Gabapentyna w leczeniu fibromialgii u dorosłych
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, and avoid problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. Below we summarise some of the recent insights that we must consider in this new Cochrane review.
-
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011b; Moore 2011c), back pain (Moore 2010d), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
-
Consequently, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In people with arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
-
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014c; Straube 2008; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia, and lower in central pain and fibromyalgia; Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are grounds for doing so.
-
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, which affects the quality of life in a significant way (Moore 2010b; Moore 2014b).
-
Imputation methods, such as LOCF, used when participants withdraw from clinical trials, can overstate drug efficacy, especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. History of earlier versions of this review
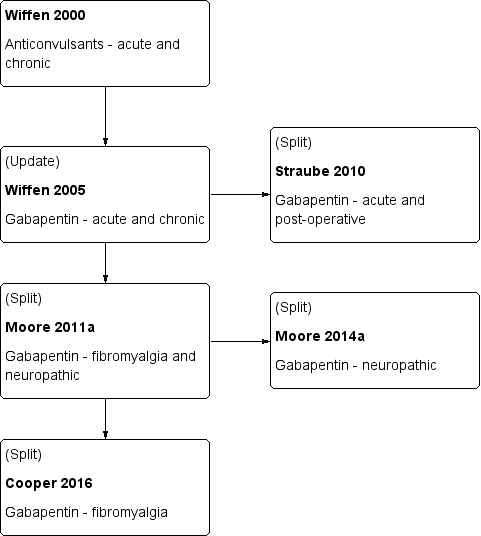
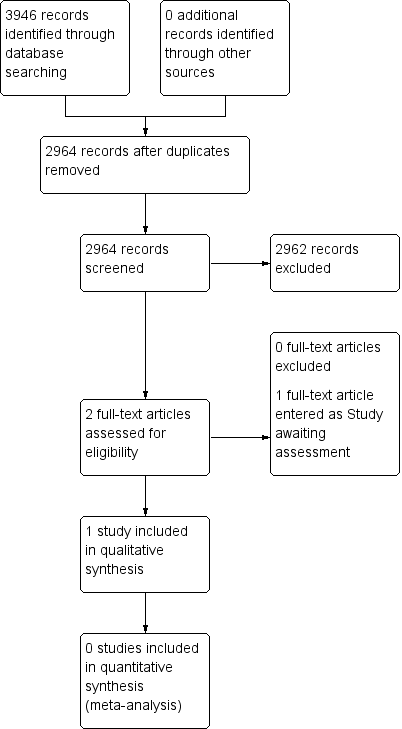
A flow diagram of this history is available in Figure 1.
This is an update of a Cochrane review published in 2011 (Moore 2011a). That review was an update of a previous Cochrane review titled 'Gabapentin for acute and chronic pain' (Wiffen 2005), which itself was an extension to a review previously published in the Cochrane Library on 'Anticonvulsant drugs for acute and chronic pain' (Wiffen 2000). The effects of gabapentin in established acute postoperative pain have been published as a separate review in 2010 (Straube 2010).
The decision to split the review in 2011 was undertaken after discussions with the Editor‐in‐Chief of Cochrane at a meeting in Oxford in early 2009. That meeting was in response to controversy in the USA over the effectiveness of gabapentin as an analgesic (Landefeld 2009), together with calls for the 2005 Cochrane review to be updated with the inclusion of unpublished information made available through litigation (Vedula 2009). It was agreed to update the review by splitting the earlier one into two components: this review which looks at the role of gabapentin in chronic neuropathic pain (including neuropathic pain of any cause, and fibromyalgia), and a second one to determine the effects of gabapentin in acute postoperative pain (Straube 2010). Other reviews may examine gabapentin in chronic musculoskeletal pain. After the Cochrane review on gabapentin for acute and chronic pain was published in 2005 (Wiffen 2005), the licence holders of the first gabapentin product to be marketed released unpublished data, and the Moore 2011a review included these data. The latest update (Cooper 2017, in press) has an expanded background, in line with other reviews of antiepileptic drugs used to treat neuropathic pain and fibromyalgia, and includes three new studies for oral gabapentin plus additional information on an already included study. We have also identified a number of ongoing studies.
The 2011 update (Moore 2011a) included 29 studies in 29 reports with 3571 participants with neuropathic pain and fibromyalgia. The Moore 2014a review included 36 studies in neuropathic pain (5483 participants) and one study in fibromyalgia (150 participants).
Appendix 3. CENTRAL search strategy
-
MESH DESCRIPTOR Pain EXPLODE ALL TREES (30244)
-
MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES (2585)
-
MESH DESCRIPTOR Somatosensory Disorders EXPLODE ALL TREES (709)
-
MESH DESCRIPTOR Fibromyalgia EXPLODE ALL TREES (533)
-
MESH DESCRIPTOR Myofascial Pain Syndromes EXPLODE ALL TREES (339)
-
MESH DESCRIPTOR Polymyalgia Rheumatica EXPLODE ALL TREES (44)
-
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)): TI,AB,KY (19856)
-
(fibromyalgi* or fibrosti* or FM or FMS): TI,AB,KY (1953)
-
((neur* or nerv*) and (compress* or damag*)): TI,AB,KY (2135)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 (48230)
-
(gabapentin* or neurontin* or neurotonin*):TI,AB,KY (1018)
-
10 AND 11 (371)
Appendix 4. MEDLINE (via Ovid) search strategy
-
exp PAIN/ (328984)
-
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (123022)
-
exp SOMATOSENSORY DISORDERS/ (17645)
-
FIBROMYALGIA/ (6749)
-
exp MYOFASCIAL PAIN SYNDROMES/ (5920)
-
POLYMYALGIA RHEUMATICA/ (2248)
-
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (74639)
-
(fibromyalgi* or fibrosti* or FM or FMS).mp. (24358)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (55239)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (528383)
-
(gabapentin* or neurontin* or neurotonin*).mp. (5166)
-
randomized controlled trial.pt. (411494)
-
controlled clinical trial.pt. (91674)
-
randomized.ab. (333337)
-
placebo.ab. (168066)
-
drug therapy.fs. (1836743)
-
randomly.ab. trial.ab. (240444)
-
trial.ab. (347248)
-
groups.ab. (1499758)
-
12 or 13 or 14 or 15 or 16 or 17 or 19 (3657357)
-
10 and 11 and 20 (1655)
Appendix 5. Embase (via OVID) search strategy
-
exp PAIN/ (928121)
-
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (54585)
-
exp SOMATOSENSORY DISORDERS/ (71019)
-
FIBROMYALGIA/ (14722)
-
exp MYOFASCIAL PAIN SYNDROMES/ (6839)
-
POLYMYALGIA RHEUMATICA/ (3640)
-
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (128563)
-
(fibromyalgi* or fibrosti* or FM or FMS).mp. (33777)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (72816)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (1095698)
-
Gabapentin/ (22468)
-
(gabapentin* or neurontin* or neurotonin*).mp (23231)
-
11 or 12 (23231)
-
crossover‐procedure/ (44583)
-
double‐blind procedure/ (126205)
-
randomized controlled trial/ (387066)
-
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1380427)
-
14 or 15 or 16 or 17 (1463359)
-
10 and 13 and 18 (1912)
Appendix 6. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
-
High: randomised trials; or double‐upgraded observational studies.
-
Moderate: downgraded randomised trials; or upgraded observational studies.
-
Low: double‐downgraded randomised trials; or observational studies.
-
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
-
limitations in the design and implementation of available studies suggesting high likelihood of bias;
-
indirectness of evidence (indirect population, intervention, control, outcomes);
-
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
-
imprecision of results (wide confidence intervals).
-
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
-
large magnitude of effect;
-
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
-
dose‐response gradient.
History of Earlier Reviews
Study flow diagram.
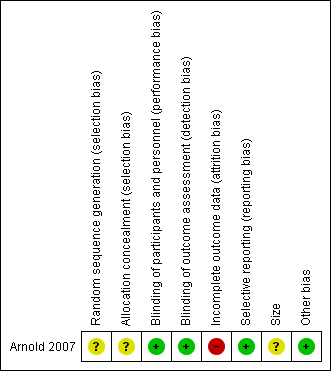
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect | Number of | Quality of the evidence | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
| PGIC ‐ any category of "better" at 12 weeks | 68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||

