European general practice, except for one carried out in the USA (Gonzales 2011). The trials included a total of 2570 participants; six of the trials included adults only, and one included both adults and children (Diederichsen 2000).
Four trials assessed CRP testing and were identified by both Aabenhus 2014 and Huang 2013.
Four trials, of which three were RCTs (one quasi‐RCT was not included), assessed rapid viral diagnostics in the management of children (aged less than 18 years) with ARI in the emergency department: Bonner 2003 recruited patients aged 2 to 21 years who presented to an ED in the USA with fever and ARI (n = 391); Poehling 2006 recruited patients age less than 5 years presenting with RTI in a US ED (˜20% of patients were at high risk from asthma; 5% had a pre‐diagnosis that required exclusion from the analysis (n = 300)); Doan 2009 recruited patients aged 3 to 36 months presenting in an ED in Canada with febrile ARI (n = 200).
Schuetz 2012 searched three databases up to May 2011 and included 14 RCTs assessing procalcitonin in EDs, hospital wards, and intensive care units. We extracted data for nine of the 14 trials undertaken in outpatient settings, and included 3613 adult patients aged ≥ 18 years. Two trials were undertaken with patients with ARI in general practice in Switzerland and Germany (Briel 2008; Burkhardt 2010); the remaining seven took place in the ED. Of the seven which took place in the ED, four trials were conducted in Switzerland (Christ‐Crain 2004; Christ‐Crain 2006; Schuetz 2009; Stolz 2007), two in China (Long 2009; Long 2011), and one in Denmark (Kristoffersen 2009). Three included participants with lower respiratory tract infections (LRTIs) (some studies with X‐ray confirmation) (Christ‐Crain 2004; Kristoffersen 2009; Schuetz 2009); three included participants with community‐acquired pneumonia (CAP), with some participants undergoing X‐ray confirmation (Christ‐Crain 2006; Long 2009; Long 2011); and one included participants with COPD exacerbation (Stolz 2007).
Doan 2014 and Schuetz 2012 both included trials that recruited patients with chronic and more serious ARIs or complications (CAP, asthma, and COPD). However, we assumed that these trials focussed on ARIs because their aim was to reduce antibiotic prescribing, therefore we included them in this overview.
Communication strategies
Shared decision making
Coxeter 2015 searched four databases up to December 2014 and included 10 reports of nine original RCTs. All trials explored the effect of interventions that aim to facilitate shared decision making (SDM) on antibiotic prescribing for ARIs in primary care. Interventions were included if they explicitly stated that they incorporated SDM, or if the intervention included one of the elements of SDM described by Makoul 2006. The trials included a total of 490,083 participants; all trials were carried out in European general practice, except for two trials conducted in Canada (Légaré 2011; Légaré 2012); all trials were cluster‐RCTs with the unit of randomisation as the individual general practitioner or practice group; four trials included patients of any age, while four included only adults, and one only children. Trials included patients with ARI, LRTI (and upper respiratory tract infection (URTI)), and acute cough.
Patient information leaflets used by clinicians in consultations
de Bont 2015 searched two databases up to April 2014 and included eight studies (seven RCTs, one non‐RCT) assessing the effect of patient information leaflets on antibiotic use and reconsultation; two trials undertaken in UK general practice were relevant because they measured clinician antibiotic prescribing: Francis 2009 tested the effectiveness of an interactive patient booklet on management of children with acute RTI (n = 558); Macfarlane 1997 tested the effectiveness of a patient information leaflet on management of adults (aged more than 15 years) with acute LRTI (n = 1014).
Educational materials for clinician and reminders
Boonacker 2010 searched three databases up to February 2009 and included 10 studies, all of which assessed interventions to promote evidence‐based practice for the management of children with URTI, which we regarded as largely synonymous with ARIs. The review included RCTs, non‐RCTs, and controlled before‐and‐after studies. Two RCTs were relevant to this overview: Christakis 2001 assessed a computerised decision support system on clinicians’ management of acute otitis media in a paediatric practice in the USA; Wilson 2002 studied the effect of collaborative development of guidelines and educational materials on clinicians’ management of children aged less than 2 years with ARI in general practice in Australia. In both trials the intervention was targeted at individual clinicians, and patient numbers were not reported.
Multifaceted interventions (multiple interventions used within one approach)
Arnold 2005 searched three databases up to May 2000 and December 2002 and included 39 studies assessing professional interventions, as defined by the Cochrane Effective Practice and Organisation of Care Group (EPOC), to improve the selection, dose, and duration of antibiotics prescribed in the outpatient setting. The review included RCTs, non‐RCTs, interrupted time series analysis, and controlled before‐and‐after studies. Five RCTs were relevant to this overview: three included only children (Finkelstein 2001; Flottorp 2002; Mainous 2000), and two included patients aged more than 15 years or aged more than 3 years (McIsaac 1998; McIsaac 2002). Two trials were undertaken in primary care settings in the USA (Finkelstein 2001; Mainous 2000), two in family practice in Canada (McIsaac 1998; McIsaac 2002), and one in general practice in Norway (Flottorp 2002). Both Canadian trials included interventions with printed educational materials for the clinician and reminders (McIsaac 1998; McIsaac 2002). Mainous 2000 included patient educational materials and audit and feedback for the clinician. Flottorp 2002 included clinician educational materials, reminders, patient education materials, computer decision support, and opportunities to gain continued professional development credit, and increased the price of telephone consultations. Finkelstein 2001 included clinician educational materials, audit and feedback, patient educational materials, and input from local opinion leaders. The number of patients seen within trials was not reported.
Methodological quality of included reviews
Quality of included reviews
Three reviews (all Cochrane Reviews) scored low risk across all of the ROBIS domains in Phase 2 and low risk of bias overall (Aabenhus 2014; Doan 2014; Schuetz 2012). The remaining five reviews scored high risk on Domain 4 of Phase 2, specifically on point 4.6 (whether biases in primary studies were minimal or addressed in the synthesis, because the 'Risk of bias' assessment had not been specifically considered and discussed in the review Results and Conclusions) (Table 1) (Arnold 2005; Boonacker 2010; Coxeter 2015; de Bont 2015; Huang 2013).
Quality of evidence in included reviews
Risk of bias of individual trials
Review authors assessed the risk of bias of trials within reviews using the Cochrane 'Risk of bias' tool (Higgins 2011); the results are presented in Table 4. One review used EPOC criteria instead, which is available on the EPOC website as "suggested risk of bias criteria" (epoc.cochrane.org/epoc‐specific‐resources‐review‐authors) (Arnold 2005). This mostly replicated the Cochrane 'Risk of bias' tool, except that it did not assess random sequence generation, blinding of participants, or selective reporting of data.
| Review ID | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| (6 RCTs) | 5 studies* (83%1) | 1 study (17%) | None (0%) | 5 studies*2 (83%) | 6 studies (100%) | 4 studies (67%) | 1 study (17%) |
| (5 RCTs) | Not reported | 1 study (20%) | Not reported | 3 studies (60%) | 2 studies (20%) | Not reported | Not reported |
| (2 RCTs) | 2 studies (100%) | 1 study (50%) | 2 studies (100%) | 2 studies (100%) | 2 studies (100%) | 2 studies (100%) | 1 study (34%) |
| (10 RCTs2) | 10 studies (100%) | 7 studies (70%) | 1 study (10%) | 7 studies (70%) | 9 studies (90%) | 10 studies (100%) | 7 studies (70%) |
| (2 RCTs) | 1 study (50%) | 2 studies (100%) | None (0%) | 1 study (50%) | 2 studies (100%) | 2 studies (100%) | Not reported |
| (3 RCTs) | 3 studies (100%) | 1 study (34%) | None (0%) | None (0%) | 2 studies (67%) | 3 studies (100%) | 3 studies (100%) |
| (7 studies3) | 1 study* (14%) | 1 study (14%) | None (0%) | None* (0%) | 5 studies (71%) | 7 studies (100%) | None (0%) |
| (9 RCTs) | 7 studies (78%) | 4 studies (33%) | None (0%) | 4 studies (33%) | 9 studies (100%) | 9 studies (100%) | 3 studies (43%) |
| RCT: randomised controlled trial | |||||||
*Results differ between reviews where RCTs had significant overlap. Aabenhus 2014 provided more detailed reporting of risk of bias than Huang 2013.
1Percentages report the proportion of RCTs within each review judged as at low risk of bias on the relevant item.
2Aabenhus 2014 reported blinding for primary outcome of antibiotic prescribing separately to blinding for other outcomes.
3Coxeter 2015 and Huang 2013 reported results for 10 and 7 publications of 9 and 6 original RCTs, respectively.
Trials within reviews were generally scored as at low risk of bias on random sequence generation, incomplete outcome data, and selective reporting. Allocation concealment and blinding of outcome assessment were more often judged to be at unclear or high risk of bias, and blinding of participants was infrequently reported due to the nature of the interventions being delivered, and was thus judged as at high risk of bias. Review authors did not often report risk of other bias, or they reported that information from individual trials was unclear. Aabenhus 2014 and Huang 2013 included four trials that appeared in both reviews. However, the 'Risk of bias' assessment reported in each review was distinctly different for random sequence generation and blinding of outcome assessment. Additional information about 'Risk of bias' assessment in Huang 2013 was not available. However, Aabenhus 2014 (a Cochrane Review) provided more detail and assessed the risk of bias for each trial.
GRADE assessments for each comparison/outcome
Only three included reviews (all Cochrane Reviews) used GRADE criteria to summarise the quality of evidence for each of their outcomes (Table 5) (Aabenhus 2014; Coxeter 2015; Schuetz 2012). Aabenhus 2014 reported that evidence for the effect of CRP testing on four outcomes was of moderate quality as assessed by the GRADE criteria. Evidence was downgraded primarily based on imprecision of the estimated effect. Coxeter 2015 and Schuetz 2012 reported that the evidence for the effect of shared decision‐making interventions and procalcitonin‐guided management on antibiotic prescribing was also of moderate quality. Evidence was downgraded primarily due to high risk of bias in included trials.
| Interventions to reduce antibiotic prescribing in the management of acute respiratory infections for patients presenting in primary care | |||||||
| Outcome | Intervention and comparison intervention | Contributing reviews | Relative effect (95% CI) of an antibiotic being prescribed1 | Number of participants (RCTs) | Quality of the evidence (GRADE)* | Comments | |
| Change in antibiotic prescriptions for ARI (at consultation) | CRP point‐of‐care test / usual care | RR 0.78 (0.66 to 0.92) | 3284 (6) | Moderate6 | CRP point‐of‐care testing probably reduces antibiotic prescribing in general practice compared to usual care. However, CRP testing may have little or no effect on prescribing in emergency departments. | ||
| General practice setting (individual trials reported): RR 0.57 (0.44 to 0.74) RR 0.58 (0.45 to 0.74) Emergency department setting: RR 1.23 (0.76 to 1.99) | 330 (1) 379 (1) 131 (1) | Moderate3* Low3,6* | |||||
| Rapid viral diagnosis / usual care | RR 0.86 (0.61 to 1.22) | 891 (3) | Low3,6* | Rapid viral diagnosis may have little or no effect on antibiotic prescribing compared to usual care. | |||
| Procalcitonin‐guided management / usual care | General practice setting: adjusted OR 0.10 (0.07 to 0.14) Emergency department setting: adjusted OR 0.34 (0.28 to 0.43) | 1008 (2) 2605 (7) | Moderate3 Moderate3 | Procalcitonin‐guided management probably reduces antibiotic prescribing in general practice and the emergency department compared to usual care. | |||
| Clinician education and decision support / usual care | Difference in behaviour change ‐12% (0.095) OR 0.60 (0.43 to 0.83) | Not reported (1) Not reported (1) | Very low3,5,6* | We are uncertain about whether clinician education and decision support reduces antibiotic prescribing compared to usual care. | |||
| Patient information leaflets / usual care | RR 0.47 (0.36 to 0.64) RR 1.15 (0.89 to 1.48) | 558 (1) 1014 (1) | Very low3,4,6* | We are uncertain as to whether patient information leaflets reduce antibiotic prescribing compared to usual care. | |||
| Shared decision making / usual care | No pooled analysis of all trials: OR 0.44 (0.26 to 0.75) RR 0.64 (0.49 to 0.84) adjusted risk difference ‐18.44 (‐27.24 to ‐9.65) | 3274 (3) 4623 (2) 481,807 (4) | Moderate3 | Shared decision making probably reduces antibiotic prescribing compared to usual care. | |||
| Multifaceted interventions | |||||||
| Printed educational materials for clinicians and reminders / usual care | Individual trials reported: OR 0.44 (0.21 to 0.92) OR 0.57 (0.27 to 1.17) | Not reported (1) Not reported (1) | Very low3,4,6* | We are uncertain as to whether printed educational materials reduce antibiotic prescribing compared to usual care. | |||
| Audit and feedback (with patient education materials) / usual care | Difference in behaviour change: ‐7.3% (audit and feedback alone) Difference in behaviour change: ‐7.2% (audit and feedback with patient materials) | Not reported (1) | Low3,6* | Audit and feedback may reduce antibiotic prescribing compared to usual care. | |||
| Educational materials and educational meetings for clinician and patient education materials / usual care | Difference in behaviour change: 16% (8% to 23%) (age 3 to 36 months) Difference in behaviour change: 12% (2% to 21%) (age 36 to 72 months) | Not reported (1) | Low3,6* | Educational materials and meetings may reduce antibiotic prescribing compared to usual care. | |||
| Educational materials, computerised decision support, professional development, financial incentive, and patient education materials / usual care | Difference in behaviour change: ‐3.0% (P = 0.03) | Not reported (1) | Low3,6* | These interventions may slightly reduce antibiotic prescribing compared to usual care. | |||
| Change in antibiotic prescriptions for ARI (within 28 days of consultation) | CRP point‐of‐care test / usual care | RR 0.80 (0.67 to 0.96) | 3284 (6) | Moderate6 | CRP point‐of‐care testing probably reduces antibiotic prescribing for up to 28 days following consultation compared to usual care. | ||
| Adverse events | CRP point‐of‐care test / usual care | RR 2.45 (0.65 to 9.19) | 4264 (1) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in adverse events compared to usual care. | ||
| Symptom duration or severity | CRP point‐of‐care test / usual care | At 7 days: RR 1.03 (0.93 to 1.14) At 28 days: RR 0.94 (0.69 to 1.28) | 1309 (3) 849 (3) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in symptom duration or severity compared to usual care. | ||
| Health‐related quality of life | Procalcitonin‐guided management / usual care | Adjusted difference in days 0.05, ‐0.46 to 0.56, P = 0.854 | 1008 (2) | Moderate3 | Procalcitonin‐guided management probably results in little or no difference in health‐related quality of life compared to usual care. | ||
| Patient satisfaction | CRP point‐of‐care test / usual care | RR 0.79 (0.57 to 1.08) | 689 (2) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in patient satisfaction compared to usual care. | ||
| Shared decision making / usual care | RR 0.86 (0.57 to 1.30) | 1110 (2) | Low3,6 | Shared decision making may result in little or no difference in patient satisfaction compared to usual care. | |||
| Management failure – reconsultation and treatment failure | CRP point‐of‐care test / usual care | RR 1.08 (0.93 to 1.27) | 5132 (4) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in reconsultation compared with usual care at 28 days' follow‐up. | ||
| Shared decision making / usual care | RR 0.87 (0.74 to 1.03) | 1860 (4) | Moderate3 | Shared decision making probably results in little or no difference in reconsultation compared to usual care. | |||
| Patient information leaflets / usual care | Individual trials reported: RR 0.80 (0.52 to 1.21) RR 0.70 (0.53 to 0.91) | 558 (1) 1014 (1) | Very low3,4,6* | We are uncertain as to whether patient intervention leaflets result in a difference in reconsultation compared to usual care. | |||
| Rapid viral diagnosis / usual care | RR 0.86 (0.59 to 1.25) | 200 (1) | Low3,6* | Rapid viral diagnostics may result in little or no difference in reconsultation relative to usual care. | |||
| Procalcitonin‐guided management / usual care | Treatment failure in general practice2: adjusted OR 0.95 (0.73 to 1.24) Treatment failure in emergency department2: adjusted OR 0.76 (0.61 to 0.95) | 1008 (2) 2605 (7) | Moderate3 | Procalcitonin‐guided management probably results in little or no difference in treatment failure in general practice compared to normal care. Procalcitonin‐guided management probably reduces treatment failure in the emergency department compared to usual care. | |||
| GRADE quality of evidence and definitions High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: Any estimate of effect is very uncertain. *GRADE criteria were applied retrospectively to outcomes when GRADE was not used by the original review authors. ARI: acute respiratory infection; CI: confidence interval; CRP: C‐reactive protein; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | |||||||
1Effect estimates are shown as reported in the original reviews. Multiple effect estimates are reported for some outcomes when reviews did not pool data from trials but reported individual trials separately.
2Schuetz 2012 defined treatment failure in primary care as death, hospitalisation, ARI‐specific complications (e.g. empyema for lower ARIs, meningitis for upper ARIs), recurrent or worsening infection, and still having ARI‐associated discomfort at 30 days. Treatment failure in the emergency setting was defined as death, intensive care unit admission, rehospitalisation after index hospital discharge, ARI‐associated complications (e.g. empyema or acute respiratory distress syndrome for lower ARIs), and recurrent or worsening infection within 30 days of follow‐up.
3Quality of evidence was downgraded one level because of risk of bias: inadequate methods of sequence generation, lack of allocation concealment, and/or lack of blinding of participants.
4Quality of evidence was downgraded one level because of inconsistency: heterogeneity in results likely due to differences in the interventions trialled across studies.
5Quality of evidence was downgraded one level because of risk of publication bias: review only reported effective interventions.
6Quality of evidence was downgraded one level due to imprecision because trials included relatively few patients or when the confidence interval showed substantial variation in the effect of the intervention.
We used GRADE to summarise the quality of the evidence for each type of intervention and each primary outcome for the remaining reviews (Table 5) (Guyatt 2008; Higgins 2011). We assessed the quality of evidence for outcomes reported by Doan 2014 as low, downgrading because of high risk of bias in included trials due to lack of allocation concealment and lack of blinding of participants, and due to imprecision as a result of wide confidence intervals. We assessed three of the trials reported in Huang 2013 individually due to overlap with Aabenhus 2014. We assessed two trials, undertaken in general practice, to be of moderate quality, downgrading because of high risk of bias due to inadequate methods of sequence generation, lack of allocation concealment, and lack of blinding of participants. We downgraded the third trial, carried out in the ED, because of high risk of bias and imprecision as a result of wide confidence intervals and small sample size. We assessed the quality of evidence for outcomes reported by Boonacker 2010 as very low, downgrading because of high risk of bias in included trials, reported publication bias, and imprecision due to sample size not being reported. We assessed the quality of evidence for outcomes reported by de Bont 2015 and Arnold 2005 as low or very low, downgrading because of high risk of bias in included trials, inconsistency in results, and imprecision due to wide confidence intervals and sample size not being reported (Table 5).
Effect of interventions
A summary of results is presented in Table 5.
Subgroup analyses were not possible due to the heterogeneity of included reviews and the data available.
Change in antibiotic prescriptions for ARI
Point‐of‐care tests
CRP testing
Aabenhus 2014 and Huang 2013 both presented moderate‐quality evidence that CRP testing probably reduces antibiotic prescribing in general practice compared to usual care.
Aabenhus 2014 found an overall effect of CRP testing showing that antibiotic prescribing is probably decreased at the initial consultation: risk ratio (RR) 0.78, 95% confidence interval (CI) 0.66 to 0.92, 3284 participants, 6 trials, moderate‐quality evidence). Aabenhus 2014 reported that the effect of CRP testing on prescribing is probably maintained at 28 days postconsultation, meaning that patients did not receive a prescription from the same practice at a later date (RR 0.80, 95% CI 0.67 to 0.96, 3284 participants, 6 trials, moderate‐quality evidence).
Huang 2013 reported seven trials that investigated the effects of CRP testing in both general practice and EDs. We have reported the three trials that were not included in the review by Aabenhus 2014. Two trials in general practice showed that CRP testing probably led to a decrease in antibiotic prescriptions compared to usual care (Cals 2011 (RR 0.57, 95% CI 0.44 to 0.74; 330 participants) and Cals 2013 (RR 0.58, 95% CI 0.45 to 0.74; 379 participants) (moderate‐quality evidence)).
Huang 2013 presented low‐quality evidence from a single small trial showing that CRP testing may have little or no effect on antibiotic prescribing in EDs compared to usual care (RR 1.23, 95% CI 0.76 to 1.99, 131 participants, 1 trial, low‐quality evidence) (Gonzales 2011).
Rapid viral diagnostics
Doan 2014 found that rapid viral diagnostics may have little or no effect on antibiotic prescribing in the ED compared to usual care (RR 0.86, 95% CI 0.61 to 1.22, 891 participants, 3 trials, low‐quality evidence).
Procalcitonin‐guided management
Schuetz 2012 reported the effect of procalcitonin measurement on the initiation of antibiotics and found that it probably decreased antibiotic initiation in both general practice (adjusted odds ratio (OR) 0.10, 95% CI 0.07 to 0.14, 1008 participants, 2 trials, moderate‐quality evidence) and the ED (adjusted OR 0.34, 95% CI 0.28 to 0.43, 2605 participants, 7 trials, moderate‐quality evidence) compared to usual care.
Shared decision making
Coxeter 2015 reported moderate‐quality evidence showing that shared decision making probably reduces antibiotic prescribing compared to usual care. They pooled the results of trials using three sets of adjusted effect estimates as part of a sensitivity analysis: set one (OR 0.44, 95% CI 0.26 to 0.75, 3274 participants, 3 trials); set two (recalculating the adjusted RR) (RR 0.64, 95% CI 0.49 to 0.84, 4623 participants, 2 trials); and set three (risk difference (RD) ‐18.44, 95% CI ‐27.24 to ‐9.65, 481,807 participants, 4 trials). This overview reports only these analyses from Coxeter 2015, which examined the effect of shared decision making on antibiotic prescribing by the clinician. Other analyses focussed on “antibiotics prescribed, dispense or decision to use” were not extracted as these included assessing the effect of shared decision making on patient behaviour.
Patient information leaflets used by clinicians in consultations
de Bont 2015 reported very low‐quality evidence from two trials assessing the effectiveness of patient information leaflets on the antibiotic prescribing of general practitioners, one showing a subsequent reduction in prescribing (RR 0.47, 95% CI 0.36 to 0.64, 558 participants) (Francis 2009), and one showing no evidence of an effect (RR 1.15, 95% CI 0.89 to 1.48, 1014 participants) (Macfarlane 1997). We are therefore uncertain about whether patient information leaftlets reduce antibiotic prescribing compared to usual care.
Educational materials for clinicians and reminders
Boonacker 2010 reported very low‐quality evidence from two trials on the effect of computerised decision support on antibiotic prescribing (Christakis 2001; Wilson 2002), therefore we are uncertain as to whether this has an effect compared to usual care. One of the included trials, Christakis 2001, presented evidence of an increase in antibiotic prescribing by both the intervention and control groups, but suggested that the intervention had a preventive effect by avoiding further increases in the rate of antibiotic prescribing (RD ‐12%, CI not reported, P = 0.095, participant number not reported). The second included trial, Wilson 2002, reported evidence that collaborative development of guidelines and education materials resulted in a reduction in antibiotic prescribing for ARI episodes (adjusted OR 0.60, 95% CI 0.43 to 0.83, participant number not reported).
Multifaceted interventions (multiple interventions used within one approach)
Arnold 2005 reported five trials using multifaceted interventions (Finkelstein 2001; Flottorp 2002; Mainous 2000; McIsaac 1998; McIsaac 2002).
McIsaac 1998 and McIsaac 2002 reported very low‐quality evidence on the effect of printed educational materials for clinicians with or without reminders compared to usual care, meaning that we are uncertain about their effects on antibiotic prescribing compared to usual care. One trial found reduced antibiotic prescribing (OR 0.44, 95% CI 0.21 to 0.92, participant number not reported) (McIsaac 1998), while the other showed no effect (OR 0.57, 95% CI 0.27 to 1.17, participant number not reported) (McIsaac 2002).
Mainous 2000 reported low‐quality evidence that audit and feedback alone or with patient education materials may reduce clinicians' antibiotic prescribing compared to usual care. They reported an increase in antibiotic prescribing for all groups, although groups that received patient education materials (with or without audit and feedback) prescribed significantly fewer antibiotics than the control (T = 2.374, P < 0.05 (exact P value not given), participant number not reported).
Finkelstein 2001 presented low‐quality evidence that educational materials and educational meetings for clinicians with patient education materials may reduce antibiotic prescribing compared to usual care in populations aged 3 to 36 months (16%, 8% to 23%) and 36 to 72 months (12%, 2% to 21%) (participant numbers not reported).
Flottorp 2002 also presented low‐quality evidence that a multifaceted intervention containing five component interventions may slightly reduce antibiotic prescribing compared to usual care (‐3.0% compared with control, P = 0.03, participant number not reported). The intervention included education materials for the clinician, computerised decision support, professional development, financial incentives, and patient education materials.
Proportion of patients with an ARI given an antibiotic prescription for immediate use and proportion of patients with an ARI given a delayed antibiotic prescription
None of the trials in the included reviews reported the proportion of patients who were provided with antibiotic prescriptions for immediate or delayed use.
Proportion of patients with an ARI colonised or infected with antibiotic‐resistant bacteria
None of the trials in the included reviews reported the proportion of patients colonised or infected with antibiotic‐resistant bacteria.
Adverse events
Only two of the eight reviews reported on adverse events.
Aabenhus 2014 reported moderate‐quality evidence that CRP point‐of‐care testing probably results in little or no difference in adverse events compared to usual care. One of the trials in this review, Little 2013, found evidence of increased hospitalisation in patients for the trial arm using the CRP test (crude RR 2.53, 95% CI 1.13 to 5.66), although after adjusting for the trial’s cluster design, the difference was not significant (RR 2.45, 95% CI 0.65 to 9.19, 4264 participants, moderate‐quality evidence). Aabenhus 2014 reported no deaths in any of the six trials evaluating CRP testing.
Coxeter 2015 reported that six of the nine trials evaluating shared decision making reported serious adverse events requiring hospitalisation, but the review authors reported that there was no difference between intervention and control groups. One trial reported a death due to myocardial infarction following pneumonia for an elderly patient in the control arm of the trial (Briel 2006). No trials reported on all‐cause mortality.
Symptom duration or severity
Aabenhus 2014 was the only review to report on symptom duration or severity, from three trials evaluating CRP testing (either as a median symptom duration to full recovery, or resolution of symptoms rated moderately bad or worse) (Cals 2009; Cals 2010; Little 2013). The review authors presented moderate‐quality evidence that CRP point‐of‐care testing probably results in little or no difference in symptom duration or severity compared to usual care at seven days (RR 1.03, 95% CI 0.93 to 1.14, 1309 participants, 3 trials) or at 28 days (RR 0.94, 95% CI 0.69 to 1.28, 849 participants, 3 trials).
Health‐related quality of life
Schuetz 2012 was the only review to report on health‐related quality of life of participants taking part in a trial of procalcitonin on prescribing, finding moderate‐quality evidence that procalcitonin‐guided management probably results in little or no difference in days of restricted activities after 14 days (adjusted difference in days 0.05, 95% CI ‐0.46 to 0.56, P = 0.854, 1008 participants, 2 trials, moderate‐quality evidence).
Patient satisfaction
Three reviews reported on patient satisfaction. Aabenhus 2014 and Huang 2013 reported the same two trials evaluating CRP testing (Cals 2009; Cals 2010), finding moderate‐quality evidence that CRP point‐of‐care testing probably results in little or no difference in patient satisfaction compared to usual care (RR 0.79, 95% CI 0.57 to 1.08, 689 participants, 2 trials, moderate‐quality evidence). Coxeter 2015 reported patient satisfaction for two trials evaluating shared decision making, finding that shared decision making may result in little or no difference in patient satisfaction compared to usual care (RR 0.86, 95% CI 0.57 to 1.30, 1110 participants, 2 trials, low‐quality evidence).
Management failure
This outcome was most often reported as reconsultation for the same illness episode. Aabenhus 2014 found CRP point‐of‐care testing probably results in little or no difference in reconsultation compared with usual care at 28 days' follow‐up (RR 1.08, 95% CI 0.93 to 1.27, 5132 participants, 4 trials, moderate‐quality evidence). Likewise, Coxeter 2015 found that shared decision making probably results in little or no difference in reconsultation for the same illness compared to usual care (RR 0.87, 95% CI 0.74 to 1.03, 1860 participants, 4 trials, moderate‐quality evidence). de Bont 2015 reported very low‐quality evidence from two trials that measured reconsultations for interventions with patient information leaflets. One trial showed evidence of a reduction in reconsultation in patients who had received the intervention (RR 0.70, 95% CI 0.53 to 0.91, 1014 participants) (Macfarlane 1997); however, the second trial showed no evidence of an effect (RR 0.80, 95% CI 0.52 to 1.21, 558 participants). We are therefore uncertain about the effect of patient intervention leaflets on reconsultation compared to usual care (Francis 2009). Doan 2014 reported low‐quality evidence from one trial on the effect of rapid viral diagnosis on doctors’ visits within two weeks of discharge following patients’ visits to the ED. The review authors found that testing may have little to no effect on reconsultation compared to usual care (RR 0.86, 95% CI 0.59 to 1.25, 200 participants, 1 trial, low‐quality evidence) (Doan 2009).
Schuetz 2012 found moderate‐quality evidence that procalcitonin‐guided management probably results in little or no difference in treatment failure in general practice compared to normal care (adjusted OR 0.95, 95% CI 0.73 to 1.24, 1008 participants, 2 trials, moderate‐quality evidence). For patients seen in the ED, there was moderate‐quality evidence that procalcitonin‐guided management probably reduces treatment failure in the ED compared to usual care (adjusted OR 0.76, 95% CI 0.61 to 0.95, 2605 participants, 7 trials, moderate‐quality evidence).
Management costs for any medication for the treatment of an ARI or associated complications
None of the trials in the included reviews reported on management costs for the treatment of an ARI or any associated complications.
Discussion
Summary of main results
This overview identified eight reviews assessing clinician‐focussed interventions to influence antibiotic prescribing for ARIs in primary care. There was moderate‐quality evidence indicating that point‐of‐care CRP testing (two reviews, nine trials), procalcitonin‐guided management (one review, nine trials), and shared decision making (one review, nine trials) probably safely reduce antibiotic prescribing in the management of ARIs compared to usual care.
The overall effect of these interventions was small (few achieving greater than 50% reduction in antibiotic prescribing, most about a quarter or less), but is likely to be clinically important. The interventions we have reported likely influence different mechanisms of behaviour change, so it is possible that combining interventions will result in greater effects.
For the other interventions, including multifaceted interventions, those centred on clinician education, patient information leaflets, and the use of rapid viral diagnostics, the evidence was of low or very low quality across outcomes, and we could not confidently draw any conclusions about the effects of these interventions compared to usual care. Further primary research is necessary to improve the evidence base in order to be able to make informed decisions about the value of these interventions.
None of the trials in the included reviews reported on management costs for the treatment of an ARI or any associated complications.
Overall completeness and applicability of evidence
The inclusion of both Cochrane and non‐Cochrane reviews provides a comprehensive summary of all eligible systematic reviews. The reviews reported on a wide range of interventions, although for each intervention there were few reviews. Most reviews addressed the effects of diagnostic tests on antibiotic prescribing. One of the included reviews investigated multifaceted interventions containing multiple components, including clinician and patient education, audit and feedback, and reminders. Since no review studied these interventions alone, the separate effects of each are unknown.
The descriptions of point‐of‐care diagnostics and shared decision making interventions were detailed enough to enable replication in clinical practice. The use of CRP testing and shared decision making in general practice was explored frequently in trials and reviews, and there appears to be sufficient evidence for policymakers and clinicians to decide whether these interventions would be useful in their own contexts. However, sufficient detail for replication was lacking for the other intervention types under the categories of clinician education, patient information leaflets, and reminders. This was reflected in the original trials. Similarly, ‘usual care’ – the control arm of most trials – was also poorly described.
There is a risk that in categorising interventions in the way that we did for this overview, we inadvertently grouped those with different underlying mechanisms of behaviour change. For example, of the two trials of patient information leaflets, effectiveness was only demonstrated when the leaflet was used “interactively” as part of the intervention. Future investigators of trials and reviews should describe interventions more comprehensively. Other review authors have further considered and classified the different components of interventions categorised in this way (Davey 2017).
It is possible that a combination of intervention types would have an additive (or even multiplicative) effect because they probably act with quite separate mechanisms of action (notwithstanding the unknown nature of these). However, ideally these should be tested separately as well as in combination.
Few trials compared interventions against one another, so we had no data to assess the relative performance of different interventions.
Although our search identified reviews of delayed‐prescribing interventions, none measured antibiotic prescribing behaviour as an outcome, which focusses on both clinicians (to change their behaviour to writing delayed, rather than immediate, prescriptions, and provide accompanying advice to patients) as well as patient behaviour (by giving them responsibility about whether or when they access antibiotics). The trials randomised patients to immediate, delayed, or no‐prescription arms, and since consumption of antibiotics was the most common primary outcome, there was no measure of only the clinicians’ behaviour.
Three of our prespecified outcomes were not reported in the contributing reviews. These outcomes were the proportion of patients receiving an antibiotic prescription for immediate or delayed use, the proportion of patients with an ARI colonised or infected with antibiotic‐resistant bacteria, and the management cost of any medication to treat an ARI or any associated complications. Delayed prescriptions are often not easily identifiable in general practice records, as they most often reflect a change in the verbal instructions given to a patient rather than a change in the issuing of the prescription; as such, it is a difficult outcome for trials to measure. Similarly, identifying antibiotic‐resistant bacteria requires lab samples, which adds complexity and cost to an RCT. The cost‐effectiveness of interventions in this area is not commonly examined, and further research in this field is needed.
Most of the trials in the included reviews were undertaken in high‐income countries, in particular countries in Europe and North America, and their findings may not be applicable in other settings.
Quality of the evidence
The reviews included in this overview were conducted to a high standard, although five of the eight reviews were marked down on one ROBIS domain because potential biases in primary studies were not considered in the interpretation of findings or in the discussion. The three reviews that scored low risk on all four ROBIS domains were all Cochrane Reviews, although a further Cochrane Review was also marked down on domain 4 of ROBIS, as were the non‐Cochrane reviews (Table 1).
The quality of evidence in included reviews was rated according to the GRADE criteria. The quality varied, with no outcomes rated as having high‐quality evidence in any included review. The most common reason for downgrading quality of evidence for each outcome was when there was judged to be a high risk of bias in the relevant trials and/or when there was imprecision in the main effect. The unavoidable lack of blinding of participants increased the risk of bias in trials. However, blinding of outcome assessment and allocation concealment was also generally not reported, which increased the risk of bias, particularly for trials investigating the effect of POCTs used by clinicians.
For the reviews in which the quality of evidence for outcomes was rated as low, evidence was downgraded by one level when there was judged to be high risk of bias, and a second level due to inconsistency in results, imprecision, or reported publication bias. Where evidence was rated as very low, evidence was downgraded another level due to imprecision in the effect estimate. The GRADE ratings for each outcome in each review are presented in Table 5, along with footnotes describing the rationale for each downgrading decision.
Potential biases in the overview process
Our methods of independent assessment of bias and data extraction (with arbitration by a third overview author) should have reduced the risk of bias in generating this overview. However, we could not formally assess the risk of publication bias in this overview because there were too few reviews, nor was this assessed in the included reviews. If studies that were narrative reviews (i.e. without quantification of the results) were more likely to be negative, our exclusion of them might have introduced publication bias.
Where reviews did not report quality of evidence according to GRADE criteria, we applied this retrospectively. This approach was limited as it used 'Risk of bias' assessments made by the review authors, and the depth of reporting between reviews varied. When reviews did report quality of evidence according to GRADE criteria, we reported the findings of the review authors.
Some overview authors are also authors on included reviews, which had the potential to introduce bias when presenting the results. However, the data extraction, narrative summary, and reporting of the overview was led by three overview authors who had no connection to previous reviews in order to minimise any bias.
Agreements and disagreements with other studies or reviews
We excluded many reviews because they did not meet our inclusion criteria. However, we are not aware of any other quantitative published overviews of reviews of clinician‐targeted interventions to reduce antibiotic prescribing for acute ARIs.

Study flow diagram.
| Intervention | Review | RCTs contributed and sample size (n) | ROBIS assessment | ||||
| Phase 2 domains | Phase 3 | ||||||
| 1. Study eligibility criteria | 2. Identification and selection of studies | 3. Data collection and study appraisal | 4. Synthesis and findings | 5. Risk of bias of review | |||
| POCT: C‐reactive protein | Andreeva 2014 (179) Cals 2009 (431) Cals 2010 (258) Diederichsen 2000 (812) Little 2013 (4264) Melbye 1995 (239) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Cals 2009 (431) Cals 2010 (258) Cals 2011 (330) Cals 2013* (379) Diederichsen 2000 (812) Gonzales 2011 (131) Melbye 1995 (229) | Low risk | Low risk | Low risk | High risk | Low risk | ||
| POCT: rapid viral detection test | Bonner 2003 (391) Doan 2009 (200) Poehling 2006 (300) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| POCT: procalcitonin | Briel 2008 (458) Burkhardt 2010 (550) Christ‐Crain 2004 (243) Christ‐Crain 2006 (302) Kristoffersen 2009 (210) Long 2009 (127) Long 2011 (156) Schuetz 2009 (1359) Stolz 2007 (208) | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Educational materials for clinician or decision support, or both | Christakis 2001 (NR) Wilson 2002 (NR) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Interventions to support shared decision making | Altiner 2007 (2164) Briel 2006 (552) Butler 2012 (479,502) Cals 2009 (431) Cals 2013* (379) Francis 2009 (558) Légaré 2011 (151) Légaré 2012 (359) Little 2013 (4264) Welschen 2004 (1723) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Patient information leaflets to be used by clinician | Francis 2009 (558) Macfarlane 1997 (1014) | Low risk | Low risk | Low risk | High risk | Low risk | |
| Multifaceted interventions | Finkelstein 2001 (NR) Flottorp 2002 (NR) McIsaac 1998 (NR) McIsaac 2002 (NR) Mainous 2000 (NR) | Low risk | Low risk | Low risk | High risk | Low risk | |
| *Cals 2013 is a follow‐up study of the trial reported in Cals 2009. NR: not reported | |||||||
| Reason for exclusion | Reviews excluded (n = 15) |
| Does not include RCTs including parallel‐group, cluster, or factorial RCTs | |
| Does not include studies that include patients presenting to primary care with acute respiratory infection | |
| Does not include interventions aimed at health professional with the primary goal of reducing antibiotic prescribing | |
| Does not investigate the effect of the intervention on antibiotic prescribing compared to usual care or control | Andrews 2012; Arroll 2003; Rausch 2009; Schuetz 2011; Spurling 2013 |
| Duplication of included review | Schuetz 2013 (duplicate of included Cochrane review Schuetz 2012) |
| Data were not reported at an individual‐study basis. | |
| No novel coverage in addition to included Cochrane Review | Cooke 2015; Engel 2012 (both fully overlap with Aabenhus 2014 in terms of included trials) |
| Rated as high risk in ROBIS quality assessment | |
| RCT: randomised controlled trial | |
| Review | Date assessed as up‐to‐date | Included RCTs (n)* | Population* | Intervention | Comparison intervention | Primary outcome* | Limitations |
| Around January 2014 | 6 | Patients presenting with ARI in general practice | Point‐of‐care tests including C‐reactive protein, procalcitonin, and white blood cell count | Usual care | Number of patients given antibiotic prescription at index consultation and at 28 days' follow‐up | Small number of included studies | |
| December 2002 | 5 | Patients presenting with RTI in primary care | Professional interventions in the Cochrane Effective Practice and Organisation of Care Group. To include: education materials for clinician, educational meetings, local consensus processes, educational outreach visits, local opinion leaders, patient‐mediated interventions, audit and feedback, reminders, marketing, mass media, financial interventions | Usual care or other intervention | Decision to prescribe an antibiotic or not | None reported. | |
| February 2009 | 2 | Children presenting with URTI in primary care | Computerised evidence‐based decision support, development of clinical practice guidelines, and educational materials for clinician | Usual care | Antibiotic prescribing and reduced proportion of prescribed antibiotic courses | Potential for publication bias in search. Cost‐effectiveness not evaluated. Insufficient information to judge risk of bias in some areas | |
| December 2014 | 9 (reported in 10 papers) | Patients presenting with ARI in primary care | Shared decision making | Usual care or other intervention | Prescription of antibiotics | Intraclass correlation was imputed for 2 studies. | |
| April 2014 | 2 | Patients presenting with ARI in general practice | Patient information leaflets (PIL) for use by clinicians in consultations | No PIL | Antibiotic prescribing | Studies were at high risk of bias as blinding to the intervention was not possible. Patients included and outcomes assessed were diverse. No heterogeneity analysis was conducted. | |
| Around July 2014 | 3 | Children presenting with ARI in the emergency department | Rapid viral diagnosis by testing | No test or test result not made known to clinician | Antibiotic prescribing rate | None reported. | |
| June 2013 | 6 (reported in 7 papers) | Patients presenting with ARI in general practice | C‐reactive protein point‐of‐care test | No test or usual care, or both | Antibiotic prescribing at index consultation | Meta‐analysis is based on aggregate data rather than individual‐participant data, so difficult to explore sources of heterogeneity. | |
| 2011 | 9 | Adults presenting with ARI in primary care | Strategies to initiate or discontinue antibiotic therapy based on procalcitonin cut‐off ranges | No use of procalcitonin | Initiation of antibiotics | Variation in patient populations/settings and treatment failure was defined differently for each context. | |
| *relevant to this overview | |||||||
| Review ID | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| (6 RCTs) | 5 studies* (83%1) | 1 study (17%) | None (0%) | 5 studies*2 (83%) | 6 studies (100%) | 4 studies (67%) | 1 study (17%) |
| (5 RCTs) | Not reported | 1 study (20%) | Not reported | 3 studies (60%) | 2 studies (20%) | Not reported | Not reported |
| (2 RCTs) | 2 studies (100%) | 1 study (50%) | 2 studies (100%) | 2 studies (100%) | 2 studies (100%) | 2 studies (100%) | 1 study (34%) |
| (10 RCTs2) | 10 studies (100%) | 7 studies (70%) | 1 study (10%) | 7 studies (70%) | 9 studies (90%) | 10 studies (100%) | 7 studies (70%) |
| (2 RCTs) | 1 study (50%) | 2 studies (100%) | None (0%) | 1 study (50%) | 2 studies (100%) | 2 studies (100%) | Not reported |
| (3 RCTs) | 3 studies (100%) | 1 study (34%) | None (0%) | None (0%) | 2 studies (67%) | 3 studies (100%) | 3 studies (100%) |
| (7 studies3) | 1 study* (14%) | 1 study (14%) | None (0%) | None* (0%) | 5 studies (71%) | 7 studies (100%) | None (0%) |
| (9 RCTs) | 7 studies (78%) | 4 studies (33%) | None (0%) | 4 studies (33%) | 9 studies (100%) | 9 studies (100%) | 3 studies (43%) |
| RCT: randomised controlled trial | |||||||
| *Results differ between reviews where RCTs had significant overlap. Aabenhus 2014 provided more detailed reporting of risk of bias than Huang 2013. | |||||||
| Interventions to reduce antibiotic prescribing in the management of acute respiratory infections for patients presenting in primary care | |||||||
| Outcome | Intervention and comparison intervention | Contributing reviews | Relative effect (95% CI) of an antibiotic being prescribed1 | Number of participants (RCTs) | Quality of the evidence (GRADE)* | Comments | |
| Change in antibiotic prescriptions for ARI (at consultation) | CRP point‐of‐care test / usual care | RR 0.78 (0.66 to 0.92) | 3284 (6) | Moderate6 | CRP point‐of‐care testing probably reduces antibiotic prescribing in general practice compared to usual care. However, CRP testing may have little or no effect on prescribing in emergency departments. | ||
| General practice setting (individual trials reported): RR 0.57 (0.44 to 0.74) RR 0.58 (0.45 to 0.74) Emergency department setting: RR 1.23 (0.76 to 1.99) | 330 (1) 379 (1) 131 (1) | Moderate3* Low3,6* | |||||
| Rapid viral diagnosis / usual care | RR 0.86 (0.61 to 1.22) | 891 (3) | Low3,6* | Rapid viral diagnosis may have little or no effect on antibiotic prescribing compared to usual care. | |||
| Procalcitonin‐guided management / usual care | General practice setting: adjusted OR 0.10 (0.07 to 0.14) Emergency department setting: adjusted OR 0.34 (0.28 to 0.43) | 1008 (2) 2605 (7) | Moderate3 Moderate3 | Procalcitonin‐guided management probably reduces antibiotic prescribing in general practice and the emergency department compared to usual care. | |||
| Clinician education and decision support / usual care | Difference in behaviour change ‐12% (0.095) OR 0.60 (0.43 to 0.83) | Not reported (1) Not reported (1) | Very low3,5,6* | We are uncertain about whether clinician education and decision support reduces antibiotic prescribing compared to usual care. | |||
| Patient information leaflets / usual care | RR 0.47 (0.36 to 0.64) RR 1.15 (0.89 to 1.48) | 558 (1) 1014 (1) | Very low3,4,6* | We are uncertain as to whether patient information leaflets reduce antibiotic prescribing compared to usual care. | |||
| Shared decision making / usual care | No pooled analysis of all trials: OR 0.44 (0.26 to 0.75) RR 0.64 (0.49 to 0.84) adjusted risk difference ‐18.44 (‐27.24 to ‐9.65) | 3274 (3) 4623 (2) 481,807 (4) | Moderate3 | Shared decision making probably reduces antibiotic prescribing compared to usual care. | |||
| Multifaceted interventions | |||||||
| Printed educational materials for clinicians and reminders / usual care | Individual trials reported: OR 0.44 (0.21 to 0.92) OR 0.57 (0.27 to 1.17) | Not reported (1) Not reported (1) | Very low3,4,6* | We are uncertain as to whether printed educational materials reduce antibiotic prescribing compared to usual care. | |||
| Audit and feedback (with patient education materials) / usual care | Difference in behaviour change: ‐7.3% (audit and feedback alone) Difference in behaviour change: ‐7.2% (audit and feedback with patient materials) | Not reported (1) | Low3,6* | Audit and feedback may reduce antibiotic prescribing compared to usual care. | |||
| Educational materials and educational meetings for clinician and patient education materials / usual care | Difference in behaviour change: 16% (8% to 23%) (age 3 to 36 months) Difference in behaviour change: 12% (2% to 21%) (age 36 to 72 months) | Not reported (1) | Low3,6* | Educational materials and meetings may reduce antibiotic prescribing compared to usual care. | |||
| Educational materials, computerised decision support, professional development, financial incentive, and patient education materials / usual care | Difference in behaviour change: ‐3.0% (P = 0.03) | Not reported (1) | Low3,6* | These interventions may slightly reduce antibiotic prescribing compared to usual care. | |||
| Change in antibiotic prescriptions for ARI (within 28 days of consultation) | CRP point‐of‐care test / usual care | RR 0.80 (0.67 to 0.96) | 3284 (6) | Moderate6 | CRP point‐of‐care testing probably reduces antibiotic prescribing for up to 28 days following consultation compared to usual care. | ||
| Adverse events | CRP point‐of‐care test / usual care | RR 2.45 (0.65 to 9.19) | 4264 (1) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in adverse events compared to usual care. | ||
| Symptom duration or severity | CRP point‐of‐care test / usual care | At 7 days: RR 1.03 (0.93 to 1.14) At 28 days: RR 0.94 (0.69 to 1.28) | 1309 (3) 849 (3) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in symptom duration or severity compared to usual care. | ||
| Health‐related quality of life | Procalcitonin‐guided management / usual care | Adjusted difference in days 0.05, ‐0.46 to 0.56, P = 0.854 | 1008 (2) | Moderate3 | Procalcitonin‐guided management probably results in little or no difference in health‐related quality of life compared to usual care. | ||
| Patient satisfaction | CRP point‐of‐care test / usual care | RR 0.79 (0.57 to 1.08) | 689 (2) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in patient satisfaction compared to usual care. | ||
| Shared decision making / usual care | RR 0.86 (0.57 to 1.30) | 1110 (2) | Low3,6 | Shared decision making may result in little or no difference in patient satisfaction compared to usual care. | |||
| Management failure – reconsultation and treatment failure | CRP point‐of‐care test / usual care | RR 1.08 (0.93 to 1.27) | 5132 (4) | Moderate6 | CRP point‐of‐care testing probably results in little or no difference in reconsultation compared with usual care at 28 days' follow‐up. | ||
| Shared decision making / usual care | RR 0.87 (0.74 to 1.03) | 1860 (4) | Moderate3 | Shared decision making probably results in little or no difference in reconsultation compared to usual care. | |||
| Patient information leaflets / usual care | Individual trials reported: RR 0.80 (0.52 to 1.21) RR 0.70 (0.53 to 0.91) | 558 (1) 1014 (1) | Very low3,4,6* | We are uncertain as to whether patient intervention leaflets result in a difference in reconsultation compared to usual care. | |||
| Rapid viral diagnosis / usual care | RR 0.86 (0.59 to 1.25) | 200 (1) | Low3,6* | Rapid viral diagnostics may result in little or no difference in reconsultation relative to usual care. | |||
| Procalcitonin‐guided management / usual care | Treatment failure in general practice2: adjusted OR 0.95 (0.73 to 1.24) Treatment failure in emergency department2: adjusted OR 0.76 (0.61 to 0.95) | 1008 (2) 2605 (7) | Moderate3 | Procalcitonin‐guided management probably results in little or no difference in treatment failure in general practice compared to normal care. Procalcitonin‐guided management probably reduces treatment failure in the emergency department compared to usual care. | |||
| GRADE quality of evidence and definitions High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: Any estimate of effect is very uncertain. *GRADE criteria were applied retrospectively to outcomes when GRADE was not used by the original review authors. ARI: acute respiratory infection; CI: confidence interval; CRP: C‐reactive protein; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | |||||||
| 1Effect estimates are shown as reported in the original reviews. Multiple effect estimates are reported for some outcomes when reviews did not pool data from trials but reported individual trials separately. | |||||||

