Ubat berasaskan ganja untuk kesakitan neuropatik kronik pada orang dewasa
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Disease: plexus root avulsion with ≥ 1 root affected Study setting: single‐centre (orthopedic clinic), UK; 2001‐2002 Study design: cross‐over Study duration: 2‐week baseline, 3 cross‐over periods for 10‐14 days, no washout periods | |
| Participants | Inclusion criteria: pain ≥ 4 on 0‐10 scale, no cannabis use for 7 days prior to inclusion, Exclusion criteria: schizophrenia, other psychotic illness or significant psychiatric illness, other than depression associated with chronic illness; serious cardiovascular disease; significant renal or hepatic impairment; epilepsy or convulsions; significant history of substance abuse; known adverse reaction to cannabis or the product excipients; surgery within 2 months (6 months for nerve repair). Female patients who were pregnant, lactating or at risk of pregnancy were also excluded. Participants: N = 48, 46 male, 2 female, mean age 39 (23‐63 years). Pain baseline 7.5 (no SD reported) (scale 0‐10). 45.8% had used cannabis medicinally, 60.4 % recreationally. | |
| Interventions | Study medication: oromucosal spray THC only (27 mg/mL), THC/CBD mix (27/25 mg/mL), maximum 48 sprays/d; placebo spray Rescue medication: none Allowed co‐therapies: stable analgesic medication over 4 weeks (fentanyl not allowed, amitriptyline max. 75 mg/d, no further details provided) | |
| Outcomes | Participant‐reported pain relief ≥ 50%: reported (NRS 0‐10, average of the last 7 days) PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE attributed to medication: reported Participant‐reported pain relief ≥ 30%: not reported; calculated by imputation method (NRS 0‐10, average of the last 7 days) Mean pain intensity: NRS 0‐10, average of the last 7 days; SD calculated from P value HRQoL: Pain Disability Index 0‐70; SD calculated from P value Sleep problems: sleep quality 10‐0; SD calculated from P value Fatigue: not assessed Psychological distress: General Health Questionnaire‐12; SD calculated from P value Withdrawals due to lack of efficacy: reported Nervous system disorders‐related AE: incompletely reported (not suited for analysis) Psychiatric disorders‐related AE: incompeletely reported (not suited for analysis) Any adverse event: open question at each visit; VAS intoxication score for AE | |
| Notes | Funding: GW Pharmaceuticals and the Royal National Orthopaedic Hospital NHS Trust Conflicts of interest: not declared "No washout period was used between the three treatment periods. Any carry over effect was unlikely to be for greater than 2–3 days so the first week of titration for each period would be sufficient to counteract any carry over with efficacy comparisons being made by averaging the variables over the last 7 days of treatment". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated by a computer generated list to the six possible sequences of receiving the three study medications" |
| Allocation concealment (selection bias) | Low risk | "Although the treatment sequence was blinded, sealed code break envelopes, one for each patient, containing information on the treatment sequence were available if necessary. Blinding was maintained throughout the study". |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Group similarity at baseline | Low risk | Identical demographic and clinical characteristics due to study design |
| Sample size bias | High risk | < 50 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: HIV neuropathy Study setting: single‐centre, university, USA; years of study not reported Study design: cross‐over Study duration: 2 weeks with 5 treatment days per each period, 2 weeks washout | |
| Participants | Inclusion criteria: adults with documented HIV infection, neuropathic pain refractory to ≥ 2 previous analgesics, and an average score of ≥ 5 on the pain intensity subscale of the Descriptor Differential Exclusion criteria: (1) current DSM‐IV substance use disorders; (2) lifetime history of dependence on cannabis; (3) previous psychosis with or intolerance to cannabis‐based medicines; (4) concurrent use of approved cannabinoid medications (i.e. Marinol); (5) positive urine toxicology screen for cannabis‐based medicines during the wash‐in week before initiating study treatment; and (6) serious medical conditions that might affect participant safety or the conduct of the trial. Individuals with a previous history of alcohol or other drug dependence were eligible provided that criteria for dependence had not been met within the last 12 months. Participants were excluded if urine toxicology demonstrated ongoing use of non prescribed, recreational drugs such as methamphetamine and cocaine Treatment group (delta‐9‐THC)/placebo group: N = 34 participants, mean age 49.1 years (SD 6.9); male 100%; pain baseline 11.1 (no SD reported) on a 0‐20 scale; 91% with previous cannabis experience | |
| Interventions | Study medication: smoked cannabis with THC ranging from 4% to 8% provided by the National Institute on Drug Abuse, depending on efficacy and tolerability. Cigarettes without THC. 4 smoking sessions in the 8‐h study day Rescue medication: not reported Allowed co‐therapies: stable regimen of opioids, anticonvulsants, antidepressants and analgesics | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported and not calculable by imputation method PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE: incompletely reported (not suited for meta‐analysis) Participant‐reported pain relief ≥ 30%: pain quality and impact descriptor differential scale 0‐20; NNTB reported; number of participants extracted from Andreae 2015 Mean pain intensity: pain quality and impact descriptor differential scale 0‐20; SD calculated from P values HRQoL: Sickness Impact profile; no details reported (not suited for meta‐analysis)** Sleep problems: not assessed Fatigue: not assessed Psychological distress: BSI** Withdrawals due to lack of efficacy: not reported Any adverse event: no details of assessment reported Nervous system disorders‐related AE: incompletely reported (not suited for meta‐analysis) Psychiatric disorders‐related AE: incompletely reported (not suited for meta‐analysis) | |
| Notes | Funding: Grant C00‐SD‐104 from the University of California, Center for Medicinal Cannabis Research Conflicts of interest: Heather Bentley and Ben Gouaux are employees of the Center for Medicinal Cannabis Research at the University of California, San Diego, the study sponsor. Ms Bentley is Project Manager for the CMCR and assisted the investigator with regulatory issues, oversight/monitoring, and preparation of the manuscript. Mr. Gouaux is a Research Associate with the CMCR and assisted the investigator with regulatory issues, oversight/monitoring, data preparation and analysis, and preparation and submission of the article. The study authors declare that over the past 3 years Dr. Atkinson has received compensation from Eli Lilly Pharmaceuticals. "There was no evident sequence effect" **No data shown; "As measured by the SIP and BSI, there were similar improvements in total mood disturbance, physical disability and quality of life for the cannabis and placebo treatment" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number generator" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by a research pharmacist ... and the key to study assignment was withheld from investigators until completion statistical analyses". |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Completer analysis of 30% pain reduction as reported by Andreae 2015 |
| Selective reporting (reporting bias) | High risk | Some outcomes were not reported |
| Group similarity at baseline | Low risk | Identical clinical and demographic characteristics due to study design |
| Sample size bias | High risk | < 50 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: chronic central and PNP (radiculopathy, CRPS, diabetic neuropathy, posttraumatic or postsurgery, trigeminal neuralgia, PHN) Study setting: outpatient units of 3 hospitals in the UK, 2001‐2002 Study design: cross‐over Study duration: 1 week pre study, 6 weeks treatment, washout 2 weeks, 6 weeks treatment | |
| Participants | Inclusion criteria: pain, such as burning, stabbing, or paraesthesia within the distribution of a peripheral nerve and a clear clinical history of its cause (sensory abnormality, allodynia, burning pain, lancinating pain, sympathetic dysfunction), pain ≥ 40 on a 100 mm VAS, stable medication Exclusion criteria: DHC not stopped 2 weeks prior to inclusion, antipsychotics, benzodiazepines (except for night sedation), MAO inhibitors, legal action, ongoing cannabis‐based medicines, severe hepatic or renal disease, epilepsy, bipolar disorder, psychosis, or a history of substance misuse Participants: DHC then nabilone: N = 48 participants, mean age 50.6 (SD 15.2) years. 23 female. Mean pain baseline 69.6 (range 29‐95) on a 0‐100 scale. No reports on prior use of cannabis. Participants: nabilone then DHC: N = 48, mean age 49.7 (SD 12.0), 27 male, 21 female; Mean pain baseline 69.6 (range 29‐95) on a 0‐100 scale. No reports on prior use of cannabis. | |
| Interventions | Study medication: dose adjustment every week (twice first week) from 30‐240 mg DHC and 0.25‐2 mg nabilone Rescue medication: paracetamol 500 mg and codeine 30 mg throughout washout up to 8 times/d Allowed co‐therapies: "Stable analgesics" | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported, calculated by imputation method (daily pain score summarised as last bi‐weekly means VAS 0‐100) PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE attributed to medication: reported Participant‐reported pain relief ≥ 30%: not reported, calculated by imputation method (daily pain score summarised as last bi‐weekly means VAS 0‐100) HRQoL: SF‐36 physical functioning 50‐0 Sleep problems: NRS 0‐10; data reported not suited for meta‐analysis (P = 0.20) Fatigue: not assessed Psychological distress: SF‐36 Mental Health 50‐0; data reported not suited for meta‐analysis (P = 0.20) Withdrawals due to lack of efficacy: not reported Withdrawal due to AE: reported Any adverse event: "At each visit the patients filled in a side effects assessment form" Nervous system disorders‐related AE: incompletely reported, not suited for meta‐analysis Psychiatric disorders‐related AE:incompletely reported, not suited for meta‐analysis | |
| Notes | Funding: grant from Cambridge Laboratories Conflict of Interest: BF’s salary was provided as part of the above research grant although he was employed by the Newcastle upon Tyne University Hospitals Trust. "We excluded carry over by basing the analyses from the last two weeks of each treatment period". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment was allocated by random permuted blocks of 10, stratified by centre." |
| Allocation concealment (selection bias) | Low risk | "The pharmacies at the treatment centres, the patients, and all clinical personnel involved in the trial were unaware of treatment allocation at all times." Code breaking envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | "The pharmacy at St Mary’s Hospital supplied identical white capsules containing 250 μg nabilone or 30 mg dihydrocodeine." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Available cases analysis (all participants randomised, which provided data in each treatment period) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as outlined in the study protocol ISRCTN15330757 |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: central neuropathic pain in multiple sclerosis (MS) Study setting: multicentre, 33 sites in UK, Canada, Spain, France, Czech Republic; 2006‐2008 Study design: Patients who had failed to gain adequate analgesia from existing medication were treated with THC/CBD spray or placebo as an add‐on treatment in a double‐blind manner, for 14 weeks to investigate the efficacy of the medication in MS‐induced neuropathic pain. This parallel‐group phase of the study was then followed by an 18‐week randomised withdrawal study (14‐week, open‐label treatment period plus a double‐blind, 4‐week, randomised‐withdrawal phase) Study duration: Phase A: 1‐week baseline, 14‐week treatment. Phase B: 14‐week, open treatment phase with 2 weeks' titration and 12 weeks' stable dose, followed by a randomised withdrawal phase of four weeks (only in France and Czech Republic) | |
| Participants | Inclusion criteria: chronic neuropathic pain due to MS, of at least 3 months' duration. Participants were also to have a sum score of at least 24 on a pain 0–10 point NRS on the last 6 days during the baseline period. In addition, their analgesic regimen was to be stable for at least 2 weeks preceding the study entry day. For Phase B also: ≥ 3 sprays/d in last 7 days of phase A, and tolerability (that means no AE), stable medication Exclusion criteria: other somatic pain causes with severe pain, including PNP, significant psychiatric (except depression related to pain), renal, hepatic, cardiovascular, or convulsive disorders, sensitivity to cannabis‐based medicines Phase A, treatment group: N = 167, female/male (54/113), mean age 48.42 (SD 10.43), 11 (7%) with cannabis experience Placebo group: N = 172, male/female (55/117), mean age 49.51 (SD 10.50) 10 (6%) with cannabis experience Phase B, treatment group: N = 21; female/male (11/10), mean age 46.2 (10.39), 0 patients with cannabis experience Placebo group: N = 21, female/male 14/7, mean age 49.82 (9.75), 1 patient with cannabis experience | |
| Interventions | Study medication: THC/CBD oromucosal spray. Each actuation of active medication delivered 2.7 mg of THC and 2.5 mg of CBD to the oral mucosa. Placebo delivered the excipient plus colorants. Max. 12 sprays/24 h Rescue medication: paracetamol Allowed co‐therapies: pain medication: stable for at least 2 weeks | |
| Outcomes | Participant‐reported pain relief ≥ 50% (parallel): only OR reported, calculated by imputation method (NRS 0‐10 for mean daily chronic neuropathic pain, average over 7 days at baseline and final 7 days) PGIC much or very much improved (parallel): reported Withdrawal due to AE (parallel): reported Serious AE (parallel and EERW): reported Participant‐reported pain relief ≥ 30%:: reported Mean pain intensity (parallel): NRS 0‐10 for mean daily chronic neuropathic pain, average over 7 days at baseline and final 7 days; SD calculated from P value HRQoL (parallel): EQ‐5D VAS 0‐100 Sleep problems (parallel): NRS 0‐10; SD calculated from P value Fatigue: NRS 0‐10; SD calculated from P value Psychological distress (parallel): SF‐36 mental health: SD calculated from P value Withdrawals due to lack of efficacy (parallel): reported Any adverse event (parallel and EERW): reported. Details of assessment of AEs not reported. Nervous system disorders‐related AE (parallel and EERW): reported Psychiatric disorders‐related AE (parallel and EERW): reported | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: R. Langford, J. Mares, A. Novotna, M. Vachora, I. Novakova, W. Notcutt, and S. Ratcliffe were all investigators in this study and their organizations received investigator fees from GW Pharma Ltd. accordingly for their participation in the study. R. Langford, W. Notcutt, and S. Ratcliffe have received consultancy and speaker fees from GW Pharma Ltd. to attend meetings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization occurred using a pre‐determined computer generated randomisation code in which treatment allocation was stratified by centre, and used randomly permuted blocks of variable sizes. Separate randomisation schemes, using the same strategy, were produced for each part of the study." |
| Allocation concealment (selection bias) | Low risk | Separate randomisation schemes |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | Data reported as outlined in the study protocol NCT00391079 available |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: chemotherapy‐induced neuropathic pain Study setting: single centre, Canada; year of study not reported Study design: cross‐over design Study duration: 4 weeks each and 2 weeks washout | |
| Participants | Inclusion criteria: neuropathic pain persisting for 3 months after completing chemotherapy with paclitaxel, vincristine, or cisplatin. The average 7‐day intensity of pain had to be ≥ 4 on an 11‐point NRS. Participants also exhibited sensory abnormalities comprising allodynia, hyperalgesia, or hypethesia. Concurrent analgesics had to be stable for 14 days before entry into the trial. Exclusion criteria: ischaemic heart disease, ongoing epilepsy, a personal or family history of schizophrenia, or psychotic disorder or substance abuse or dependency within the previous 2 years. Exclusion criteria also included pregnancy or other medical condition that might compromise safety in the trial. Both groups: N = 18; mean age 58 (SD 11.34) years; 15/18 female; previous cannabis use 5/18 | |
| Interventions | Study medication: THC/CBD oromucosal spray. Each actuation of active medication delivered 2.7 mg of THC and 2.5 mg of CBD to the oral mucosa. Placebo delivered the excipient plus colorants. Max. 12 sprays/24 h Rescue medication: not reported Allowed co‐therapies: pain medication (anticonvulsants, antidepressants, NSAIDs, opioids): stable for at least 2 weeks | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported, calculated by imputation method. NRS (0‐10) for mean daily chronic neuropathic pain, average over 7 days at baseline and final 7 days PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE: reported Participant‐reported pain relief ≥ 30%: not reported, calculated by imputation method Mean pain intensity: reported HRQoL (parallel): SF‐36 physical component summary score 50‐0 Sleep problems: not assessed Fatigue: not assessed Psychological distress: SF‐36 mental health summary score 50‐0 Withdrawals due to lack of efficacy: not reported Any adverse event: not reported. No details of assessment of AEs reported. Nervous system disorders‐related AE: reported (summarised by the authors of the review) Psychiatric disorders‐related AE: reported (summarised by the authors of the review) | |
| Notes | Funding: none Conflicts of interest: the study authors declare no conflicts of interest "Thus, the two week washout was chosen to assure no carry over effect between study arms" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | "Participants and study staff were blinded to the randomisation code, which was not broken until the completion of the study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Unclear risk | No protocol reported |
| Group similarity at baseline | Low risk | Identical baseline characteristics due to study design |
| Sample size bias | High risk | < 50 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: painful diabetic neuropathy Study setting: multicentre international trial, UK, Czech Republic, Romania; July 2005‐2006 Study design: parallel Study duration: 1 week baseline, 14 weeks treatment | |
| Participants | Inclusion criteria: ciagnosed with Type 1 or 2 diabetes mellitus as diagnosed according to the WHO criteria. Diagnosed with neuropathic pain due to distal symmetrical diabetic neuropathy of at least 6 months' duration, as defined by a NDS score of ≥ 4, and in whom pain was not wholly relieved with their current therapy. The NDS score must be attained from ≥ 2 different test parameters and not only the ankle jerk reflex. The last 6 daily diary 0‐10 NRS pain scores before randomisation summed to at least 24. Stable dose of regular pain medication and non‐pharmacological therapies (including TENS) for ≥ 14 days prior to the screening visit and willingness for these to be maintained throughout the study. Exclusion criteria: uncontrolled diabetes with HbA1c blood levels of > 11% at Visit 1, Day B1. Had used cannabinoid‐based medications within 60 days of study entry and were unwilling to abstain for the duration for the study. History of schizophrenia, other psychotic illness, severe personality disorder or other significant psychiatric disorder other than depression associated with their underlying condition, known or suspected history of alcohol or substance abuse. History of epilepsy or recurrent seizure, postural drop of 20 mmHg or more in systolic blood pressure at screening. Evidence of cardiomyopathy, MI, cardiac disease. QT interval; of > 450 ms (men) or > 470 ms (women) at Visit 1. Secondary or tertiary atrioventricular block or sinus bradycardia (HR < 50 bpm) or sinus tachycardia (HR > 110 bpm) at Visit 1. Diastolic blood pressure of < 50 mmHg or >105 mmHg in a sitting position at rest for 5 min prior to randomisation. Impaired renal hepatic function Treatment group: N = 149: mean age 60.8 (10.38 SD) years; female/male 56/93. No reports on baseline pain scores and on previous cannabis use. Placebo group: N = 148; mean age 58.2 (10.57 SD) years; female/male 58/90. No reports on baseline pain scores and on previous cannabis use. | |
| Interventions | Study medication: Sativex (DHC 27 mg/mL/CBD25 mg/mL), delivered in 100 µL actuations by mucosal spray, maximum max per 24 h: 65 mg TC/60 mg cannabidiol); placebo Rescue medication: no information provided Allowed co‐therapies: no information provided | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported and not calculable by imputation method. Mean Diabetic Neuropathy Pain 0‐10 NRS score at the end of treatment (average of last 7 days' treatment) (Your nerve pain over the last 24 h from 0‐10); PGIC much or very much improved: reported Withdrawal due to AE: reported Serious AE: reported Participant‐reported pain relief ≥ 30%:reported Mean pain intensity: Mean Diabetic Neuropathy Pain 0‐10 NRS score at the end of treatment (average of last 7 days' treatment) HRQoL:: EQ‐5D 0 ‐100 Sleep problems: NRS 0‐10 Fatigue: not assessed Psychological distress: not assessed Withdrawals due to lack of efficacy: reported Any adverse event: mean intoxication score. No details of assessment reported Nervous system disorders‐related AE: reported Psychiatric disorders‐related AE: reported | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Identical placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Group similarity at baseline | Low risk | Similar demographic characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: MS and other defects of neurological function Study setting: multicentre trial in the UK, no year of study reported Study design: parallel Study duration: baseline period, 3‐week treatment period | |
| Participants | Inclusion criteria: chronic refractory pain due to multiple sclerosis or other defects of neurological function. Neuropathic pain with a mean severity NRS score at ≥ 4 during last 7 days of the baseline period. Relatively stable neurology during the preceding 6 months. Stable medication regimen during the preceding 4 weeks. Had not used cannabis‐based medicines for at least the preceding 7 days and willing to abstain from any use of cannabis‐based medicines during the study Exclusion criteria: history of schizophrenia, other psychotic illness, severe personality disorder or other significant psychiatric disorder other than depression associated with their underlying condition. History of alcohol or substance abuse. Severe cardiovascular disorder, such as ischaemic heart disease, arrhythmias (other than well‐controlled atrial fibrillation), poorly controlled hypertension or severe heart failure. History of autonomic dysreflexia. History of epilepsy. Renal and liver problems Treatment group (delta‐9‐THC): N = 36, female/male 20/16, mean age 51.72 (SD 12.11), 24 in MS‐subset. No baseline pain scores reported. No reports on previous cannabis use Placebo group: N = 34, female/male 21/13, mean age 57.61 (SD 10.28), 19 in MS‐subset. No baseline pain scores reported. No reports on previous cannabis use | |
| Interventions | Study medication: each actuation of oromucosal spray delivers 2.5 mg THC and 2.5 mg CBD. The maximum permitted dose of was 8 actuations in any 3‐hour period, and 48 actuations in any 24‐h period (THC 120 mg:CBD 120 mg). Placebo same number of actuations possible Rescue medication: no details provided, but percentage of days with uses recorded as secondary outcome (less in active group) Allowed co‐therapies: no details provided | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported and not calculable by imputation method. NRS 0‐10, 3 measures/day, average of the last 7 days PGIC much or very much improved: reported Withdrawal due to AE: reported, systematic assessment Serious AE: reported, systematic assessment HRQoL: Spitzer Quality of life index 15‐0 Participant‐reported pain relief ≥ 30%: not reported and not calculable by imputation method Mean pain intensity: NRS 0‐10, 3 measures/day, average of the last 7 days Sleep problems: NRS 0‐10 Fatigue: not assessed Psychological distress: not assessed Withdrawals due to lack of efficacy: not reported Any adverse event: reported; systematic assessment, no details reported Nervous system disorders‐related AE: reported; systematic assessment Psychiatric disorders‐related AE: reported; systematic assessment | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | "Identical placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Group similarity at baseline | Low risk | Similar demographic characteristics at baseline |
| Sample size bias | High risk | < 50 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: intractable neuropathic pain associated with spinal cord Injury Study setting: multicentre study UK, Romania; no years of study reported Study design: parallel Study duration: 7‐21 days baseline period, 3 weeks treatment | |
| Participants | Inclusion criteria: diagnosis of non‐acute spinal cord injury, with central neuropathic pain not wholly relieved by current therapy. Central neuropathic pain with a mean severity NRS score ≥ 4 during last 7 days of the baseline period. Relatively stable neurology during the preceding 6 months. Stable medication regimen during the preceding 4 weeks. Had not used cannabis‐based medicines for at least the preceding 7 days and willing to abstain from any use of cannabis‐based medicines during the study Exclusion criteria: history of schizophrenia, other psychotic illness, severe personality disorder or other significant psychiatric disorder other than depression associated with their underlying condition. History of alcohol or substance abuse. Severe cardiovascular disorder, such as ischaemic heart disease, arrhythmias (other than well‐controlled atrial fibrillation), poorly controlled hypertension or severe heart failure. History of autonomic dysreflexia. History of epilepsy. Renal and liver problems Treatment group (delta‐9‐THC): N = 56, age 48.7 (12.97), female/male 13/43. No reports on pain baseline scores and on previous cannabis use Placebo group: N = 60, age 47.6 (12.69), female/male 12/48. No reports on pain baseline scores and on previous cannabis use | |
| Interventions | Study medication: THC (27 mg/mL): CBD (25 mg/mL) as extract of Cannabis sativa L., with peppermint oil, 0.05%, in ethanol:propylene glycol (50:50) excipient. Each actuation delivered 100 μL (THC 2.7 mg and CBD 2.5 mg). The maximum permitted dose of study medication was 8 actuations in any 3‐h period, and 48 actuations in any 24‐h period Rescue medication: paracetamol 500 mg Allowed co‐therapies: stable medication regimen | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported and not calculable by imputation method. NRS 0‐10 Neuropathic Pain Scale PGIC much or very much improved: reported Withdrawal due to AE: reported Serious AE: Participant‐reported pain relief ≥ 30%:: not reported and not calculable by imputation method Mean pain intensity: NRS 0‐10 Neuropathic Pain Scale HRQoL: Spitzer Quality of Life Index Score 15‐0 Sleep problems: sleep disturbance NRS 0‐10 Fatigue: not assessed Psychological distress: not assessed Withdrawals due to lack of efficacy: not reported Any adverse event: reported. No details of assessment reported Nervous system disorders‐related AE: reported Psychiatric disorders‐related AE: reported | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided, but identical placebo |
| Blinding of participants and personnel (performance bias) | Low risk | "Identical placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Group similarity at baseline | Low risk | Similar demographic characteristics of the study groups at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: pain and allodynia patients with unilateral neuropathic pain of peripheral origin of various aetiologies Study setting: multicentre (5 UK, 1 Belgium); study period not reported Study design: parallel Study duration: baseline 7‐10 days, therapy 5 weeks | |
| Participants | Inclusion criteria: unilateral PNP and allodynia, at least 6 months with identifiable nerve lesion, unilateral PNP and allodynia, CRPS type II, ≥ 4 on a NRS for spontaneous pain 4 out of 7 days during baseline. A stable medication regimen of analgesics for at least 2 weeks prior to study entry. Exclusion criteria: cannabinoid use < 7 days, failure to abstain, schizophrenia, psychosis, or other major psychiatric condition beyond depression with underlying condition. Concomitant severe non‐neuropathic pain or the presence of cancer‐related neuropathic pain or from diabetes mellitus, known history of alcohol or substance abuse, severe cardiovascular condition, poorly controlled hypertension, epilepsy, pregnancy, lactation, significant hepatic or renal impairment Treatment group (delta‐9‐THC): N = 63, female 35, mean age 52.4 (SD 15.8) years. Pain baseline 7.3 (SD 1.4) on 0‐10 scale. 13 (21%) prior cannabis use | |
| Interventions | Study medication: spray for sublingual and oro‐pharyngeal administration. Each 100 μL spray delivers 2.7 mg of THC and 2.5 mg of CBD, identically appearing placebo spray. Participants were allowed a maximum dose of 8 sprays per 3‐h interval and a maximum of 48 sprays per 24 h. Rescue medication: none Allowed co‐therapies: stable dose regimen | |
| Outcomes | Participant‐reported pain relief ≥ 50%: reported. NRS 0‐10 over 7 days PGIC much or very much improved: only average scores reported (not suited for meta‐analysis) Withdrawal due to AE: assessed Serious AE: assessed; only psychiatric serious AEs reported Participant‐reported pain relief ≥ 30%: reported. NRS 0‐10 over 7 days Mean pain intensity: neuropathic pain scale total score 0‐60 HRQoL: not assessed Sleep problems: NRS 0‐10; SD calculated from P value Fatigue: not assessed Psychological distress: General Health Questionnaire 0‐48: SD calculated from P value Any adverse event: not reported (details of assessment of AE not reported) Nervous system disorders‐related AE: incompeletely reported (not suited for analysis) Psychiatric disorders‐related AE: incompeletely reported (not suited for analysis) | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After eligibility was confirmed, patients were assigned to the next sequential randomisation number within each centre. The randomisation schedule had a 1:1 treatment allocation ratio with randomly permuted blocks stratified by centre and was generated using a computer based pseudo‐random number algorithm". |
| Allocation concealment (selection bias) | Low risk | "The randomisation schedule was held by the sponsor with a copy in patient‐specific sealed envelopes sent to the pharmacy in each centre." |
| Blinding of participants and personnel (performance bias) | Low risk | "That the smell and taste of the cannabinoid preparation might lead to unblinding was averted by disguising them with addition of peppermint oil to both preparations." |
| Blinding of outcome assessment (detection bias) | Low risk | "The analyses were verified by an independent statistician. The principal investigator had full access to all the data and carried out further confirmatory analyses" |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Group similarity at baseline | Low risk | Similar clinical and demographic characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: central pain in MS Study setting: UK, single‐centre; study period not reported Study design: parallel, randomised, placebo‐controlled, parallel‐group study Study duration: 5 weeks, including 1 week baseline | |
| Participants | Inclusion criteria: at least 6 months after MS diagnosis, at least 3 months central pain with unlikely other cause, both with dysaesthetic characteristics or painful spasm, 2 weeks of stable analgesic regimen, no cannabinoid use the last 7 days Exclusion criteria: spasticity‐related pain, visceral pain, headache, acute MS‐related pain, major psychiatric disorder, other than pain‐related depression, severe concomitant illness, seizures, history or suspicion of substance abuse, diabetes mellitus, levodopa use, hypersensitivity to cannabis‐based medicines Treatment group (delta‐9‐THC/CBD): N = 34; 6 male/28 female, mean age 50.3 (SD 6.7) years; 15 with previous cannabis exposure Placebo group: N = 32; 8 male/24 female; mean age 48.1 (SD 9.7) years; 21 with previous cannabis exposure | |
| Interventions | Study medication: Oromucosal spray containing 2.7 mg THC and 2.5 mg CBD per 100 µL spray, max 48 sprays in 48 h, identically appearing placebo Rescue medication: not reported Allowed co‐therapies: amitriptylin maximally 75 mg/d | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported, calculated by imputation method. NRS 0‐10 for most troublesome neuropathic pain at daily maximum, mean of 7 days PGIC much or very much improved: reported Withdrawal due to AE: reported Serious AE attributed to medication: reported Participant‐reported pain relief ≥ 30%: not reported, calculated by imputation method Mean pain intensity: NRS 0‐10 for most troublesome neuropathic pain at daily maximum, mean of 7 days HRQoL: not assessed Sleep problems: sleep quality 10‐0; SD calculated from P value Fatigue: not assessed Psychological distress: General Health Questionnaire 0‐48: SD calculated from P value Withdrawals due to lack of efficacy: not reported Any adverse event: not reported. No details of assessment reported Nervous system disorders‐related AE: reported Psychiatric disorders‐related AE: reported | |
| Notes | Funding: GW Pharmaceuticals Conflicts of interest: Rog, Young and Nurmikko received funding and/or honoraria from GW pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐determined randomisation code that remained unknown to study personnel throughout the trial. Randomised permuted blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Pharmacist dispensed medication |
| Blinding of participants and personnel (performance bias) | Low risk | Identically appearing placebo also for smell |
| Blinding of outcome assessment (detection bias) | Low risk | Secondary outcomes assessed by blinded nurses |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | Consistent reporting of all outcomes |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | High risk | < 50 participants per study arm |
| Study characteristics | ||
| Methods | Disease: central neuropathic pain in MS Study setting: single‐centre (Neurology Department), Germany, study period 2007‐2010 Study design: parallel Study duration: dose titration of study medication over 2 weeks, 2 weeks' titration, followed by a 10‐week maintenance phase. 32 weeks open label | |
| Participants | Inclusion criteria: aged 18–70 years, met the McDonald criteria for definite MS and had stable disease symptoms and moderate‐severe central neuropathic pain (CNP) at maximal pain area for at least 3 months as reported by participants (Numerical Rating Scale (NRS) for pain ≥ 4). CNP was defined as initiated or caused by a primary lesion or dysfunction of the CNS. Exclusion criteria: any peripheral pain syndromes, pre‐existing psychotic disorders, severe cardiac diseases, or known substance abuse; dronabinol intake within the last 12 months prior to study entry or Marijuana use within 1 month prior to study entry Treatment group (dronabinol): N = 124, mean age 48.4 (SD 9.6) years, 88% female, time since CNP diagnosis 130 (96) months, pain score baseline (extracted from figure 6.6), previous cannabis use not reported Placebo group: N = 116, mean age 47.0 (SD 9.7) years, 87% female, time since CNP diagnosis 138 (98) months, pain score baseline (extracted from figure 6.8), previous cannabis use not reported | |
| Interventions | Study medication: dosing was increased every 5 days by 2.5 mg to reach a daily dose between 7.5 and 15.0 mg Rescue medication: oral intake of tramadol Allowed co‐therapies: amitriptyline and gabapentin, if started at least 3 months earlier with a stable dose | |
| Outcomes | Participant‐reported pain relief ≥ 50%:: not reported. NRS 0‐10 mean weekly pain score. Calculated by imputation method. Baseline pain scores extracted from figure PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE attributed to medication: reported Participant‐reported pain relief ≥ 30%:: not reported. NRS 0‐10 mean weekly pain score. Calculated by imputation method. Baseline pain scores extracted from figure Mean pain intensity: NRS 0‐10 mean weakly pain score HRQoL: Short form health survey SF‐36. Mean changes without SD or P value reported* Sleep problems: not assessed Fatigue: not assessed Psychological distress: not assessed Withdrawals due to lack of efficacy: reported Any adverse event: for safety analysis, vital signs, laboratory parameters, (serious) AEs (SAEs) including (serious) adverse reactions (SARs) were regularly assessed during all 3 periods. Furthermore, participantss rated the global tolerability on a 4‐point rating scale (1 = very good to 4 = poor). If study medication intake was interrupted, the investigator documented withdrawal symptoms such as restlessness, irritability, sleep interference, decreased appetite, excessive sweating, or other drug‐dependence‐related symptoms Nervous system disorders‐related AE: not reported Psychiatric disorders‐related AE: not reported | |
| Notes | Funding: Bionorica research GmbH (Innsbruck, Austria) Conflicts of interest: CN, EMK, GW, and DA‐S are employees of Bionorica SE, Germany. SS has received grant support and speaker honoraria from Bayer Vital, Bionorica, Biogen, BMS, DIAMED, Genzyme, Novartis, Pfizer, Teva. MM has received lecture fees, travel grants and honoraria for consulting from Bayer Health Care AG, Biogen GmbH, Bionorica, Merck Serono, Novartis Pharma GmbH, Sanofi‐Aventis (Genzyme), and Teva *no significant difference | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization code |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No details reported ("Full analysis set") |
| Selective reporting (reporting bias) | Low risk | All outcomes as outlined in protocol NCT00959218 reported |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: Chronic painful diabetic peripheral polyneuropathy in diabetes mellitus type 1 and 2 Study setting: Single‐centre (Diabetes Research Department), UK; study period not reported Study design: Parallel Study duration: Dose titration of study medication over 2 weeks, followed by a 10‐week maintenance phase | |
| Participants | Inclusion criteria: Neuropathy Total Symptom Score 6 > 4 and < 16 for at least 6 months with stable glycaemic control (A1C 11%), persistent pain, despite an adequate trial of tricyclic antidepressants Exclusion criteria: Not reported Treatment group (delta‐9‐THC/CBD): N = 15, Mean age 58.2 (SD 8.8) years, 4 female, mean diabetes duration 11.2 ± 8.4 years, 2 with previous cannabis use Placebo group: N = 15, 7 female, mean age 54.4 (SD 11.6) years, mean diabetes duration 13.7 (SD 6) years; 2 with previous cannabis use | |
| Interventions | Study medication: Sativex (tetrahydrocannabinol (27 mg/mL) and CBD (25 mg/mL)) as a pump‐action spray, sublingually, up to 4 doses per day Rescue medication: Not reported Allowed co‐therapies: Not reported | |
| Outcomes | Participant‐reported pain relief ≥ 50%: Reported. VAS 0‐10 PGIC much or very much improved: Not assessed Withdrawal due to AE: Reported, but not the proportion of patients in each group Serious AE attributed to medication: Not reported Participant‐reported pain relief ≥ 30%:: Not reported, calculated by imputation method (VAS 0‐10) Mean pain intensity: Neuropathic pain scale (VAS 0‐100) HRQoL: EQ‐5D health status index Sleep problems: Sleep quality 10‐0; SD calculated from P value Fatigue: Not assessed Psychological distress: General Health Questionnaire 0‐48: SD calculated from P value Withdrawals due to lack of efficacy: Not reported Any adverse event: Not reported. No details of assessment reported Nervous system disorders‐related AE: Not reported Psychiatric disorders‐related AE: Not reported | |
| Notes | Funding: Diabetes UK grant Conflicts of interest: The authors declared that they have no conflicts of interest relevant to the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | High risk | One patient excluded from ITT‐analysis |
| Selective reporting (reporting bias) | High risk | Tolerability and safety outcomes not reported |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | High risk | < 50 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: post‐herpetic neuralgia, peripheral neuropathy, focal nerve lesion, radiculopathy or CRPS type 2 associated with allodynia Study setting: 21 centres in the UK, 7 centres in Czech Republic, 6 centres in Romania, 4 centres in Belgium 1 one centre in Canada; 2005‐2006 Study design: parallel Study duration: 15‐week (1‐week baseline and 14‐week treatment period) | |
| Participants | Inclusion criteria: age ≥18 years, mechanical allodynia within the territory of the affected nerve(s) (confirmed by either a positive response to stroking the allodynic area with a SENSELABTM Brush 05 (Somedic AB, Hörby, Sweden) or to force applied by a 5.07 g Semmes‐Weinstein monofilament), at least a 6‐month disease history (post‐herpetic neuralgia, peripheral neuropathy, focal nerve lesion, radiculopathy or CRPS CRPS type 2), receiving the appropriate treatment, sum score of at least 24 on a pain 0–10 NRS for more than 6 days (baseline days 2–7) during the baseline period (average 0–10 NRS score of 4/10), and pain that was not wholly relieved by their current therapy. Stable analgesic regimen for at least 2 weeks preceding study entry. Exclusion criteria: severe pain from other concomitant conditions; history of significant psychiatric, renal, hepatic, cardiovascular or convulsive disorders, or with a known hypersensitivity to the study medication; CRPS type 1, cancer‐related PNP or pain resulting from diabetes mellitus; receiving a prohibited medication (including cannabis or cannabinoid‐based medications (in the last year), any analgesics taken on a ‘PRN’ (when required) basis, the introduction of any new analgesic medication, or any alteration to the dosage of the patient’s concomitant analgesic medication (other than the rescue analgesia provided), or all paracetamol‐containing medications (stopped on the day the patient entered the baseline period)), patients unwilling to abstain for the study duration; patients with a known history of alcohol or substance abuse; women of child‐bearing potential or their partners unless willing to ensure effective contraception was used throughout the study, participants who had received an investigational medicinal product within 12 weeks of screening; pregnant or lactating women and those planning a pregnancy; people with any physical abnormality at screening (i.e. any abnormalities that, in the opinion of the investigator, would prevent the participant from safely participating in the study), or those intending to travel or donate blood during the study Treatment group (delta‐9‐THC): N = 128; 66% female; mean age 57.6 (mean age 14.4) years; 99% white; duration of neuropathic pain 6.3 (SD 6.7 years), 13 with cannabis exposure (10%) Placebo group: N = 118, 55% female; mean age 57 (SD 14.1) years; 98% white; duration of neuropathic pain 6.3 (SD 6.4) years, 12 with cannabis exposure (10%) | |
| Interventions | Study medication: pump action oromucosal spray, each 100 μL spray of THC/CBD delivered 2.7 mg of THC and 2.5 mg of CBD, each spray of placebo delivered the excipients plus colorants, both THC/CBD spray and placebo contained peppermint oil to blind the smell and taste, maximum of eight sprays in a 3‐h period up to a maximum of 24 sprays per 24‐h period Rescue medication: paracetamol 500 mg, max. Single dose 1 g, max. Daily dose 4 g Allowed co‐therapies: concomitant analgesic medication, with the exception of paracetamol (acetaminophen), provided that a stable dose was maintained throughout the study | |
| Outcomes | Participant‐reported pain relief ≥ 50%: NRS 0‐10. Only OR reported: not suited for meta‐analysis (P = 0.157) PGIC much or very much improved: reported Withdrawal due to AE: reported Serious AE: reported; systematic assessment Participant‐reported pain relief ≥ 30%: NRS 0‐10; only OR reported: not suited for meta‐analysis (P = 0.021) Mean pain intensity: Neuropathic pain scale: data not suited for meta‐analysis (P = 0.069) HRQoL: EQ‐5D Health Status 100 to 0 Sleep problems: sleep quality 10‐0; SD calculated from P value Fatigue: not assessed Psychological distress: General Health Questionnaire 0‐48: SD calculated from P value Withdrawals due to lack of efficacy: reported Any adverse event: reported; "systematic assessment" Nervous system disorders‐related AE: reported; systematic assessment Psychiatric disorders‐related AE: reported; systematic assessment | |
| Notes | Funding: GW Pharmaceuticals. GW Pharmaceuticals was involved in the study design, data collection and analysis, as well as in the preparation of this manuscript and publication decisions Conflicts of interest: all authors received investigator fees from GW Pharma Ltd (GW) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by GW Biometrics department; sealed code break envelopes for each partcipant |
| Blinding of participants and personnel (performance bias) | Low risk | THC/CBD and placebo spray contained peppermint oil to blind to taste and smell |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Low risk | Study protocol (NCT 00710554) available; all predefined outcomes reported |
| Group similarity at baseline | Low risk | Similar demographic and clinical characteristics at baseline |
| Sample size bias | Unclear risk | 50‐199 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: MS (central pain) Study setting: outpatient clinic, University Hospital of Aarhus, Denmark; study period 2001 Study design: cross‐over Study duration: 15‐20 days with washout period of at least 21 days (actually 19‐57), 1 week baseline, 3 weeks intervention, 3 weeks washout, 3 weeks intervention | |
| Participants | Inclusion criteria: diagnosed with MS, aged 18‐55 years, pain ≥ 3 on 0‐10 NRS, investigators assessed central pain examination, central pain being a pain in a body territory with abnormal sensation to pinprick, touch, warmth, cold, ability to differentiate central from spasticity‐related pain Exclusion criteria: musculoskeletal disorders, PNP, visceral pain at max. pain site, hypersensitivity to cannabis‐based medicines or sesame oil, heart disease, mania, depression or schizophrenia, alcohol or drug misuse, no antidepressants, anticholinergic, antihistaminic agents or CNS depressants, use of analgesic drugs, (medications had to be stopped 1 week before first visit) pregnancy or lactation, sexually active women without reliable contraception, other clinical trials, lack of co‐operation, use of marijuana within 3 months before the study, unwillingness to abstain from marijuana use Treatment group (dronabinol) and placebo group: N = 24; 41.7% male, mean age 50 (23‐55) years, no ethnic group, current cannabis use not reported | |
| Interventions | Study medication: dronabinol starting with 1 x 2.5 mg capsules up to 2 x 5 mg/d Rescue medication: paracetamol Allowed co‐therapies: spasmolytic drugs and paracetamol | |
| Outcomes | Participant‐reported pain relief ≥ 50%: reported. NRS 0‐10 (end of treatment period) PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE: reported Participant‐reported pain relief ≥ 30%: not reported.Not calculable by imputation method because baseline values not reported Mean pain intensity: median spontaneous pain intensity NRS 0‐10 during the last week of treatment HRQoL: SF‐36 physical functioning (50‐0); data of first treatment period used for analysis; SD calculated from P value Sleep problems: not assessed Fatigue: not assessed Psychological distress: SF‐36 mental health (50‐0). Data of first treatment period used for analysis; SD calculated from P value Withdrawals due to lack of efficacy: reported Any adverse event: reported. "Patient used their own words to record AEs in diaries" Nervous system disorders‐related AE: reported Psychiatric disorders‐related AE: reported | |
| Notes | Funding: the study was supported by grants from the Danish Multiple Sclerosis Society (grant no 2002/71045), grant 900035 from manager Ejnar Jonasseon and his wife’s memorial grant, and the Warwara Larsen Foundation (grant no 664.28), Denmark. Solvay Pharmaceuticals provided study medication (dronabinol (Marinol) and placebo capsules), labelling, and packaging. In addition, the company provided financial support for study monitoring and data analysis. IPC‐Nordic, Denmark, packaged and labelled the study medication and monitored the study. These companies were not involved in the design or execution of the study or writing the manuscript. Conflicts of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We assigned patients to treatment sequence by using a computer generated randomisation code with a block size of six prepared by IPC‐Nordic" |
| Allocation concealment (selection bias) | Low risk | "Investigators allocated patients consecutively by time of inclusion at the study site. One investigator (KBS) enrolled all participants and allocated them to treatment". |
| Blinding of participants and personnel (performance bias) | Low risk | "We administered both active treatment and placebo as white capsules (soft gelatin capsules) in identical containers. The taste and smell of the capsules did not differ." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | All participants terminated the study |
| Selective reporting (reporting bias) | Unclear risk | No study protocol reported |
| Group similarity at baseline | Low risk | No significant differences in demographic and clinical characteristics between the study groups because of study design |
| Sample size bias | High risk | < 25 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: diabetic peripheral polyneuropathy Study setting: single‐centre, Canada; study period not reported Study design: EERW Study duration: single‐blind for 4 weeks, double‐blind randomised withdrawal for 5 weeks | |
| Participants | Inclusion criteria: DPN pain questionnaire score ≥ 4, pain duration at least 3 months, pain severity with averaged scores of P40 mm on the 100‐mm VAS of the short‐Form McGill Pain Questionnaire Exclusion criteria: participants with other causes of pain, including PHN, lumbar radiculopathy, central neuropathic pain, CRPSs I or II, or significant osteoarthritis, were excluded. Any skin conditions over the area of DPN which could hinder examination, led to exclusion. Any current diagnoses of schizophrenia, psychotic disorder, bipolar affective disorder, obsessive compulsive disorder, or major depressive disorder were also exclusionary. Clinically significant unstable medical conditions that could compromise participation, such as with poor diabetic control (haemoglobin A1C ≥ 11%), history of substance abuse or dependence, malignancy other than squamous cell carcinoma in the last 2 years, elevation of liver enzymes above 3 times the upper limit of normal, or an anticipated need for surgery or hospitalisation within the next 16 weeks after screening led to exclusion at the discretion of the investigator. Those participants previously exposed to nabilone were excluded. Any use of self‐obtained cannabis‐based medicines or other illicit drugs during the study was prohibited, and participants with a positive urinary illicit drug screen (including detection of 11‐nor‐delta‐9‐ tetrahydrocannabinol‐9‐carboxylic acid) were excluded at screening. Treatment group (nabilone (delta‐9‐THC)): N = 13; mean age 61.6 (SD 14.6) years; 69% male; 92% white; duration of diabetes 10 (SD 12.6) years. No reports on previous cannabis use Placebo group: N = 13; mean age 60.8 (SD 15.2) years; 38% male; 92% white; duration of diabetes 9.7 (SD 13.1) years. No reports on previous cannabis use | |
| Interventions | Study medication: nabilone 1 mg‐5 mg/d orally Rescue medication: placebo drug Allowed co‐therapies: no details provided | |
| Outcomes | Participant‐reported pain relief ≥ 50%: reported (NRS 0‐10 over the preceding 24 h) PGIC much or very much improved: reported (in figure) Withdrawal due to AE: reported Serious AE: reported Participant‐reported pain relief ≥ 30%: reported Mean pain intensity: average pain intensity (VAS 0‐10) HRQoL: Euro‐QOL VAS 100‐0 Sleep problems: Medical Outcomes Study Sleep problems index: reported Fatigue: not assessed Withdrawals due to lack of efficacy: reported Any adverse event: reported; "All spontaneously reported and observed AEs were recorded at each clinic visit and during telephone follow‐up visits" Nervous system disorders‐related AE: incompletely reported. Not suited for meta‐analysis Psychiatric disorders‐related AE: reported | |
| Notes | Funding: Valeant Conflicts of interest: Dr. Toth received honoraria from Valeant Canada for educational lectures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Electronic randomization system was used to randomise individual subjects without block randomisation as developed by an outside coordinator" |
| Allocation concealment (selection bias) | Low risk | "Randomization was concealed from subjects, clinical coordinator, and assessing physicians" |
| Blinding of participants and personnel (performance bias) | Low risk | "Medication was blinded for placebo using capsules of identical size, colour, taste, and smell." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT by LOCF |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available (NCT01035281) but no outcomes reported |
| Group similarity at baseline | High risk | Significant difference in sex ratio at baseline |
| Sample size bias | High risk | < 25 participants per treatment arm |
| Study characteristics | ||
| Methods | Disease: non HIV neuropathy > 3 months duration caused by trauma, surgery; with pain ≥ 40/100 VAS, stable analgesic regimen Study setting: single‐centre university, Canada; 2003‐2006 Study design: 4 periods cross‐over Study duration: 2 weeks with 5 treatment days per each period, 9 days' washout | |
| Participants | Inclusion criteria: men and women aged ≥ 18 years with neuropathic pain of at least 3 months in duration caused by trauma or surgery, with allodynia or hyperalgesia, and with an average weekly pain intensity score > 4 on a 10‐cm VAS. Participants had a stable analgesic regimen and reported not having used cannabis during the year before the study Potential participants had to have normal liver function (defined as aspartate aminotransferase < 3 times normal), normal renal function (defined as a serum creatinine level < 133 μmol/L), normal haematocrit (> 38%) and a negative result on β human chorionic gonadotropin pregnancy test (if applicable). Exclusion criteria: pain due to cancer or nociceptive causes, presence of significant cardiac or pulmonary disease, current substance abuse or dependence (including abuse of or dependence on cannabis), history of psychotic disorder, current suicidal ideation, pregnancy or breastfeeding, participation in another clinical trial within 30 days of enrolment, and ongoing insurance claims Treatment group (delta‐9‐THC)/placebo group): N = 23 participants, mean age: 45.4 years (SD 12.3); gender (male/female): 11/12; 18 (81%) with previous cannabis exposure, but not within the year prior to the study | |
| Interventions | Study medication: 3 different potencies of THC (2.5%, 6%, 9.4%) from whole herb in gelatine capsules inhaled through pipe. Placebo cigarettes underwent ethanolic extraction. Dose estimate: 0, 1.625, 3.9 and 5.85 mg/d (average) THC per period Rescue medication: not reported Allowed co‐therapies: "Stable regimen" | |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported and not calculable by imputation method. Average daily pain Intensity on 0‐10 NRS average over 5 treatment days PGIC much or very much improved: not assessed Withdrawal due to AE: reported Serious AE attributed to study medication: reported Participant‐reported pain relief ≥ 30%: not reported and not calculable by imputation method Mean pain intensity: average daily pain intensity on 0‐10 NRS HRQoL: EQ‐5D state of health VAS 100‐0 Sleep problems: sleep quality Leeds Sleep Evaluation Questionnaire 0‐10 Fatigue: not assessed Psychological distress: Profile of Mood States total mood disturbance 0‐200 Withdrawals due to lack of efficacy: not reported Any adverse event: reported; No details of assessment reported Nervous system disorders‐related AE: reported Psychiatric disorders‐related AE: reported | |
| Notes | Funding: Canadian Institutes of Health (JHM 50014) and Louise and Alan Wards Foundation Conflicts of interest: the study authors declare that they have not conflict of interest. "We found no evidence of significant carry‐over effect for any outcome" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details for investigators reported. Participants correctly guessed allocation at the end of the trial |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Selective reporting (reporting bias) | Low risk | Consistent reporting according to study protocol (ISRCT68314063) |
| Group similarity at baseline | Low risk | Identical demographic and baseline characteristics due to study protocol |
| Sample size bias | High risk | < 25 participants per treatment arm |
AE: adverse events; bpm: beats per minute; BSI: Brief Symptom Inventory; CBD: cannabidiol; CNS: central nervous system; CRPS: complex regional pain syndrome; DHC: dihydrocodeine; DPN: diabetic peripheral neuropathic; DSM: Diagnostic and Statistical Manual of Mental Disorders; EERW: enriched enrolment randomised withdrawal; EQ‐5D: EuroQol quality of life instrument; HR: heart rate; HRQoL: Health‐related quality of life; ITT: intention‐to‐treat; LOCF: last observation carried forward; mg: milligrams; MAO: monoamine oxidase; MI: myocardial infarction; μL = microlitre; mL = millilitre; µmol/L: micromoles per litre; MS: multiple sclerosis; N: number; NDS: Neuropathy Disability Score; NNTB: number needed to treat for an additional beneficial outcome; NRS: numerical rating scale; NSAIDs: non‐steroidal anti‐inflammatory drugs; OR: odds ratio; PGIC: Patient Global Impression of Change; PHN: postherpetic neuralgia; PNP: peripheral neuropathic pain; SD; standard deviation; SIP: Sickness Impact Profile;SF‐36: short‐form 36 quality of life instrument; TENS: transcutaneous electrical nerve stimulation; THC: tetrahydrocannabinol; VAS: visual analogue scale; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Cannabis cigarettes or placebo cigarettes in 55 participants. HIV‐associated neuropathy; study duration < 2 weeks | |
| Smoked cannabis or placebo cigarettes in 30 participants with MS for 2 weeks; no definite statement that the pain was of neuropathic nature | |
| Synthetic THC or oral placebo in 21 participants with chronic neuropathic central and peripheral pain of various aetiologies; study duration < 2 weeks | |
| 34 ‘N of 1’ studies with THC, CBD and THC/CBD or placebo over 12 weeks; 2 participants with non‐neuropathic pain included | |
| 572 participants with MS were treated with THC/CBD spray for 12 weeks; participants were selected because of spasticity refractory to conventional treatment; no definite statement that the pain was of neuropathic nature | |
| Randomised, controlled, double‐blind, cross‐over pilot study with 7 participants with spinal cord injury and neuropathic pain comparing dronabinol with diphenhydramine; < 10 participants per treatment arm | |
| 15 participants with MS‐induced neuropathic pain were treated with nabilone as an adjunctive to gabapentin for 9 weeks; < 10 participants per treatment arm | |
| 20 participants with neurogenic symptoms due to lesions of the central or peripheral nervous system were treated with plant‐based THC/CBD for 2 weeks in a cross‐over design. 13 of 20 participants with pain. No statement or analysis that carry‐over effects were excluded | |
| 160 participants with MS treated with THC/CBD spray or placebo spray of 6 weeks; no definite statement that the pain was of neuropathic nature | |
| Inhaled cannabis in 16 participants with painful diabetic polyneuropathy for 4 single dosing sessions. Study duration < 2 weeks | |
| Vaporised cannabis (1.3% and 3.5%) or placebo in 39 participants with central and peripheral neuropathic pain for 1 day (experimental study) | |
| 38 participants with central or peripheral neuropathic pain were treated with smoked cannabis or placebo. Study duration < 1 week | |
| Nabilone or placebo in 11 participants with MS und upper motor neuron disease‐associated spasticity‐related pain for 4 weeks; no definite statement that the pain was of neuropathic nature | |
| 667 participants with MS were treated with oral cannabis extract (THC) or delta 9‐THC or placebo for 15 weeks. Spasticity was the primary outcome. Pain was a secondary outcome; only around 65% of participants had pain, with no pain intensity at baseline reported | |
| 275 patients with MS were treated for 12 weeks with plant‐derived THC 2.5‐15 mg/d orally or placebo. No definite statement that the pain was of neuropathic nature |
CBD: cannabidiol; mg: milligrams; µmol/L: micromoles per litre;MS: multiple sclerosis; THC: tetrahydrocannabinol;
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Disease: phantom limb pain Study setting: single‐centre university, Canada; 2009‐2011 Study design: parallel Study duration: 6 weeks |
| Participants | Inclusion criteria
Exclusion criteria:
Treatment group nabilone/placebo group: N = not reported |
| Interventions | Study medication: nabilone 0.5 mg at bedtime for 1 week, then 0.5 mg twice daily for 1 week. After a reassessment of the outcome measures, the dose is increased to 0.5 mg in the morning and 1 mg at hs for 1 week, followed by an increase to 1 mg twice daily in the last week of the study. Rescue medication: not reported Allowed co‐therapies: not reported |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported PGIC much or very much improved: not assessed Withdrawal due to AE: not reported Serious AE attributed to study medication: not reported Participant‐reported pain relief ≥ 30%: not reported Mean pain intensity: VAS for pain; not reported HRQoL:: SF‐36 not reported Sleep problems: Groningen Sleep Quality Scale; not reported Fatigue: not assessed Psychological distress: Hospital Anxiety and Depression Scale not reported Withdrawals due to lack of efficacy: not reported Any adverse event: reported; no details of assessment reported Nervous system disorders‐related AE: not reported Psychiatric disorders‐related AE: not reported |
| Notes | Funding: Valeant, University of Manitoba Conflicts of interest: not declared |
| Methods | Disease: diabetic neuropathic pain Study setting: single‐centre university, Canada; start 2009; the recruitment status of this study is unknown because the information has not been verified recently. Study design: EERW Study duration: all participants who experienced at least a 30% reduction in their weekly mean pain score during the 4‐week, single‐blind flexible dosing phase considered a responder, and further continued in the study. During the double‐blind portion of the study, participants randomised to nabilone continued on the dose of nabilone achieved at the completion of the single‐blind phase, and this dose was maintained throughout the double‐blind phase. Participants randomised to placebo received 1 mg of nabilone daily for 1 week, followed by 4 consecutive weeks of placebo. This dose of nabilone permitted a tapering for those participants achieving a higher daily dose of nabilone during the single‐blind phase, or maintained those who were taking only 1 mg/d in the single‐blind phase, preventing an abrupt termination of treatment in participants who were randomised into the placebo portion. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Study medication:nabilone, flexible dosing nabilone at 0.5 mg‐4 mg/d Rescue medication: not reported Allowed co‐therapies: not reported |
| Outcomes | Participant‐reported pain relief ≥ 50%: no information provided PGIC much or very much improved: no information provided Withdrawal due to AE: no information provided Serious AE attributed to study medication: no information provided Participant‐reported pain relief ≥ 30%: no information provided Mean pain intensity: no information provided HRQoL:: no information provided Sleep problems: no information provided Fatigue: no information provided Psychological distress: no information provided Withdrawals due to lack of efficacy: no information provided Any adverse event: no information provided Nervous system disorders‐related AE: no information provided Psychiatric disorders‐related AE: no information provided |
| Notes | Funding: University of Calgary Conflicts of interest: not declared |
| Methods | Disease: neuropathic pain in spinal cord injured persons Study setting: single‐centre university, Canada; 2012‐2015 Study design: cross‐over Study duration: 11 weeks each period |
| Participants | Inclusion criteria
Exclusion criteria
Treatment group nabilone/placebo group: N = not reported |
| Interventions | Study medication: nabilone 0.5 mg tablets od titrated to a maximum daily dose of 3 mg by mouth over an 11‐week phase; placebo 0.5 mg by mouth daily, dose titrated to a maximum daily dose of 3.0 mg by mouth over an 11‐week phase Rescue medication: not reported Allowed co‐therapies: not reported |
| Outcomes | Participant‐reported pain relief ≥ 50%: not reported PGIC much or very much improved: not reported Withdrawal due to AE: not reported Serious AE attributed to study medication: not reported Participant‐reported pain relief ≥ 30%: not reported Mean pain intensity: VAS for pain and Neuropathic Pain Questionnaire; not reported HRQoL:: SF‐36 not reported Sleep problems: Pittsburgh Sleep Quality Index; not reported Fatigue: not assessed Psychological distress: not assessed Withdrawals due to lack of efficacy: not reported Any adverse event: reported; no details of assessment reported Nervous system disorders‐related AE: not reported Psychiatric disorders‐related AE: not reported |
| Notes | Funding: University of Manitoba The Manitoba Spinal Cord Injury Research Fund Canadian Paraplegic Association Health Sciences Centre Foundation, Manitoba Conflicts of interest: not declared |
AE; adverse events; ALT: alanine aminotransferase; AST: aspartate aminotransferase; DSM: Diagnostic and Statistical Manual of Mental Disorders; EERW: enriched enrolment randomised withdrawal; GGT: gamma‐glutamyl transferase; HRQoL: health‐related quality of life; mg: milligrams; MI: myocardial infarction; N: number; PGIC: Patient Global Impression of Change; SF‐36: short‐form 36 quality of life instrument; THC: tetrahydrocannabinol; VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain relief of 50% or greater Show forest plot | 8 | 1001 | Risk Difference (IV, Random, 95% CI) | 0.05 [0.00, 0.09] |
| Analysis 1.1  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 1: Pain relief of 50% or greater | ||||
| 1.1.1 Central pain ‐ multiple sclerosis | 4 | 669 | Risk Difference (IV, Random, 95% CI) | 0.08 [‐0.00, 0.15] |
| 1.1.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.06, 0.28] |
| 1.1.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 30 | Risk Difference (IV, Random, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| 1.1.4 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.1.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 125 | Risk Difference (IV, Random, 95% CI) | 0.13 [0.00, 0.25] |
| 1.2 Patient Global Impression much or very much improved Show forest plot | 6 | 1092 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| Analysis 1.2  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 2: Patient Global Impression much or very much improved | ||||
| 1.2.1 Central pain ‐ multiple sclerosis | 2 | 397 | Risk Difference (IV, Random, 95% CI) | 0.06 [‐0.01, 0.14] |
| 1.2.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.34 [0.17, 0.50] |
| 1.2.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 281 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.14] |
| 1.2.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 228 | Risk Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.17] |
| 1.2.5 Central or peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.19] |
| 1.3 Withdrawals due to adverse events Show forest plot | 13 | 1848 | Risk Difference (IV, Random, 95% CI) | 0.04 [0.02, 0.07] |
| Analysis 1.3  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 3: Withdrawals due to adverse events | ||||
| 1.3.1 Central pain ‐ multiple sclerosis | 4 | 693 | Risk Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.08] |
| 1.3.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| 1.3.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.10, 0.10] |
| 1.3.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 1.3.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 68 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 1.3.6 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.3.7 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 427 | Risk Difference (IV, Random, 95% CI) | 0.08 [0.02, 0.13] |
| 1.3.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.07] |
| 1.4 Serious adverse events Show forest plot | 13 | 1876 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| Analysis 1.4  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 4: Serious adverse events | ||||
| 1.4.1 Central pain ‐ multiple sclerosis | 4 | 693 | Risk Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.06] |
| 1.4.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 1.4.3 Peripheral pain ‐ chemotherapy‐induced neuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.10, 0.10] |
| 1.4.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.05, 0.08] |
| 1.4.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 68 | Risk Difference (IV, Random, 95% CI) | 0.03 [‐0.07, 0.13] |
| 1.4.6 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 1.4.7 Peripheral pain ‐ polyneuropathies of various aetiologies | 3 | 455 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 1.4.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.06 [‐0.15, 0.03] |
| 1.5 Pain relief of 30% or greater Show forest plot | 10 | 1586 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.03, 0.15] |
| Analysis 1.5  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 5: Pain relief of 30% or greater | ||||
| 1.5.1 Central pain ‐ multiple sclerosis | 3 | 645 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.25] |
| 1.5.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.16, 0.38] |
| 1.5.3 Peripheral pain ‐ diabetic polyneuropathy | 2 | 327 | Risk Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| 1.5.4 Peripheral pain ‐ HIV polyneuropathy | 1 | 56 | Risk Difference (IV, Random, 95% CI) | 0.29 [0.05, 0.52] |
| 1.5.5 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.25] |
| 1.5.6 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 381 | Risk Difference (IV, Random, 95% CI) | 0.11 [0.03, 0.19] |
| 1.6 Mean pain intensity Show forest plot | 14 | 1837 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.60, ‐0.09] |
| Analysis 1.6  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 6: Mean pain intensity | ||||
| 1.6.1 Central pain ‐ multiple sclerosis | 4 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 1.6.2 Central pain ‐ spinal cord injury | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.41, 0.33] |
| 1.6.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.86, 0.45] |
| 1.6.4 Peripheral pain ‐ diabetic polyneuropathy | 2 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.27, 0.17] |
| 1.6.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.94, 0.12] |
| 1.6.6 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.79, ‐0.08] |
| 1.6.7 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 428 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.75, 0.44] |
| 1.6.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.71, 0.23] |
| 1.7 Health‐related quality of life Show forest plot | 9 | 1284 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.10, 0.13] |
| Analysis 1.7  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 7: Health‐related quality of life | ||||
| 1.7.1 Central pain ‐ multiple sclerosis | 2 | 363 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.27, 0.14] |
| 1.7.2 Central pain ‐ spinal cord injury | 1 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.37, 0.37] |
| 1.7.3 Peripheral pain ‐ diabetic polyneuropathy | 2 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.06, 0.39] |
| 1.7.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.42, 0.28] |
| 1.7.5 Peripheral pain of various aetiologies | 2 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 1.7.6 Central and peripheral pain ‐ various aetiologies | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.35, 0.64] |
| 1.8 Sleep problems Show forest plot | 8 | 1386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.90, ‐0.04] |
| Analysis 1.8  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 8: Sleep problems | ||||
| 1.8.1 Central pain ‐ multiple sclerosis | 1 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.22] |
| 1.8.2 Central pain ‐ spinal cord injury | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.41, 0.32] |
| 1.8.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 1.8.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.78, ‐0.07] |
| 1.8.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐2.17, 0.61] |
| 1.8.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.78, 0.16] |
| 1.9 Psychological distress Show forest plot | 7 | 779 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.61, ‐0.02] |
| Analysis 1.9  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 9: Psychological distress | ||||
| 1.9.1 Central pain ‐ multiple sclerosis | 2 | 363 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.65, 0.59] |
| 1.9.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.78, ‐0.37] |
| 1.9.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.97, 0.47] |
| 1.9.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.62, 0.08] |
| 1.9.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.80, ‐0.16] |
| 1.10 Withdrawals due to lack of efficacy Show forest plot | 9 | 1576 | Risk Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| Analysis 1.10  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 10: Withdrawals due to lack of efficacy | ||||
| 1.10.1 Central pain ‐ multiple sclerosis | 4 | 697 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 1.10.2 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 1.10.3 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 1.10.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 371 | Risk Difference (IV, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.10.5 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 1.11 Any adverse event Show forest plot | 7 | 1356 | Risk Difference (IV, Random, 95% CI) | 0.19 [0.12, 0.27] |
| Analysis 1.11  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 11: Any adverse event | ||||
| 1.11.1 Central pain ‐ multiple sclerosis | 3 | 627 | Risk Difference (IV, Random, 95% CI) | 0.22 [0.05, 0.39] |
| 1.11.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.34 [0.18, 0.50] |
| 1.11.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.12 [0.02, 0.22] |
| 1.11.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 246 | Risk Difference (IV, Random, 95% CI) | 0.15 [0.05, 0.25] |
| 1.11.5 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.21 [0.06, 0.36] |
| 1.12 Specific adverse event: nervous system disorders Show forest plot | 9 | 1304 | Risk Difference (IV, Random, 95% CI) | 0.38 [0.18, 0.58] |
| Analysis 1.12  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 12: Specific adverse event: nervous system disorders | ||||
| 1.12.1 Central pain ‐ multiple sclerosis | 3 | 453 | Risk Difference (IV, Random, 95% CI) | 0.33 [0.09, 0.58] |
| 1.12.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.53 [0.38, 0.68] |
| 1.12.3 Peripheral pain ‐chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 1.12.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.26 [0.15, 0.37] |
| 1.12.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 332 | Risk Difference (IV, Random, 95% CI) | 0.29 [0.19, 0.39] |
| 1.12.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.37 [0.15, 0.58] |
| 1.13 Specific adverse event: psychiatric disorders Show forest plot | 9 | 1314 | Risk Difference (IV, Random, 95% CI) | 0.10 [0.06, 0.15] |
| Analysis 1.13  Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 13: Specific adverse event: psychiatric disorders | ||||
| 1.13.1 Central pain ‐ multiple sclerosis | 3 | 453 | Risk Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.16] |
| 1.13.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 1.13.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.06, 0.28] |
| 1.13.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 1.13.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 342 | Risk Difference (IV, Random, 95% CI) | 0.21 [0.14, 0.29] |
| 1.13.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.27] |
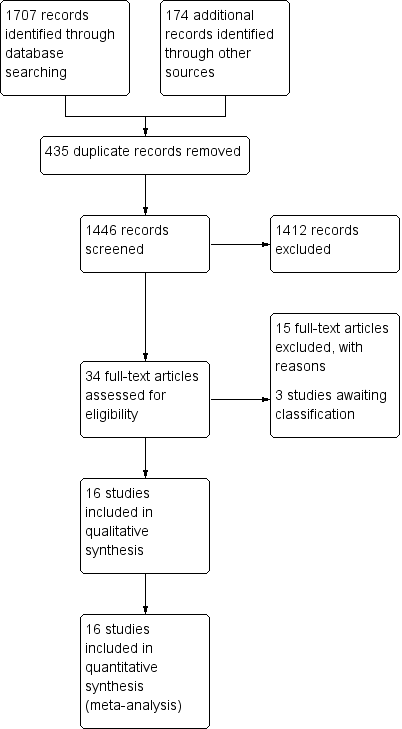
Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 1: Pain relief of 50% or greater

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 2: Patient Global Impression much or very much improved

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 3: Withdrawals due to adverse events

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 4: Serious adverse events

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 5: Pain relief of 30% or greater

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 6: Mean pain intensity

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 7: Health‐related quality of life

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 8: Sleep problems

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 9: Psychological distress

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 10: Withdrawals due to lack of efficacy

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 11: Any adverse event

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 12: Specific adverse event: nervous system disorders

Comparison 1: Cannabis‐based medicines versus placebo at final treatment, Outcome 13: Specific adverse event: psychiatric disorders
| Cannabis‐based medicines compared with placebo for chronic neuropathic pain | ||||||
| Patient or population: adults with chronic neuropathic pain Settings: outpatient study centres and hospitals in Europe and North America Intervention: cannabis‐based medicines (smoked cannabis; oral plant‐based (dronabinol) or synthetic tetrahydrocannabinol (THC) (nabilone); oromucosal spray of THC and cannabidiol (CBD)) Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention 95% CI | Probable outcome with placebo | Relative effect Risk difference (95% CI) | No. of participants | Quality of the evidence | Comments |
|---|---|---|---|---|---|---|
| Participant‐reported pain relief of 50% or greater | 209 per 1000 (196 to 222) | 173 per 1000 | 0.05 (0.00 to 0.09) | 1001 (8 studies) | ⊕⊕⊝⊝ low1,2 | NNTB 20 (11 to 100) |
| Patient Global Impression of Change much or very much improved | 261 per 1000 (246 to 276) | 211 per 1000 | 0.09 (0.01 to 0.17) | 1092 (6 studies) | ⊕⊝⊝⊝ very low1,3,4 | NNTB 11 (6 to 100) |
| Withdrawals due to adverse events | 104 per 1000 (99 to 107) | 47 per 1000 | 0.04 (0.02 to 0.07) | 1848 (13 studies) | ⊕⊕⊕⊝ moderate1 | NNTH 25 (16 to 50) |
| Serious adverse events | 66 per 1000 (63 to 69) | 52 per 1000 | 0.01 (‐0.01 to 0.03) | 1876 (13 studies) | ⊕⊕⊝⊝ low1,2 | NNTH not calculated |
| Participant‐reported pain relief of 30% or greater | 377 per 1000 (358 to 396) | 304 per 1000 | 0.09 (0.03 to 0.15) | 1586 (10 studies) | ⊕⊕⊕⊝ moderate1 | NNTB 11 (7 to 33) |
| Specific adverse events:nervous system disorder | 611 per 1000 (576 to 644) | 287 per 1000 | 0.38 (0.18 to 0.58) | 1304 (9 studies) | ⊕⊕⊝⊝ low1,3 | NNTH 3 (2 to 6) |
| Specific adverse events:psychiatric disorders | 165 per 1000 (156 to 174) | 49 per 1000 | 0.10 (0.06 to 0.15) | 1314 (9 studies) | ⊕⊕⊝⊝ low1,3 | NNTH 10 (7 to 16) |
| Abbreviations: | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| 1 Downgraded once: indirectness. People with current or historical substance abuse, or both, and major medical diseases excluded. 4 Downgraded once: Publication bias. All studies funded by the manufacturer of the drug. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain relief of 50% or greater Show forest plot | 8 | 1001 | Risk Difference (IV, Random, 95% CI) | 0.05 [0.00, 0.09] |
| 1.1.1 Central pain ‐ multiple sclerosis | 4 | 669 | Risk Difference (IV, Random, 95% CI) | 0.08 [‐0.00, 0.15] |
| 1.1.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.06, 0.28] |
| 1.1.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 30 | Risk Difference (IV, Random, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| 1.1.4 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.1.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 125 | Risk Difference (IV, Random, 95% CI) | 0.13 [0.00, 0.25] |
| 1.2 Patient Global Impression much or very much improved Show forest plot | 6 | 1092 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| 1.2.1 Central pain ‐ multiple sclerosis | 2 | 397 | Risk Difference (IV, Random, 95% CI) | 0.06 [‐0.01, 0.14] |
| 1.2.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.34 [0.17, 0.50] |
| 1.2.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 281 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.14] |
| 1.2.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 228 | Risk Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.17] |
| 1.2.5 Central or peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.19] |
| 1.3 Withdrawals due to adverse events Show forest plot | 13 | 1848 | Risk Difference (IV, Random, 95% CI) | 0.04 [0.02, 0.07] |
| 1.3.1 Central pain ‐ multiple sclerosis | 4 | 693 | Risk Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.08] |
| 1.3.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.17] |
| 1.3.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.10, 0.10] |
| 1.3.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 1.3.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 68 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 1.3.6 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.3.7 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 427 | Risk Difference (IV, Random, 95% CI) | 0.08 [0.02, 0.13] |
| 1.3.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.07] |
| 1.4 Serious adverse events Show forest plot | 13 | 1876 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 1.4.1 Central pain ‐ multiple sclerosis | 4 | 693 | Risk Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.06] |
| 1.4.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 1.4.3 Peripheral pain ‐ chemotherapy‐induced neuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.10, 0.10] |
| 1.4.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.05, 0.08] |
| 1.4.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 68 | Risk Difference (IV, Random, 95% CI) | 0.03 [‐0.07, 0.13] |
| 1.4.6 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 1.4.7 Peripheral pain ‐ polyneuropathies of various aetiologies | 3 | 455 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 1.4.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | ‐0.06 [‐0.15, 0.03] |
| 1.5 Pain relief of 30% or greater Show forest plot | 10 | 1586 | Risk Difference (IV, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 1.5.1 Central pain ‐ multiple sclerosis | 3 | 645 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.25] |
| 1.5.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.16, 0.38] |
| 1.5.3 Peripheral pain ‐ diabetic polyneuropathy | 2 | 327 | Risk Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| 1.5.4 Peripheral pain ‐ HIV polyneuropathy | 1 | 56 | Risk Difference (IV, Random, 95% CI) | 0.29 [0.05, 0.52] |
| 1.5.5 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.25] |
| 1.5.6 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 381 | Risk Difference (IV, Random, 95% CI) | 0.11 [0.03, 0.19] |
| 1.6 Mean pain intensity Show forest plot | 14 | 1837 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.60, ‐0.09] |
| 1.6.1 Central pain ‐ multiple sclerosis | 4 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 1.6.2 Central pain ‐ spinal cord injury | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.41, 0.33] |
| 1.6.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.86, 0.45] |
| 1.6.4 Peripheral pain ‐ diabetic polyneuropathy | 2 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.27, 0.17] |
| 1.6.5 Peripheral pain ‐ HIV polyneuropathy | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.94, 0.12] |
| 1.6.6 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.79, ‐0.08] |
| 1.6.7 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 428 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.75, 0.44] |
| 1.6.8 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.71, 0.23] |
| 1.7 Health‐related quality of life Show forest plot | 9 | 1284 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.10, 0.13] |
| 1.7.1 Central pain ‐ multiple sclerosis | 2 | 363 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.27, 0.14] |
| 1.7.2 Central pain ‐ spinal cord injury | 1 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.37, 0.37] |
| 1.7.3 Peripheral pain ‐ diabetic polyneuropathy | 2 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.06, 0.39] |
| 1.7.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.42, 0.28] |
| 1.7.5 Peripheral pain of various aetiologies | 2 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 1.7.6 Central and peripheral pain ‐ various aetiologies | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.35, 0.64] |
| 1.8 Sleep problems Show forest plot | 8 | 1386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.90, ‐0.04] |
| 1.8.1 Central pain ‐ multiple sclerosis | 1 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.22] |
| 1.8.2 Central pain ‐ spinal cord injury | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.41, 0.32] |
| 1.8.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 1.8.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.78, ‐0.07] |
| 1.8.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 3 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐2.17, 0.61] |
| 1.8.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.78, 0.16] |
| 1.9 Psychological distress Show forest plot | 7 | 779 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.61, ‐0.02] |
| 1.9.1 Central pain ‐ multiple sclerosis | 2 | 363 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.65, 0.59] |
| 1.9.2 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.78, ‐0.37] |
| 1.9.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.97, 0.47] |
| 1.9.4 Peripheral pain ‐ plexus injury | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.62, 0.08] |
| 1.9.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.80, ‐0.16] |
| 1.10 Withdrawals due to lack of efficacy Show forest plot | 9 | 1576 | Risk Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 1.10.1 Central pain ‐ multiple sclerosis | 4 | 697 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 1.10.2 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 1.10.3 Peripheral pain ‐ plexus injury | 1 | 141 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 1.10.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 371 | Risk Difference (IV, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.10.5 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 1.11 Any adverse event Show forest plot | 7 | 1356 | Risk Difference (IV, Random, 95% CI) | 0.19 [0.12, 0.27] |
| 1.11.1 Central pain ‐ multiple sclerosis | 3 | 627 | Risk Difference (IV, Random, 95% CI) | 0.22 [0.05, 0.39] |
| 1.11.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.34 [0.18, 0.50] |
| 1.11.3 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.12 [0.02, 0.22] |
| 1.11.4 Peripheral pain ‐ polyneuropathy of various aetiologies | 1 | 246 | Risk Difference (IV, Random, 95% CI) | 0.15 [0.05, 0.25] |
| 1.11.5 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.21 [0.06, 0.36] |
| 1.12 Specific adverse event: nervous system disorders Show forest plot | 9 | 1304 | Risk Difference (IV, Random, 95% CI) | 0.38 [0.18, 0.58] |
| 1.12.1 Central pain ‐ multiple sclerosis | 3 | 453 | Risk Difference (IV, Random, 95% CI) | 0.33 [0.09, 0.58] |
| 1.12.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.53 [0.38, 0.68] |
| 1.12.3 Peripheral pain ‐chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 1.12.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.26 [0.15, 0.37] |
| 1.12.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 332 | Risk Difference (IV, Random, 95% CI) | 0.29 [0.19, 0.39] |
| 1.12.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.37 [0.15, 0.58] |
| 1.13 Specific adverse event: psychiatric disorders Show forest plot | 9 | 1314 | Risk Difference (IV, Random, 95% CI) | 0.10 [0.06, 0.15] |
| 1.13.1 Central pain ‐ multiple sclerosis | 3 | 453 | Risk Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.16] |
| 1.13.2 Central pain ‐ spinal cord injury | 1 | 116 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 1.13.3 Peripheral pain ‐ chemotherapy‐induced polyneuropathy | 1 | 36 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.06, 0.28] |
| 1.13.4 Peripheral pain ‐ diabetic polyneuropathy | 1 | 297 | Risk Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 1.13.5 Peripheral pain ‐ polyneuropathy of various aetiologies | 2 | 342 | Risk Difference (IV, Random, 95% CI) | 0.21 [0.14, 0.29] |
| 1.13.6 Central and peripheral pain ‐ various aetiologies | 1 | 70 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.27] |

