扑热息痛治疗腰痛
Appendices
Appendix 1. CENTRAL search strategy
Last searched 07 August 2015
1. acetaminophen.mp. or exp Acetaminophen/
2. Analgesics, Non‐Narcotic/tu [Therapeutic Use]
3. (paracetamol or tylenol or panadol).mp.
4. 1 or 2 or 3
5. exp Osteoarthritis, Hip/ or exp Osteoarthritis/ or exp Osteoarthritis, Spine/ or exp Osteoarthritis, Knee/
6. Low back pain.mp. or exp Low Back Pain/
7. Neck pain.mp. or exp Neck Pain/
8. ("low back pain" or "back pain" or "neck pain" or backache or lumbago or "neck ache" or "spin* pain" or "knee pain" or "hip pain").mp.
9. 5 or 6 or 7 or 8
10. 4 and 9
Appendix 2. MEDLINE search strategy
Last searched 07 August 2015
1. acetaminophen.mp. OR exp acetaminophen/
2. *Analgesics, Non‐Narcotic/tu, th [Therapeutic Use, Therapy]
3. analgesic*.ab,ti.
4. (aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolOR OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben).tw.
5. OR/1‐4
6. osteoarthritis.mp. OR exp osteoarthritis/
7. exp low back pain/
8. exp back pain/
9. exp neck pain/
10. ("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
11. OR/6‐10
12. 5 AND 11
13. randomized controlled trial.pt. OR exp randomized controlled trial/
14. "randomized controlled trial".mp.
15. exp random allocation/
16. placebo.mp. OR exp placebos/ OR exp placebo effect/
17. (random* adj3 trial).ab,ti.
18. "controlled clinical trial".mp. OR exp controlled clinical trial/
19. Random*.ab,ti.
20. OR/13‐19
21. 12 AND 20
22. limit 21 to humans
Appendix 3. EMBASE search strategy
Last searched 07 August 2015
1. 'acetaminophen'/exp OR 'acetaminophen'
2. (aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolOR OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben)
3. 1 OR 2
4. 'osteoarthritis'/exp OR 'osteoarthritis'
5. 'low back pain'/exp OR 'low back pain'
6. 'backache'/exp OR 'backache'
7. 'neck pain'/exp OR 'neck pain'
8. 'low back pain' OR 'back pain' OR 'neck pain' OR backache OR lumbago OR 'neck ache' OR 'spin$ pain' OR 'knee pain' OR 'hip pain'
9. OR (4‐8)
10. 3 AND 9
11. 'randomized controlled trial (topic)'/exp OR 'randomized controlled trial (topic)'
12. 'randomization'/exp OR 'randomization'
13. 'placebo'/exp OR 'placebo'
14. randomized:ab
15. placebo:ab
16. randomly:ab
17. OR (11‐16)
18. 10 AND 17
Appendix 4. CINAHL search strategy
Last searched 07 August 2015
S15. S7 AND S14
S14. S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13. "backache"
S12. "hip pain"
S11. (MH "Knee Pain+") OR "knee pain"
S10. (MH "Neck Pain") OR "neck pain"
S9. (MH "Low Back Pain") OR "low back pain" OR (MH "Back Pain+")
S8. (MH "Osteoarthritis+") OR "osteoarthritis" OR (MH "Osteoarthritis, Spine+") OR (MH "Osteoarthritis, Knee") OR (MH "Osteoarthritis, Hip")
S7. S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6. "panadol"
S5. "tylenol"
S4. "paracetamol"
S3. "analgesic$"
S2. (MH "Analgesics+/TU")
S1. (MH "Acetaminophen") OR "acetaminophen"
Appendix 5. AMED search strategy
Last searched 07 August 2015
1. exp Acetaminophen/ OR acetaminophen.mp.
2. exp Analgesics/ OR Analgesics.mp.
3. exp Drug therapy/ OR drug therapy.mp.
4. analgesic*.ab,ti.
5. (aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolor OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben).tw.
6. OR/1‐5
7. exp Osteoarthritis/ OR osteoarthritis.mp.
8. exp Low back pain/ OR low back pain.mp.
9. back pain.mp. OR exp Backache/
10. exp Neck pain/ OR neck pain.mp.
11. ("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
12. OR/7‐11
13. 6 AND 12
14. exp Randomized controlled trials/ OR randomized controlled trial.mp.
15. randomized controlled trial.pt.
16. exp Random allocation/ OR random allocation.mp.
17. exp Placebos/ OR placebo.mp.
18. (random* adj3 trial).ab,ti.
19. Random*.ab,ti.
20. OR/14‐19
21. 13 AND 20
Appendix 6. Web of Science search strategy
Last searched 07 August 2015
16. #15 AND #9
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
15. #14 OR #13 OR #12 OR #11 OR #10
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
14. TOPIC: (Random*)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
13. TOPIC: (controlled clinical trial)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
12. TOPIC: (placebo)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
11. TOPIC: (random allocation)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
10. TOPIC: (randomized controlled trial)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
9. #3 AND #8
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
8. #7 OR #6 OR #5 OR #4
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
7. TOPIC: ((spin* pain" OR "knee pain" OR "hip pain"))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
6. TOPIC: (neck pain)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
5. TOPIC: (back pain)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
4. TOPIC: (osteoarthritis)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
3. #2 OR #1
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
2. TOPIC: (Paracetamol OR tylenol OR panadol)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
1. TOPIC: (acetaminophen)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=All years
Appendix 7. LILACS search strategy
Last searched 07 August 2015
((Acetaminophen OR paracetamol OR tylenol OR panadol) AND (osteoarthritis OR back pain OR lumbago OR backache OR neck pain OR knee pain OR hip pain))
Appendix 8. IPA search strategy
Last searched 07 August 2015
1. acetaminophen.mp.
2. (aceta or actimin or anacin or apacet or "aspirin free anacin" or acamol or acetalgin or adol or aldolOR or alvedon or apiretal or atamel or atasol or benuron or biogesic or "biogesic kiddielets" or buscapina or banesin or "ben u ron" or calpol or captin or cemol or coldex or cotibin or crocin or dafalgan or daleron or "dawa ya magi" or depon or dexamol or dolex or dolgesic or doliprane or dolorol or dolprone or "duiyixian anjifen pian" or dapa or dolo or datril or duatrol or dayquil or efferalgan or enelfa or europain or febrectal or febricet or febridol or fensum or feverall or fibi or "fibi plus" or gelocatil or gripin or gesic or genapap or genebs or hedex or hedanol or herron or influbene or kafa or kitadol or lekadol or lupocet or lemsip or liquiprin or pyrigesic or mexalen or milidon or minoset or momentum or napa or "neo kiddielets" or neopap or "oraphen pd" or pyrigesic or pacol or pamol or parol or panado or panadol or panamax or panda or panodil or pyrigesic or paracet or paracetamol or paracitol or paralen or paramed or paramol or parol or perdolan or perfalgan or pinex or "pyongsu cetamol" or pyrenol or pyrigesic or plicet or panadrex or paratabs or paralgin or phenaphen or revanin or rokamol or rubophen or redutemp or sara or scanol or "sinpro n" or "snaplets fr" or suppap or tachipirin or tachipirina or tafirol or tapsin or termalgin or tempra or thomapyrin or tipol or "togal classic duo" or treuphadol or triaminic or tylenol or tamen or tapanol or tipol or uphamol or vermidon or vitamol or valorin or xumadol or zolben).tw.
3. 1 OR 2
4. osteoarthritis.mp.
5. low back pain.mp.
6. back pain.mp.
7. neck pain.mp.
8. ("low back pain" or "back pain" or "neck pain" or backache or lumbago or "neck ache" or "spin* pain" or "knee pain" or "hip pain").mp.
9. OR/4‐8
10. 3 AND 9
Appendix 9. ClinicalTrials.gov and WHO ICTRP search strategy
Last searched 07 August 2015
ClinicalTrials.gov: Search: (paracetamol OR acetaminophen) AND Condition: back pain
WHO ICTRP: Title: (paracetamol OR acetaminophen) AND Condition: back pain
Appendix 10. 'Risk of bias' criteria
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number, or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (for example a list of random numbers); assignment envelopes were used without appropriate safeguards (for example if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants and personnel (performance bias)
Performance bias due to knowledge of the allocated interventions by participants or personnel/care providers during the study
There is a low risk of performance bias if blinding of participants or personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
-
for participant‐reported outcomes in which the participant was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
-
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
-
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature, or handling of incomplete outcome data
There is a low risk of attrition bias if: there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts are very large, imputation using even 'acceptable' methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is a low risk of reporting bias if the study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods, or subsets of the data (for example subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (for example study funding).
Appendix 11. The GRADE approach to evidence synthesis
The quality of evidence will be categorised as follows:
-
High (⊙⊙⊙⊙): further research is very unlikely to change the confidence in the estimate of effect.
-
Moderate (⊙⊙⊙○): further research is likely to have an important impact in the confidence in the estimate of effect.
-
Low (⊙⊙○○): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very Low (⊙○○○): any estimate of effect is very uncertain.
The evidence available to answer each subquestion will be graded on the domains in the following manner:
1. Study design
2. Risk of bias
Limitations in the study design and implementation may bias the estimates of the treatment effect. Our confidence in the estimate of the effect and in the following recommendation decreases if studies suffer from major limitations. We will examine all studies on five types of biases:
a) Selection (random sequence generation, allocation concealment, group similarities at baseline)
b) Performance (blinding of participants, blinding of healthcare providers)
c) Attrition (dropouts and intention‐to‐treat analysis)
d) Measurement (blinding of the outcome assessors and timing of outcome assessment)
e) Reporting bias (selective reporting)
3. Inconsistency
Inconsistency refers to an unexplained heterogeneity of results. Widely differing estimates of the treatment effect (i.e. heterogeneity or variability in results) across studies suggest true differences in underlying treatment effect. Inconsistency may arise from differences in: populations (e.g. drugs may have larger relative effects in sicker populations), interventions (e.g. larger effects with higher drug doses), or outcomes (e.g. diminishing treatment effect with time).
The quality of evidence will be downgraded as follows:
-
by one level: when the heterogeneity or variability in results is large.
-
by two levels: when the heterogeneity or variability in results is large AND there was inconsistency arising from populations, interventions, or outcomes.
4. Indirectness
Indirect population, intervention, comparator, or outcome: the question being addressed in this systematic review is different from the available evidence regarding the population, intervention, comparator, or an outcome in the included randomised trial.
The quality of evidence will be downgraded as follows:
-
by one level: when there is indirectness in only one area
-
by two levels: when there is indirectness in two or more areas
5. Imprecision
Results are imprecise when studies include relatively few participants and few events and thus have wide confidence intervals around the estimate of the effect. In such a case we judge the quality of the evidence to be lower than it otherwise would be because of uncertainty in the results. Each outcome is considered separately.
For dichotomous outcomes
We will consider imprecision for either of the following two reasons:
-
There is only one study (unless the study provide data from more than 300 participants). When there is more than one study, the total number of events is less than 300 (a threshold rule‐of‐thumb value) (Mueller 2007).
-
95% confidence interval around the pooled or best estimate of effect includes both 1) no effect and 2) appreciable benefit or appreciable harm. The threshold for 'appreciable benefit' or 'appreciable harm' is a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%.
The quality of the evidence will be downgraded as follows:
-
by one level: when there is imprecision due to (1) or (2)
-
by two levels: when there is imprecision due to (1) and (2)
For continuous outcomes
We will consider imprecision for either of the following two reasons:
-
There is only one study (unless the study provide data from more than 400 participants). When there is more than one study, total population size is less than 400 (a threshold rule‐of‐thumb value; using the usual α and β, and an effect size of 0.2 standard deviations, representing a small effect).
-
95% confidence interval includes no effect and the upper or lower confidence limit crosses an effect size (standardised mean difference) of 0.5 in either direction.
The quality of the evidence will be downgraded as follows:
-
by one level: when there is imprecision due to (1) or (2)
-
by two levels: when there is imprecision due to (1) and (2)
6. Publication bias
Publication bias is a systematic underestimate or an overestimate of the underlying beneficial or harmful effect due to the selective publication of studies.
The quality of evidence will be downgraded as follows:
-
by one level: when the funnel plot suggests publication bias
7. Magnitude of the effect
8. Dose response gradient
9. Influence of all plausible residual confounding

Study flow diagram.
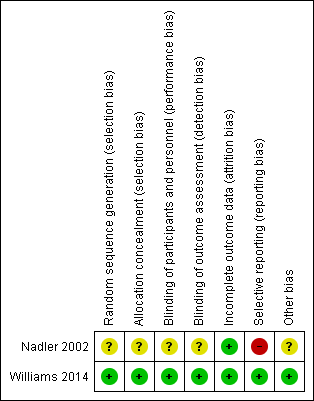
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Acute low back pain ‐ paracetamol versus placebo, outcome: 1.1 Pain.
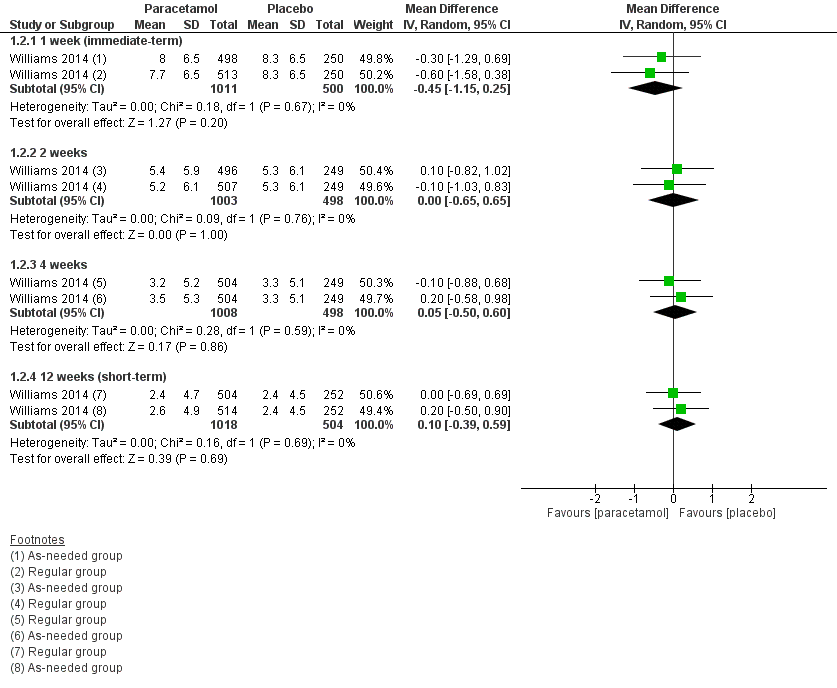
Forest plot of comparison: 1 Acute low back pain ‐ paracetamol versus placebo, outcome: 1.2 Disability
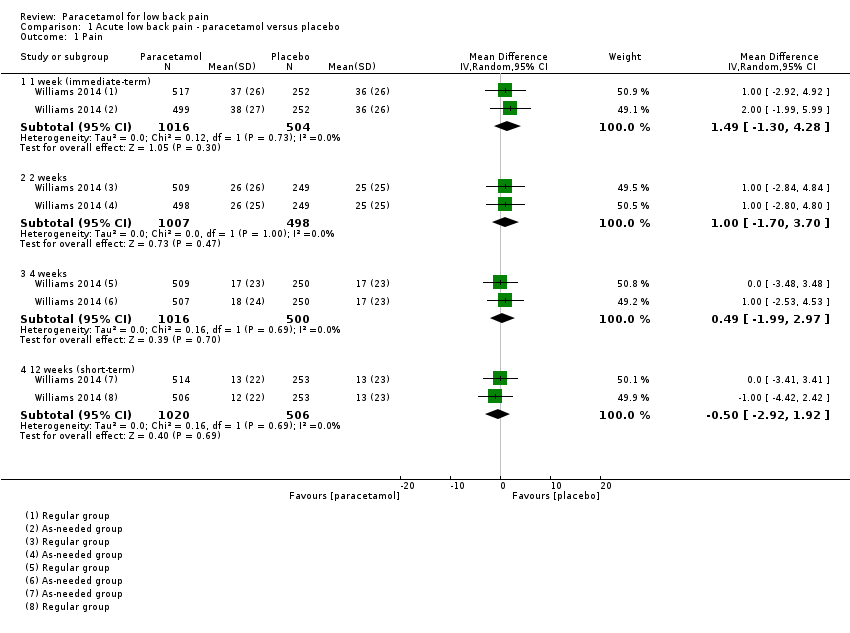
Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 1 Pain.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 2 Disability.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 3 Quality of life, physical component.
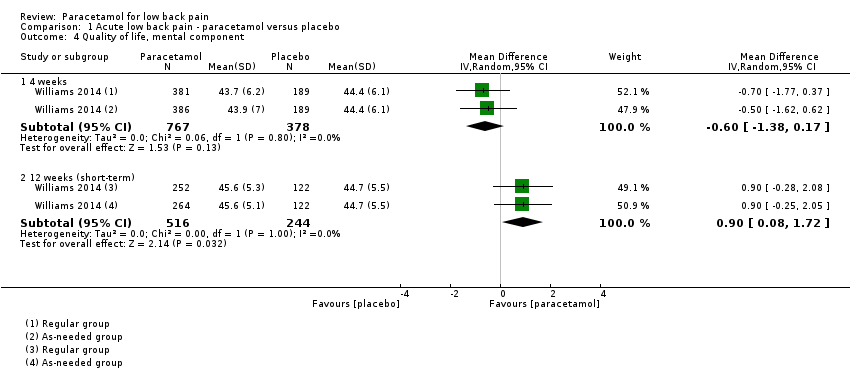
Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 4 Quality of life, mental component.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 5 Function.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 6 Adverse events.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 7 Global impression of recovery.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 8 Poor sleep quality.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 9 Patient adherence.

Comparison 1 Acute low back pain ‐ paracetamol versus placebo, Outcome 10 Use of rescue medication.
| Paracetamol compared with placebo for acute low back pain | ||||||
| Patient or population: People with acute low back pain Settings: Primary care Intervention: Paracetamol Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Paracetamol | |||||
| Pain 1 week (immediate term) (NRS 0 to 100) | The mean pain in the control group was 36 points | The mean pain in the intervention group was (1.30 lower to 4.28 higher) | ‐ | 1520 | ⊕⊕⊕⊕ | The difference is not statistically |
| Pain 12 weeks (short term) (NRS 0 to 100) | The mean pain in the control group was 13 points | The mean pain in the intervention group was (2.92 lower to 1.92 higher) | ‐ | 1526 | ⊕⊕⊕⊕ | The difference is not statistically |
| Disability 1 week (immediate term) (RMDQ 0 to 24) | The mean disability in the control group was 8.3 points | The mean disability in the intervention group was (1.15 lower to 0.25 higher) | ‐ | 1511 | ⊕⊕⊕⊕ | The difference is not statistically |
| Disability 12 weeks (short term) (RMDQ 0 to 24) | The mean disability in the control group was 2.4 points | The mean disability in the intervention group was (0.39 lower to 0.59 higher) | ‐ | 1522 | ⊕⊕⊕⊕ | The difference is not statistically |
| Any adverse events Up to 12 weeks' follow‐up | 107 per 1000 | 115 per 1000 | RR 1.07 (0.86 to 1.33) | 1624 | ⊕⊕⊕⊕ | The difference is not statistically |
| Serious adverse events Up to 12 weeks' follow‐up | 90 per 1000 | 81 per 1000 | RR 0.90 (0.30 to 2.67) | 1643 | ⊕⊕⊕ | The difference is not statistically |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded for imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week (immediate‐term) | 1 | 1520 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐1.30, 4.28] |
| 1.2 2 weeks | 1 | 1505 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.70, 3.70] |
| 1.3 4 weeks | 1 | 1516 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐1.99, 2.97] |
| 1.4 12 weeks (short‐term) | 1 | 1526 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.92, 1.92] |
| 2 Disability Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.15, 0.25] |
| 2.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.65, 0.65] |
| 2.3 4 weeks | 1 | 1506 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.50, 0.60] |
| 2.4 12 weeks (short‐term) | 1 | 1522 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.39, 0.59] |
| 3 Quality of life, physical component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.94, 0.36] |
| 3.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.91, 1.72] |
| 4 Quality of life, mental component Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 4 weeks | 1 | 1145 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.38, 0.17] |
| 4.2 12 weeks (short‐term) | 1 | 760 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.08, 1.72] |
| 5 Function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 1 week (immediate‐term) | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 5.2 2 weeks | 1 | 1499 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 5.3 4 weeks | 1 | 1502 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 5.4 12 weeks (short‐term) | 1 | 1518 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 6 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any adverse events (up to 12 weeks) | 1 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 6.2 Serious adverse events (up to 12 weeks) | 1 | 1643 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.67] |
| 7 Global impression of recovery Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 1 week (immediate‐term) | 1 | 1515 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.2 2 weeks | 1 | 1501 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 7.3 4 weeks | 1 | 1511 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 7.4 12 weeks (short‐term) | 1 | 1523 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.17] |
| 8 Poor sleep quality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 1 week (immediate‐term) | 1 | 1511 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.25] |
| 8.2 2 weeks | 1 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 8.3 4 weeks | 1 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.52] |
| 8.4 12 weeks (short‐term) | 1 | 1523 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.90, 1.76] |
| 9 Patient adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 4 weeks | 1 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.22] |
| 10 Use of rescue medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Up to 2 weeks | 1 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.16, 1.55] |

