نقش پاراستامول (استامینوفن) در تسکین کمردرد
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, actively controlled, multicentre, single‐blind study | |
| Participants | Population source: 371 participants with acute non‐specific LBP of at least moderate pain intensity (133 participants randomised to paracetamol or placebo group). The study was conducted at 11 sites. Inclusion criteria: Pain of moderate intensity (2 or more on a 6‐point scale), age 18 to 55 years (inclusive), ambulatory status, no low back trauma within the preceding 48 hours, and an answer of “yes” to the question “Do the muscles in your low back hurt?” Exclusion criteria: Any evidence or history of radiculopathy or other neurologic deficits (e.g. abnormal straight‐leg‐raise test results, patellar reflexes, or bowel or bladder function), or a history of back surgery, fibromyalgia, diabetes mellitus, peripheral vascular disease, osteoporosis, gastrointestinal ulcers, gastrointestinal bleeding or perforation, renal disease, pulmonary oedema, cardiomyopathy, liver disease, intrinsic coagulation defects, bleeding diseases or anticoagulant therapy (e.g. warfarin), daily back pain for more than 3 consecutive months, or hypersensitivity to acetaminophen, non‐steroidal anti‐inflammatory drugs, or heat | |
| Interventions | The intervention groups consisted of
All treatments were administered on 2 consecutive days | |
| Outcomes | Primary: pain relief (NRS 0 to 5), disability (Roland Morris 0 to 24). Secondary: safety. | |
| Notes | Funding: This study was funded by the Procter & Gamble Company. Conflicts of interest: Industry funds were received to support this work. 6 out of 8 study authors are employees of the Procter & Gamble Health Sciences Institute, and 1 author is a paid consultant for Procter & Gamble Company. There is no information on the dates when the study was conducted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomized to treatment by a ratio of 6:6:6:1:1". Comment: Unclear |
| Allocation concealment (selection bias) | Unclear risk | Comment: Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "single (investigator) blind". Comment: Unclear if blinding was done for the assessor or the care provider |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "single (investigator) blind". Comment: Unclear if the assessor or the care provider was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 9/113 not evaluable for primary efficacy variable in the intervention group; 1/20 not evaluable for primary efficacy variable in the control group |
| Selective reporting (reporting bias) | High risk | Comment: The placebo group is listed in the methods, but no data for this group are reported for any time point |
| Other bias | Unclear risk | Comment: Data for the placebo group are unavailable. This study received funds from a company that produces paracetamol |
| Methods | Multicentre, double‐dummy, randomised, placebo‐controlled trial | |
| Participants | Population source: 1653 participants (acetaminophen n = 550, acetaminophen as required n = 546, placebo n = 547) recruited from primary care clinicians (general practitioners, pharmacists, or physiotherapists) across Sydney, Australia, screened consecutive people who sought care for LBP directly or in response to a community advertisement. Inclusion criteria: A new episode of acute LBP (defined as pain between the 12th rib and buttock crease that was less than 6 weeks' duration and preceded by 1 month of no pain) with or without leg pain, and at least moderate‐intensity pain (measured by an adaptation of item 7 of the SF‐36). Exclusion criteria: Suspected serious spinal pathology; currently using full regular recommended doses of an analgesic; spinal surgery in the preceding 6 months; contraindication to paracetamol; using psychotropic medication for a health condition deemed to prevent reliable recording of study information; or pregnant or planning pregnancy | |
| Interventions | Participants were asked to take 2 types of tablets for up to 4 weeks: 2 tablets from the regular box every 6 to 8 hours (6 tablets per day), and 1 or 2 tablets from the as‐needed box when needed for pain relief (4 to 6 hours apart, to a maximum of 8 tablets per day). Participants in the regular group received 665 mg modified‐release paracetamol tablets in the regular box and placebo tablets in the as‐needed box. Participants in the as‐needed group received placebo tablets in the regular box and 500 mg paracetamol immediate‐release tablets in the as‐needed box. Participants in the placebo group received placebo tablets in both boxes | |
| Outcomes | Pain (0 to 10), disability (Roland Morris 0 to 24), function (Patient‐Specific Functional Scale), quality of life (SF‐12), global impression of recovery (Global Perceived Effect scale), sleep quality, adherence, adverse events | |
| Notes | Funding: National Health and Medical Research Council of Australia and GlaxoSmithKline Australia. Conflicts of interest: One author received funding for a research scholarship There is no information on the dates when the study was conducted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation schedule". Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed random allocation"; "Research staff not involved in preparation of medicine boxes collected baseline information by telephone and instructed patients to open the box and begin treatment". Comment: Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Clinicians, participants, and staff collecting outcome data, assessing outcomes, and analysing data were masked to group allocation". Comment: Adequate |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Clinicians, participants, and staff collecting outcome data, assessing outcomes, and analysing data were masked to group allocation". Comment: Adequate |
| Incomplete outcome data (attrition bias) | Low risk | 31/1099 withdrawn from intervention groups; 15/553 withdrawn from control group. The completeness of survival data, measured by the completeness index, was 94.4% |
| Selective reporting (reporting bias) | Low risk | All outcomes that are of interest in the review have been reported |
| Other bias | Low risk | This study was an investigator‐initiated trial that received supplementary funding from a pharmaceutical company and reported that the sponsor had no role in conducting the study or analysing the data. Given this background and the negative trial outcome, this study appears to be free of other sources of bias and met this criterion |
LBP: low back pain
NRS: Numeric Rating Scale
SF‐12: 12‐Item Short Form Health Survey
SF‐36: 36‐Item Short Form Health Survey
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Combination of medications | |
| No placebo group | |
| No placebo group | |
| Combination of medications | |
| No placebo group | |
| Not RCT | |
| Not RCT | |
| Commentary | |
| Commentary | |
| Not RCT | |
| Combination of medications | |
| Combination of medications | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Not RCT | |
| No placebo group | |
| No placebo group | |
| No paracetamol group | |
| No paracetamol group | |
| No placebo group | |
| Not RCT | |
| Not RCT | |
| Combination of medications | |
| No placebo group | |
| Combination of paracetamol and other medications | |
| Combination of paracetamol and other medications | |
| Combination of paracetamol and other medications | |
| Combination of paracetamol and other medications | |
| No placebo group | |
| Combination of paracetamol and other medications | |
| Combination of paracetamol and other medications | |
| Combination of paracetamol and other medications | |
| No paracetamol group | |
| Combination of medications | |
| Combination of medications | |
| Combination of medications | |
| Not low back pain | |
| No placebo group | |
| Retracted study | |
| No paracetamol group |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 1 Pain. | ||||
| 1.1 1 week (immediate‐term) | 1 | 1520 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐1.30, 4.28] |
| 1.2 2 weeks | 1 | 1505 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.70, 3.70] |
| 1.3 4 weeks | 1 | 1516 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐1.99, 2.97] |
| 1.4 12 weeks (short‐term) | 1 | 1526 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.92, 1.92] |
| 2 Disability Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 2 Disability. | ||||
| 2.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.15, 0.25] |
| 2.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.65, 0.65] |
| 2.3 4 weeks | 1 | 1506 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.50, 0.60] |
| 2.4 12 weeks (short‐term) | 1 | 1522 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.39, 0.59] |
| 3 Quality of life, physical component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 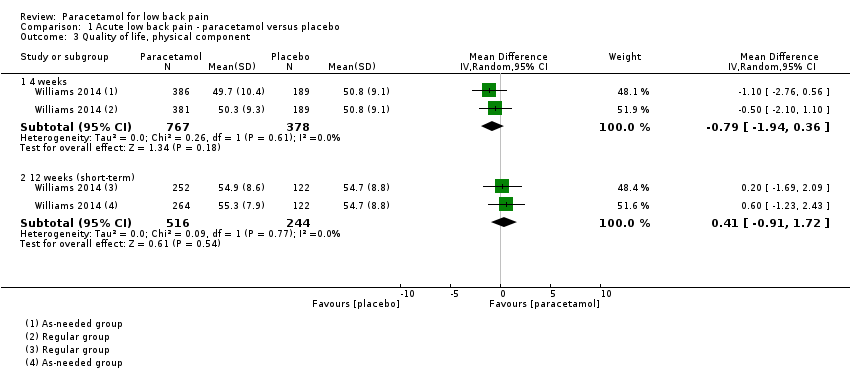 Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 3 Quality of life, physical component. | ||||
| 3.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.94, 0.36] |
| 3.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.91, 1.72] |
| 4 Quality of life, mental component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 4 Quality of life, mental component. | ||||
| 4.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.38, 0.17] |
| 4.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.08, 1.72] |
| 5 Function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 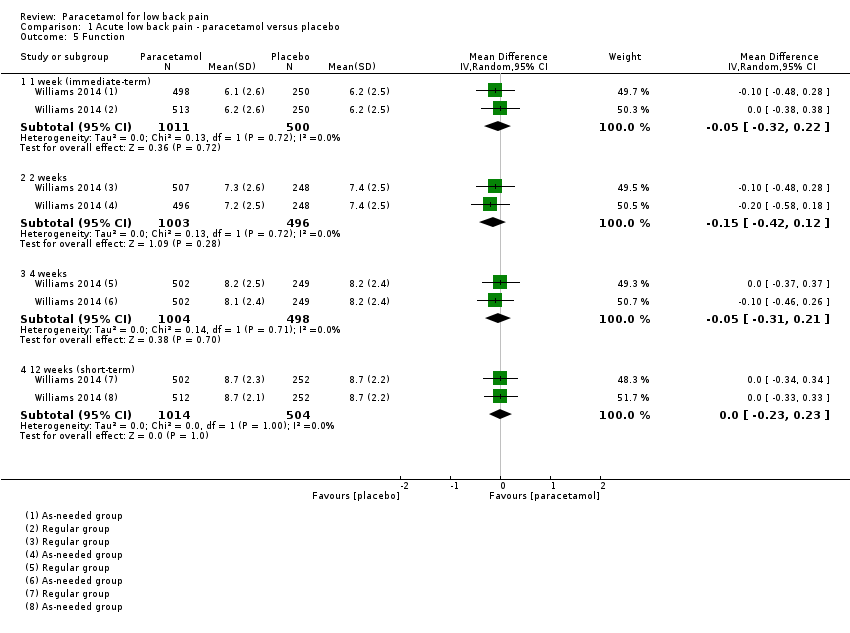 Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 5 Function. | ||||
| 5.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 5.2 2 weeks | 1 | 1499 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 5.3 4 weeks | 1 | 1502 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 5.4 12 weeks (short‐term) | 1 | 1518 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 6 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 6 Adverse events. | ||||
| 6.1 Any adverse events (up to 12 weeks) | 1 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 6.2 Serious adverse events (up to 12 weeks) | 1 | 1643 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.67] |
| 7 Global impression of recovery Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 7 Global impression of recovery. | ||||
| 7.1 1 week (immediate‐term) | 1 | 1515 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.3 4 weeks | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 7.4 12 weeks (short‐term) | 1 | 1523 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.17] |
| 8 Poor sleep quality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 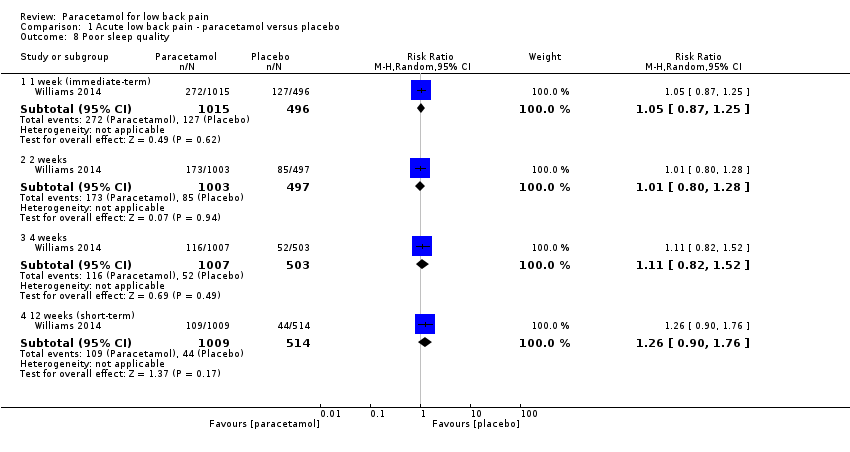 Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 8 Poor sleep quality. | ||||
| 8.1 1 week (immediate‐term) | 1 | 1511 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.25] |
| 8.2 2 weeks | 1 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 8.3 4 weeks | 1 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.52] |
| 8.4 12 weeks (short‐term) | 1 | 1523 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.90, 1.76] |
| 9 Patient adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 9 Patient adherence. | ||||
| 9.1 4 weeks | 1 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.22] |
| 10 Use of rescue medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 10 Use of rescue medication. | ||||
| 10.1 Up to 2 weeks | 1 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.16, 1.55] |

Study flow diagram.
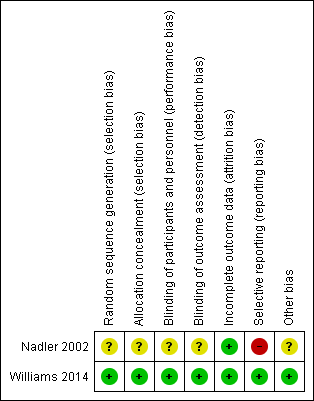
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Acute low back pain ‐ paracetamol versus placebo, outcome: 1.1 Pain.
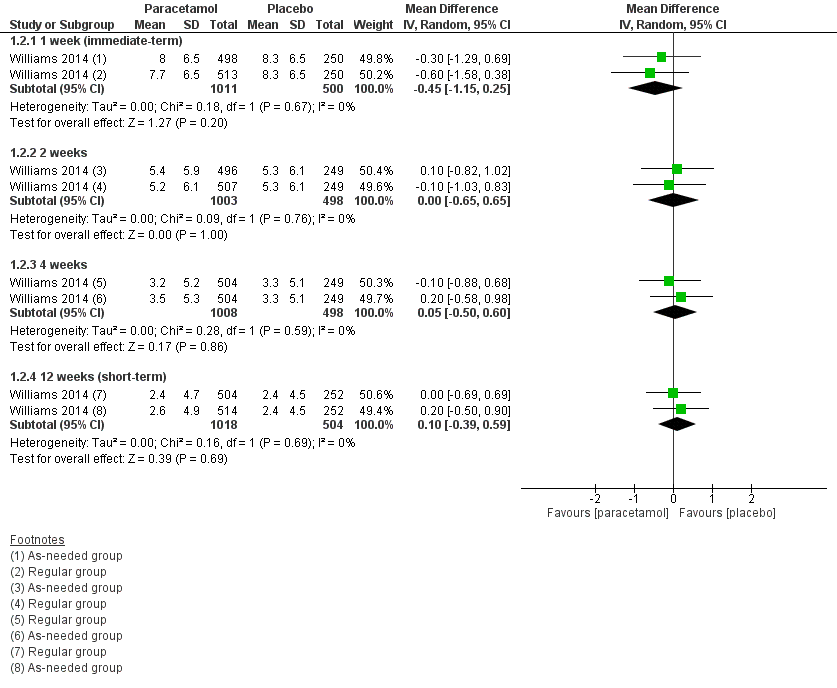
Forest plot of comparison: 1 Acute low back pain ‐ paracetamol versus placebo, outcome: 1.2 Disability
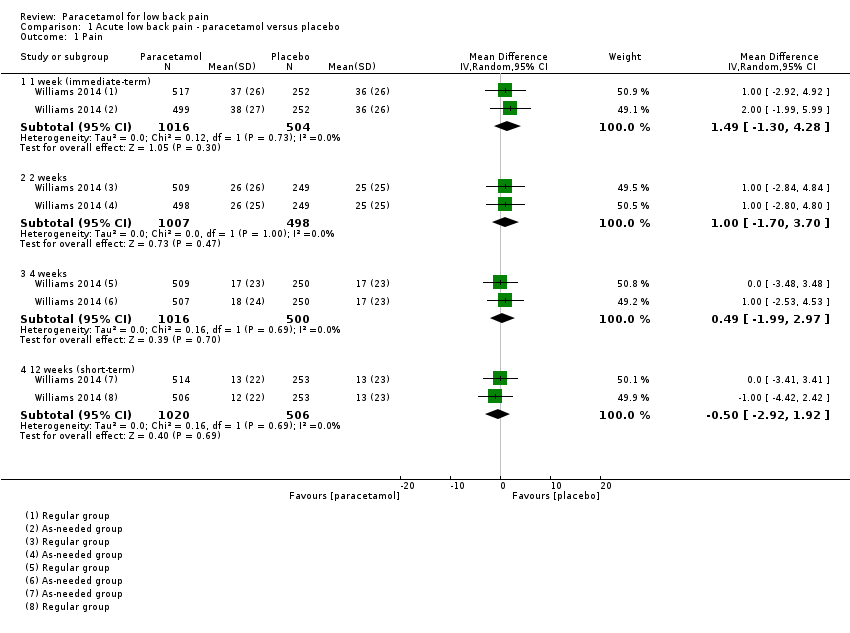
Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 1 Pain.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 2 Disability.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 3 Quality of life, physical component.
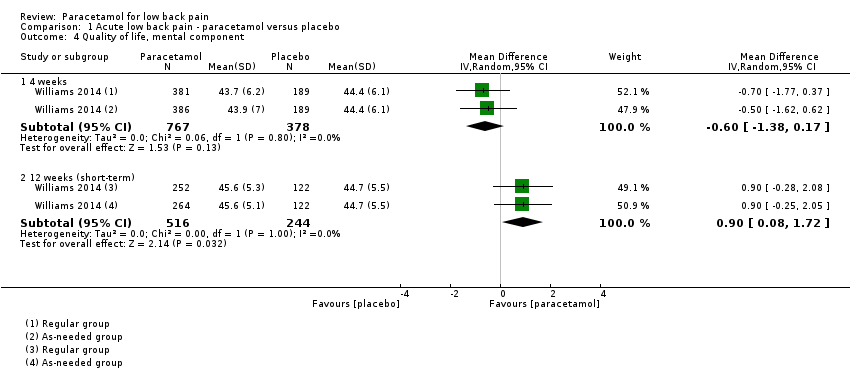
Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 4 Quality of life, mental component.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 5 Function.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 6 Adverse events.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 7 Global impression of recovery.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 8 Poor sleep quality.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 9 Patient adherence.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 10 Use of rescue medication.
| Paracetamol compared with placebo for acute low back pain | ||||||
| Patient or population: People with acute low back pain Settings: Primary care Intervention: Paracetamol Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Paracetamol | |||||
| Pain 1 week (immediate term) (NRS 0 to 100) | The mean pain in the control group was 36 points | The mean pain in the intervention group was (1.30 lower to 4.28 higher) | ‐ | 1520 | ⊕⊕⊕⊕ | The difference is not statistically |
| Pain 12 weeks (short term) (NRS 0 to 100) | The mean pain in the control group was 13 points | The mean pain in the intervention group was (2.92 lower to 1.92 higher) | ‐ | 1526 | ⊕⊕⊕⊕ | The difference is not statistically |
| Disability 1 week (immediate term) (RMDQ 0 to 24) | The mean disability in the control group was 8.3 points | The mean disability in the intervention group was (1.15 lower to 0.25 higher) | ‐ | 1511 | ⊕⊕⊕⊕ | The difference is not statistically |
| Disability 12 weeks (short term) (RMDQ 0 to 24) | The mean disability in the control group was 2.4 points | The mean disability in the intervention group was (0.39 lower to 0.59 higher) | ‐ | 1522 | ⊕⊕⊕⊕ | The difference is not statistically |
| Any adverse events Up to 12 weeks' follow‐up | 107 per 1000 | 115 per 1000 | RR 1.07 (0.86 to 1.33) | 1624 | ⊕⊕⊕⊕ | The difference is not statistically |
| Serious adverse events Up to 12 weeks' follow‐up | 90 per 1000 | 81 per 1000 | RR 0.90 (0.30 to 2.67) | 1643 | ⊕⊕⊕ | The difference is not statistically |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded for imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week (immediate‐term) | 1 | 1520 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐1.30, 4.28] |
| 1.2 2 weeks | 1 | 1505 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.70, 3.70] |
| 1.3 4 weeks | 1 | 1516 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐1.99, 2.97] |
| 1.4 12 weeks (short‐term) | 1 | 1526 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.92, 1.92] |
| 2 Disability Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.15, 0.25] |
| 2.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.65, 0.65] |
| 2.3 4 weeks | 1 | 1506 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.50, 0.60] |
| 2.4 12 weeks (short‐term) | 1 | 1522 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.39, 0.59] |
| 3 Quality of life, physical component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.94, 0.36] |
| 3.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.91, 1.72] |
| 4 Quality of life, mental component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.38, 0.17] |
| 4.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.08, 1.72] |
| 5 Function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 5.2 2 weeks | 1 | 1499 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 5.3 4 weeks | 1 | 1502 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 5.4 12 weeks (short‐term) | 1 | 1518 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 6 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any adverse events (up to 12 weeks) | 1 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 6.2 Serious adverse events (up to 12 weeks) | 1 | 1643 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.67] |
| 7 Global impression of recovery Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 1 week (immediate‐term) | 1 | 1515 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.3 4 weeks | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 7.4 12 weeks (short‐term) | 1 | 1523 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.17] |
| 8 Poor sleep quality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 1 week (immediate‐term) | 1 | 1511 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.25] |
| 8.2 2 weeks | 1 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 8.3 4 weeks | 1 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.52] |
| 8.4 12 weeks (short‐term) | 1 | 1523 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.90, 1.76] |
| 9 Patient adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 4 weeks | 1 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.22] |
| 10 Use of rescue medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Up to 2 weeks | 1 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.16, 1.55] |

