نقش پروفیلاکسی روتین آنتیبیوتیک پس از زایمان طبیعی واژینال برای کاهش موربیدیتی عفونی مادران
چکیده
پیشینه
موربیدیتیهای عفونی در ایجاد موربیدیتی و مورتالیتی قابل توجه مادری و پریناتال، از جمله در زنانی که در معرض خطر بیشتر ظاهری عفونت نیستند، نقش دارند. آنتیبیوتیکها برای کاهش بروز عفونتها، اغلب پس از زایمان بدون عارضه (uncomplicated childbirth) برای زنان تجویز میشوند، به خصوص در شرایطی که زنان بیشتر در معرض خطر ابتلا به موربیدیتیهای عفونی دوران بارداری قرار دارند.
اهداف
ارزیابی اینکه به کارگیری روتین آنتیبیوتیکهای پروفیلاکتیک در زنان پس از زایمان واژینال طبیعی (بدون عارضه)، در مقایسه با دارونما (placebo) یا عدم استفاده از آنتیبیوتیک پروفیلاکسی، موربیدیتیهای عفونی پس از زایمان (postpartum) مادری را کاهش میدهد و پیامدها را بهبود میبخشد یا خیر.
روشهای جستوجو
ما پایگاه ثبت کارآزماییهای گروه بارداری و زایمان در کاکرین (31 آگوست 2017)؛ LILACS؛ ClinicalTrials.gov، پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (ICTRP) (22 آگوست 2017) و فهرست منابع مطالعات بازیابی شده را جستوجو کردیم.
معیارهای انتخاب
مایل به انتخاب کارآزماییهای تصادفیسازی یا شبه‐تصادفیسازی شدهای بودیم که به ارزیابی استفاده از آنتیبیوتیکهای پروفیلاکتیک در برابر دارونما یا عدم استفاده از آنتیبیوتیک پروفیلاکسی پرداختند. کارآزماییهای استفاده کننده از طرح خوشهای‐تصادفیسازی شده برای ورود به این مرور واجد شرایط بودند، اما هیچ موردی از این نوع کارآزمایی نیافتیم.
در نسخههای بهروز شده آینده این مرور، فقط مطالعات منتشر شده به صورت چکیده را، در صورتی که اطلاعات کافی برای ارزیابی خطرات سوگیری (bias) موجود ارائه کنند، وارد خواهیم کرد. ما چکیده مقالات حذف شده را برای ورود به مطالعه هنگامی در نظر میگیریم که متن کامل آنها در دسترس قرار گیرد، یا نویسندگان اطلاعات بیشتری درباره آن ارائه دهند.
کارآزماییهای استفاده کننده از یک طرح متقاطع، برای ورود به این مرور واجد شرایط نیستند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل به ارزیابی کارآزماییها برای ورود و خطرات سوگیری پرداختند. آنها بهطور مستقل از هم دادهها را استخراج کرده و دقت آنها را کنترل کردند، و از طریق بحث کردن به حل تفاوتهای موجود در ارزیابیها پرداختند. آنها کیفیت روششناسی را با استفاده از معیار استاندارد کاکرین و سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی کردند.
ما خلاصهها را با توجه به خطرات نسبی (RRs) و تفاوتهای میانگین (MDs) را با استفاده از مدلهای اثر‐ثابت یا اثرات‐تصادفی ارائه میکنیم. برای یک پیامد اولیه، ناهمگونی و اثر متقابل قابل توجهی یافتیم. با استفاده از تجزیهوتحلیل زیر‐گروه برای بررسی تاثیرات واحد تصادفی شده، بیشتر تحقیق کردیم. تمام نویسندگان مرور نتایج را بررسی و تفسیر کردند.
نتایج اصلی
یک کارآزمایی تصادفیسازی و کنترل شده (RCT) و دو شبه‐RCT که به مقایسه رژیمهای مختلف آنتیبیوتیک با دارونما (placebo) یا عدم درمان پرداخته بودند، دادههای مربوط به 1779 زن را که زایمان واژینال بدون عارضه داشتند، ارائه کردند. کارآزماییهای وارد شده در دهه 1960 (یک کارآزمایی) و دهه 1990 (دو کارآزمایی) انجام گرفتند. کارآزماییها در فرانسه، ایالات متحده آمریکا و برزیل انجام شدند. آنتیبیوتیکهای به کار گرفته شده عبارت بودند از: سولفامتوکسیپیریدازین (sulphamethoxypyridazine) خوراکی یا کلرامفنیکل (chloramphenicol) برای سه تا پنج روز، و آموکسیسیلین داخل وریدی (intravenous amoxicillin) و کلاولانیک اسید (clavulanic acid) به صورت تکدوز یک ساعت پس از زایمان. خطر سوگیری را برای اکثر حوزهها، به استثنای سوگیری گزارشدهی و سایر سوگیریهای بالقوه، بالا رتبهبندی کردیم.
کیفیت شواهد، بر اساس ارزیابی کیفیت GRADE، با توجه به محدودیتهای بسیار جدی در طراحی مطالعات وارد شده، عوارض کم و فواصل اطمینان (CI) گسترده در تخمین اثرگذاری، از پائین تا بسیار پائین رتبهبندی شد.
ما متوجه کاهش در خطر ابتلا به اندومتریت (endometritis) شدیم (RR: 0.28؛ 95% CI؛ 0.09 تا 0.83؛ دو کارآزمایی، 1364 زن، کیفیتبسیار پائین). با این حال، یک کارآزمایی برای این پیامد صفر عارضه را گزارش کرد و ما شواهد را با کیفیت بسیار پائین رتبهبندی کردیم. تفاوتی اندک یا عدم تفاوت بین گروهها از نظر خطر ابتلا به عفونت مجاری ادراری (urinary tract infection; UTI) (RR: 0.25؛ 95% CI؛ 0.05 تا 1.19؛ دو کارآزمایی، 1706 زن، کیفیت پائین)، عفونت زخم پس از اپیزیوتومی (episiotomy) (در کارآزماییهای وارد شده به عنوان پارگی زخم (wound dehiscence) گزارش شد) (RR: 0.78؛ 95% CI؛ 0.31 تا 1.96؛ دو کارآزمایی، 1364 زن، کیفیت بسیار پائین) و تعداد روزهای بستری شدن مادران در بیمارستان (MD: ‐0.15؛ 95% CI؛ 0.31‐ تا 0.01؛ یک کارآزمایی، 1291 زن، کیفیت بسیار پائین) وجود داشت. در گروه کنترل هزینه خدمات مراقبت بر اساس دلار آمریکا ½2 بیشتر از گروه دریافت کننده آنتیبیوتیک پروفیلاکسی بود (3600 USD: 9000 USD؛ یک کارآزمایی، 1291 زن). تفاوتی اندک یا عدم تفاوت بین گروههای درمان و کنترل از نظر عوارض جانبی ناشی از مصرف آنتیبیوتیکها (خارش پوست) گزارش شده در یک زن در هر یک از دو کارآزمایی وجود داشت (RR: 3.03؛ 95% CI؛ 0.32 تا 28.95؛ دو کارآزمایی، 1706 زن، کیفیت بسیار پائین). بروز موربیدیتی عفونی شدید مادری، مقاومت ضد‐میکروبی یا رضایت زنان از مراقبت در هیچ یک از مطالعات وارد شده بررسی نشد.
نتیجهگیریهای نویسندگان
به کارگیری روتین آنتیبیوتیکها ممکن است خطر ابتلا به اندومتریت را پس از زایمان واژینال بدون عارضه کاهش دهد. تعداد کم و ماهیت کارآزماییها، تفسیر شواهد را برای کاربرد در عمل محدود میکند، به ویژه در شرایطی که زنان ممکن است در معرض خطر بالاتری برای ابتلا به اندومتریت پیشرفته قرار داشته باشند. استفاده از آنتیبیوتیکها بروز عفونتهای مجاری ادراری، عفونت زخم یا طول مدت بستری مادران را در بیمارستان کاهش نداد. آنتیبیوتیکها جایگزین اقدامات پیشگیرانه و کنترل میزان عفونت در دوران زایمان و دوره پس از زایمان نیستند. تصمیم برای به کارگیری روتین آنتیبیوتیکهای پروفیلاکتیک پس از زایمان واژینال طبیعی باید با ویژگیهای بیمار، شرایط زایمان و تجربه ارائه دهنده، از جمله در نظر گرفتن سهم استفاده بیرویه از آنتیبیوتیکها در افزایش مقاومت ضد‐میکروبی، هماهنگ باشد. کارآزماییهای تصادفیسازی و کنترل شده با طراحی خوب و دارای توان بالای آزمون، میتواند به ارزیابی ارزش بالای به کارگیری روتین آنتیبیوتیک در میزان پیشگیری از عفونتهای مادری پس از زایمان واژینال طبیعی کمک کند.
PICO
خلاصه به زبان ساده
درمان روتین آنتیبیوتیک پیشگیرانه پس از زایمان واژینال طبیعی برای کاهش عفونتهای مادری
موضوع چیست؟
عفونتهایی که در دوران زایمان رخ میدهند ممکن است منجر به بیماری قابل توجه و حتی مرگومیر مادر یا نوزادش شود. این باور وجود دارد که خطر ابتلا به عفونت در زایمان در مراکز مراقبت سلامت در محیطهای با سطح پائین منابع، به دلیل شرایط بهداشتی ضعیف، آب ناکافی و سیستمهای فاضلاب نامناسب، جمعیت بیش از حد و نسبت کم متخصصین حرفهای بهداشت نسبت به تعداد بیمار، نسبت به مراکزی که از شرایط با منابع خوب برخوردارند، بالاتر است. تجویز آنتیبیوتیکها پس از زایمان واژینال بدون عارضه، روندی معمول برای غلبه بر این وضعیت در برخی از محیطهای با سطح پائین منابع است.
چرا این موضوع مهم است؟
در مواجهه با افزایش مقاومت ضد‐میکروبی به دلیل سوءمصرف و تجویز بیش از حد آنتیبیوتیکها، ما به شواهدی در مورد تاثیر مصرف روتین آنتیبیوتیکها برای پیشگیری از عفونتهای پس از بارداری واژینال طبیعی بدون عوارض نیاز داریم.
ما چه شواهدی به دست آوردیم؟
این مرور این موضوع را ارزیابی کرد که درمان روتین آنتیبیوتیک پس از زایمان واژینال بدون عارضه، در مقایسه با دارونما یا عدم استفاده از آنتیبیوتیک، از عفونت مادر پیشگیری میکند یا خیر. در آگوست 2017 شواهد مربوط به سه بانک اطلاعاتی را جستوجو کردیم. سه کارآزمایی را شامل 1779 زن شناسایی کردیم. کیفیت شواهد بین پائین تا بسیار پائین بود. آنتیبیوتیکهای مختلف در سه کارآزمایی و برای مدت زمانهای مختلف به کار گرفته شدند. این کارآزماییها در دهه 1960 (یک کارآزمایی) و دهه 1990 (دو کارآزمایی)، و در فرانسه، ایالات متحده آمریکا و برزیل انجام شدند.
به کارگیری روتین آنتیبیوتیک، تعداد زنان مبتلا به عفونت رحم (اندومتریت) (2 کارآزمایی، 1364 زن) را تا 70% کاهش داد. استفاده از آنتیبیوتیکها بروز عفونتهای مجاری ادراری (2 کارآزمایی، 1706 زن)، عفونتهای زخم پس از اپیزیوتومی (2 کارآزمایی، 1364 زن) یا طول مدت بستری مادران در بیمارستان (1 کارآزمایی، 1291 زن) را کاهش نداد.
برای خارش پوست به دلیل مصرف آنتیبیوتیکها، تفاوتی بین گروهها وجود نداشت، در هر یک از دو کارآزمایی (1706 زن) یک زن دچار این عارضه گزارش شد. هزینه خدمات مراقبت در گروهی که آنتیبیوتیک پروفیلاکسی دریافت نکرد، بیشتر بود. بروز عفونتهای شدید مادری و بیماری، مقاومت ضد‐میکروبی یا رضایت زنان از مراقبت ذکر نشد.
این یافتهها چه معنایی دارند؟
تعداد کم کارآزماییها تفسیر شواهد مربوط به استفاده روتین از آنتیبیوتیکها را پس از زایمان واژینال طبیعی محدود میکنند. بروز کم اندومتریت در مطالعات نشان میدهد که برای پیشگیری از چند مورد عفونت، تعداد نسبتا زیادی از زنان باید درمان شوند.
باید میان عواملی همچون نیازهای زنان، شرایط زایمان و تجربه ارائه دهنده (به عنوان مثال، با معاینات یا مداخلات مکرر واژینال) و تهدید بهداشت عمومی مقاومت آنتیبیوتیکی تعادل برقرار شود.
پژوهشهای بیشتر درباره کارآزماییهای تصادفیسازی و کنترل شده با طراحی خوب به ارزیابی ارزش افزوده مصرف روتین آنتیبیوتیکها توسط زنان پس از زایمان واژینال طبیعی برای پیشگیری از عفونتهای مادری کمک خواهند کرد.
Authors' conclusions
Summary of findings
| Routine antibiotic prophylaxis compared to no treatment/placebo for preventing infections in women who had normal vaginal births | ||||||
| Patient or population: Women who had normal vaginal births | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment/placebo | Risk with routine antibiotic prophylaxis | |||||
| Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal intensive care unit admission, or organ failure) | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| Endometritis | Study population | RR 0.28 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 23 per 1000 | 7 per 1000 | |||||
| Urinary tract infection | Study population | RR 0.25 | 1706 | ⊕⊕⊝⊝ | ‐ | |
| 32 per 1000 | 8 per 1000 | |||||
| Wound infection (episiotomy dehiscence) | Study population | RR 0.78 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 7 per 1000 | 10 per 1000 | |||||
| Adverse effects of antibiotics (skin rash) | Study population | RR 3.03 | 1706 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Length of maternal hospital stay | The mean length of maternal hospital stay (including maternal re‐admission to hospital) was 5.12 days | MD 0.15 lower | not estimable | 1291 | ⊕⊝⊝⊝ | ‐ |
| Individual antimicrobial resistance | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Two studies with very serious design limitations (‐2). | ||||||
Background
Infections occurring during pregnancy, childbirth and the puerperium are associated with considerable maternal and perinatal morbidity and mortality. However, global data on the incidence of maternal infection morbidity are scarce. The World Health Organization (WHO) estimates the global incidence of puerperal infections at 4.4% among live births, representing over 5.7 million cases a year (WHO 2005). Important variations exist between regions, with higher incidence in low‐ and middle‐income countries (up to 7%) compared to high‐income countries where the estimated incidence is lower (1% to 2%) (Dolea 2003).
Despite a relatively low incidence overall and the availability of interventions for prevention and treatment, maternal sepsis is one of the leading causes of maternal mortality, accounting for about one‐tenth of global maternal deaths (Say 2014). Most of the deaths are recorded in Asia (12%) and Africa (10%), compared to only 2% in developed countries (Khan 2006). In addition to death and acute morbidities, infections are associated with maternal long‐term disabilities and may also have considerable impact on newborn morbidity and mortality.
Description of the condition
In general, normal (uncomplicated) birth is considered as spontaneous in onset, low‐risk at the start of labour and remaining so throughout labour and childbirth. The infant is born spontaneously in the vertex position between 37 and 42 completed weeks of pregnancy. After birth, mother and infant are in good condition (NICE 2014; WHO 1996).
Several pre‐existing maternal conditions may increase the risk of developing bacterial infections among women expected to have uncomplicated vaginal births. These include conditions such as malnutrition, anaemia, bacterial vaginosis and group B streptococcus infections (Hussein 2010; Tharpe 2008). In addition, complications during labour and childbirth (e.g. prolonged rupture of membranes, prolonged labour, lacerations of the genital tract and retained products of conception) or provider interventions (e.g. frequent vaginal examinations, operative vaginal birth (forceps, vacuum), and episiotomy) might increase the risk of infection in the puerperium (Hussein 2010; Tharpe 2008). It is therefore not easy to identify women who might be at higher risk of developing infection after normal birth, and for whom prophylactic antibiotics might be useful for preventing infections in the puerperium. Moreover, diagnosis of maternal infection is based mainly on non‐specific clinical signs and symptoms (pelvic pain, fever, abnormal smell/foul‐odour vaginal discharge or delay in uterine involution) that can manifest several days after childbirth, resulting in difficulties in prompt identification of cases, particularly after discharge from the hospital (Hussein 2010). Failure to recognise the onset of infection contributes to delays in treatment and increased risk of morbidity and mortality (Acosta 2014).
There is a general belief that the risk of infection is higher for births attended at a healthcare facility (facility‐based births) in low‐resource settings as a result of poor hygienic conditions, poor water and sanitation systems, overcrowding and low health professional‐to‐patient ratio (Hussein 2010). In addition, the prompt identification of micro‐organisms and selective antibiotic prescription is often limited in these settings.
Description of the intervention
Hygiene and infection‐control measures (hand‐washing and disinfection, single use of gloves, and cleaning and sterilisation of equipment) are the basis for prevention of infection around the time of childbirth for women with uncomplicated vaginal births (Hussein 2010).
Antibiotic prophylaxis is characterised by the use of broad‐spectrum antibiotics (e.g. ampicillin, cephalosporin, a combination of antibiotics) effective against the micro‐organism most likely to cause infections, to be given before, during or immediately after the procedure and for a short period of time (single dose or for less than 24 hours), and in the absence of any sign of infection (ACOG 2011). Antibiotic prophylaxis given after normal birth has the potential to further decrease infection risk, particularly in settings where appropriate hygiene, infection‐control measures and sanitation during labour, childbirth and the postpartum period are not ensured, or where early detection of puerperal infections and laboratory investigations is limited. The goal of antibiotic prophylaxis is to prevent infection by reaching therapeutic tissue levels at the time microbial contamination is most likely to occur (ACOG 2011).
There is growing evidence that different antibiotic regimens are routinely prescribed following uncomplicated vaginal birth in low‐resource settings to overcome failures of the health system and the high risk of infections in the postpartum period. Studies from India (Sharma 2013) and Vietnam (Ngoc 2005) show that over 90% of women giving birth vaginally receive antibiotics before hospital discharge. This includes the use of a wide range of antibiotics (amoxicillin, ampicillin, cephalosporins, fluoroquinolones or combinations of antibiotics), different routes (oral, intramuscular, intravenous), and duration of administration (average of three to four days).
How the intervention might work
Bacterial infections around the time of childbirth are generally polymicrobial, including aerobic and anaerobic bacteria, and reflect vaginal colonisation (Van Dillen 2010). Antibiotic prophylaxis after normal vaginal birth could help to prevent maternal infections by ensuring adequate antimicrobial serum and tissue concentrations during the postpartum period. To be effective, such antibiotics have to be active against the predominant organisms that cause postpartum infections and administered for the shortest period to minimise side effects and the impact of its routine use on emerging antimicrobial resistance. Indeed, exposure to antibiotics in the postpartum might cause adverse effects to the mother or the breast‐fed neonate, including disruption of the normal flora, increased risk of resistant bacterial infections, allergic reactions as well as increased healthcare costs (ACOG 2011). There are also concerns about rising resistance to antibiotics at the facility and community level (WHO 2014). This might further complicate the choice of suitable prophylactic antibiotics, as generally broad‐spectrum antibiotics are required to cover common pathogens (Newton 2008).
Why it is important to do this review
The increasing trend observed in facility‐based births may increase the risk of hospital‐acquired infections after normal vaginal birth if not accompanied by improvements in the quality of hygiene and infection‐control measures (Hussein 2011). However, the evidence is unclear about the added effect of antibiotic prophylaxis on the prevention of postpartum infections after an uncomplicated vaginal birth.
There are increasing public health concerns about emerging antibiotic resistance following misuse or overuse of antibiotics (WHO 2014). This is also applicable to the obstetric populations and the possibility of inadequate response to treatment of puerperal infections due to early exposure to undereffective antibiotic prophylactic regimens. Given the large proportion of women experiencing uncomplicated vaginal birth, a universal application of antibiotics to such women has the potential to lead to substantial clinical benefits in terms of reducing infection risk, but could also lead to direct harm for the woman and indirect harm to the general public with increasing resistance to antibiotics, as described above.
Objectives
To assess whether the routine administration of prophylactic antibiotics to women after normal (uncomplicated) vaginal birth, compared with placebo or no antibiotic prophylaxis, reduces postpartum maternal infectious morbidities and improves outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), including quasi‐randomised trials and cluster‐randomised trials, that compared the use of routine antibiotic prophylaxis after normal (uncomplicated) vaginal birth at the facility level, with either a placebo or no antibiotic prophylaxis. In future updates of this review, we will include studies published in abstract form only, provided sufficient information is available to asses risks of bias. We will consider excluded abstracts for inclusion once the full publication is available, or the authors provide more information.
We exclude trials with a cross‐over design.
Types of participants
Women who had an uncomplicated vaginal birth at term. This excludes women who presented with any complication during labour, as reported by trial authors (e.g. chorioamnionitis, prolonged prelabour rupture of membranes (PRM), prolonged second stage of labour, third‐ or fourth‐degree vaginal tears, postpartum haemorrhage), or those who have had intrapartum procedures (operative vaginal birth or manual removal of placenta). Episiotomy was not considered a complication of labour.
Types of interventions
The main comparison of the review is the use of any routine antibiotic prophylaxis after uncomplicated vaginal birth compared with either placebo or no routine antibiotic prophylaxis. We will not consider trials administering antibiotic prophylaxis during labour for inclusion.
Types of outcome measures
Primary outcomes
-
Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal intensive care unit (ICU) admission, or organ failure) as reported by trial authors.
-
Endometritis (with or without myometritis and with or without salpingitis causing maternal febrile morbidity), as reported by the trial authors.
-
Urinary tract infection (fever or dysuria and positive urine culture).
Secondary outcomes
-
Wound infection (perineal first‐ or second‐degree tear, vaginal tear or episiotomy), as reported by trial authors.
-
Adverse effects of antibiotics (Maternal: allergic reaction, nausea, vomiting, diarrhoea, skin rashes, anaphylaxis. Neonatal: allergic reaction, diarrhoea, skin rashes, anaphylaxis).
-
Length of maternal hospital stay (including maternal re‐admission to hospital).
-
Cost of care (including maternal re‐admission to hospital, cost of antibiotic treatment).
-
Individual antimicrobial resistance (e.g. no response to first‐line antibiotic treatment, culture of antibiotic‐resistant bacterial strains), as reported by trial authors.
-
Women's satisfaction with care, as reported by trial authors.
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 August 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition to the search carried out by the Information Specialist, we searched LILACS using the search strategy given in Appendix 1 (22 August 2017).
We also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports, using the search terms given in Appendix 2 (22 August 2017).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions to our search.
Data collection and analysis
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (MB and CEC) independently assessed for inclusion all the studies we identified as a result of the search strategy, resolving any disagreement through discussion.
We created a study flow diagram (Figure 1) to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data, which two review authors (MB and CEC) used for eligible studies, resolving discrepancies through discussion. We entered data into Review Manager 5 software (RevMan 2014) and checked them for accuracy. Information regarding one of the excluded papers was unclear and grouped with a population excluded from our review objective. Efforts to contact the author to retrieve segregated data and further details proved unsuccessful. In future updates, where information is unclear we will try to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (MB and CEC) independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (EO or OTO).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. In future updates, where sufficient information is reported or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data or less than 20%; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses.
Assessing the quality of the evidence using GRADE
We assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook related to the following outcome for the main comparison.
-
Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal ICU admission, or organ failure) as reported by the trial authors.
-
Endometritis (with or without myometritis and with or without salpingitis causing maternal febrile morbidity), as reported by the trial authors.
-
Urinary tract infection (fever or dysuria and positive urine culture).
-
Wound infection (perineal first‐ or second‐degree tear, vaginal tear, episiotomy), as reported by trial authors.
-
Adverse effects of antibiotics (Maternal: allergic reaction, nausea, vomiting, diarrhoea, skin rashes, anaphylaxis. Neonatal: allergic reaction, diarrhoea, skin rashes, anaphylaxis).
-
Length of maternal hospital stay (including maternal re‐admission to hospital).
-
Individual antimicrobial resistance (e.g. no response to first‐line antibiotic treatment, culture of antibiotic‐resistant bacterial strains), as reported by trial authors.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create a ’Summary of findings’ table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
For continuous data, we calculated the mean difference (MD) for outcomes measured in a similar way between trials. In future updates we will use the standardised mean difference (SMD) where necessary to combine trials that measured the same outcome, but with different methods.
Unit of analysis issues
Cluster‐randomised trials
We found no cluster‐randomised trials. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Section 16.3.4 or 16.3.6), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion in this review.
Other unit of analysis issues
Included studies had only two treatment groups. For future updates, we will consider trials with more than two treatment groups for inclusion if at least one intervention group is relevant to the review. If multiple groups from one study are relevant for inclusion, we plan to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group.
Dealing with missing data
For included studies, we have noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 is greater than 30% and either the Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
We included three studies in the meta‐analysis. For subsequent updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. We observed clinical heterogeneity for one primary outcome (urinary tract infection) and one secondary outcome (wound infection (episiotomy dehiscence)). It was sufficient to suspect that the underlying treatment effects differed between trials, as evidenced by the substantial statistical heterogeneity detected.
We used a random‐effects meta‐analysis to produce an overall summary, as we considered the average treatment effect across trials to be clinically meaningful. We treat the random‐effects summary as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
For this outcome, we used random‐effects analyses, presenting the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
We identified substantial heterogeneity, which we investigated using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
-
High‐income countries versus low‐ and middle‐income countries.
-
Short‐duration antibiotic prophylaxis regimens versus long‐duration regimens.
We were able to introduce subgroups based on duration, short‐ (single dose) versus long‐term (several days with multiple doses). The low number of eligible studies limited further assessments.
Subgroup analysis was to be restricted to this review's primary outcomes.
-
Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal ICU admission, or organ failure) as reported by trial authors.
-
Endometritis (with or without myometritis and with or without salpingitis causing maternal febrile morbidity), as reported by the trial authors.
-
Urinary tract infection (fever or dysuria and positive urine culture).
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi2 statistic, P value, and the interaction test I2 value.
Sensitivity analysis
We undertook sensitivity analysis for one primary outcome based on the 'Risk of bias' assessment for allocation concealment and attrition rates. We conducted sensitivity analyses by removing studies that were at high risk of bias (such as quasi‐randomised studies) for these domains to assess whether this made any difference to the overall result, and by trial quality. There were no cluster‐randomised trials. If this is the case in future updates of this review, we will investigate the effect of different values of the ICC.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group’s Trials Register retrieved 10 trial reports. We retrieved 259 records from LILACS, 54 from ClinicalTrials.gov and 10 from ICTRP. Among these, we identified five duplicates from the same source. We excluded reports due to lack of comparisons, randomisation and irrelevance to review objective. We retrieved 13 full texts after independent title and abstract screening by two review authors. These studies provided data on women who received antibiotics after normal vaginal births (see Figure 1).
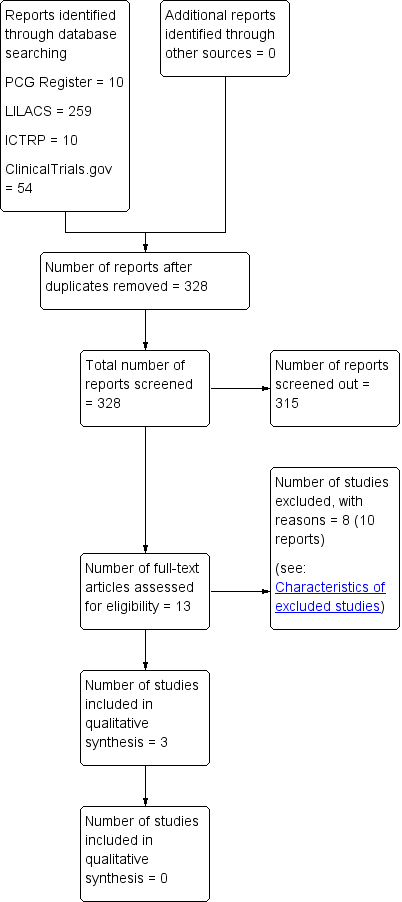
Study flow diagram.
Further full‐text evaluation identified three eligible trials which contribute data to this review. We included one randomised controlled trial (RCT) and two quasi‐RCTs. Studies provided individual data on a different class of drugs, so we could not compare differences between different drug classes.
Included studies
Design
One RCT (Fernandez 1993) and two quasi‐RCTs (Neto 1990; Turck 1962) contributed data on 1779 women who had uncomplicated vaginal births, comparing different antibiotic regimens with placebo or no treatment. Two trials specifically assessed the use of prophylactic antibiotics after in‐out catheterisation of the urethra (Turck 1962) or episiotomy (Neto 1990). In the other study women were recruited in the labour ward as soon as they delivered (Fernandez 1993). Women were followed up at 10 days (Neto 1990), two weeks (Fernandez 1993) and six weeks (Turck 1962) after birth. We provide a detailed description of studies in the table Characteristics of included studies.
In the studies, pregnant women were randomised into two groups, according to hospital registration number (Turck 1962), protocol number (odd/even number) (Neto 1990) or a random‐number table in the labour ward (Fernandez 1993).
The studies took place between 1961 and 1962 (Turck 1962), 1988 and 1989 (Neto 1990), and during 1989 (Fernandez 1993).
Sample sizes
Turck 1962 included 431 women, later excluding 16 participants. The remaining 415 women were assigned to the treated (217) or placebo groups (198). Neto 1990 included 80 women with normal birth and episiotomy, of which 73 were followed‐up and included in the analysis, 34 in the intervention arm and 39 in the control non‐treated arm. Fernandez 1993 included 1373 women, of which 82 were excluded after randomisation and 1291 analysed.
Setting
Two studies were conducted in high‐income countries, in a public hospital in France (Fernandez 1993) and a private clinic in the USA (Turck 1962). The remaining study was conducted in a public hospital in Brazil (Neto 1990).
Participants
In Turck 1962, all the women had catheters inserted just prior to childbirth, from which urine analysis was done. In the second study of 431 pregnant women (Turck 1962), 16 women were excluded prior to assigning the 415 participants to treated (217) and placebo (198) groups. Exclusions were based on allergies to sulphonamides, intake of other antibiotics and the presence of indwelling catheters (Turck 1962) prior to randomisation.
One thousand two hundred and ninety‐one women meeting the following inclusion criteria were eventually analysed in Fernandez 1993: vaginal birth, absence of pyrexia (> 38° C) during labour or the hour following delivery, an interval of less than 24 hours between rupture of membranes and the onset of labour, absence of any evidence of extragenital infection (e.g. urinary tract with absence of urination burning) and absence of known allergies to the drug of choice (Amox‐CA) or to β‐lactams. Women with evidence of amniotic fluid infection at the time of admission were included (Fernandez 1993). In addition, 82 women were excluded after randomisation. These included five in whom the antibiotic given was unknown, 71 women excluded due to antibiotic injections after uterine exploration, and six other women who had more than a 24‐hour interval between rupture of membranes and labour onset. This left 1291 women for analysis. No differences were reported between groups for maternal age, parity, body mass index, history of infection during pregnancy, other maternal morbidity (diabetes, hypertension), gestational age, type of pregnancy (singleton versus twins), time in labour ward, number of vaginal examinations, number of urethral catheterisations, temperature on admission to the labour ward or epidural anaesthesia.
Neto 1990 included data for 34 women in the intervention arm and 39 in the control no‐treatment arm. No difference was reported between groups for race, maternal age, parity, gestational age, length of labour, length of rupture of membranes, and number of vaginal examinations.
Interventions and comparisons
Treatment with antibiotic prophylaxis was compared to placebo (Turck 1962) or no treatment (Fernandez 1993; Neto 1990). Antibiotics administered included: sulphamethoxypyridazine one tablet a day from three to five days after in‐out urethral catheterisation (Turck 1962), oral chloramphenicol 500 mg four times daily for 72 hours after episiotomy repair (Neto 1990), and intravenous amoxicillin and clavulanic acid (®Augmentin, Beecham laboratory) at 1.2 g at one hour after birth (Fernandez 1993). When reported, the choice of the antibiotics by the authors was guided by their broad spectrum (Fernandez 1993), ease of administration and relatively low allergenicity (Turck 1962).
Outcomes
Outcomes addressed by the three eligible studies included two of our primary outcomes (incidence of endometritis, urinary tract infection) and four of our secondary outcomes (wound infection, adverse effects of antibiotics, length of maternal hospital stay and cost of care). The studies did not report on other important outcomes of this review (incidence of severe maternal infectious morbidity, antimicrobial resistance and women's satisfaction with care).
In one study (Fernandez 1993), endometritis was defined as pyrexia of ≥ 38° C confirmed on two separate occasions and accompanied by pain on mobilisation of the uterus or fetid lochia or both, and/or a leukocytosis of more than 10,000/mm3) that occurred within two weeks following childbirth. A slightly different definition was used by Neto 1990, as two of the following; fever, hypogastric pain, uterine involution, abnormal lochia, assessed at 10 days postpartum. In Turck 1962, urinary tract infection was diagnosed as the presence in urine of gram‐negative bacteriuria (more than 105 organisms per millilitre of urine), in addition to symptoms or signs such as fever, dysuria, frequency, or flank pain diagnosed by attending physicians. Neto 1990 defined episiotomy dehiscence as wound rupture without signs of infection, and episiotomy infection as pain, heat, redness or purulent discharge and wound rupture. No definition was provided in Fernandez 1993.
Sources of funding
Sources of trial funding were not mentioned in Fernandez 1993 or Neto 1990. Turck 1962 was supported by a training grant from the US Public Health Service, with the antibiotics and placebo provided through Lederle laboratories.
Trialist declarations of interest
Declarations of interest were not reported in any of the included studies (Fernandez 1993; Neto 1990; Turck 1962)
Excluded studies
We excluded eight studies. Studies were excluded due to lack of randomisation (Charles 1986), inclusion of women with assisted vaginal births, or third‐degree tears (Costa 1998; Yamagishi 2009), prophylactic antibiotic administration during labour (Kampikaho 1993; Oluwalana 2017) or just prior to birth (Cormier 1988; Sharma 2014) and vaginal cleansing with antibiotics (Matthias 1986). One study (Yamagishi 2009) provided non‐segregated data; our efforts to contact the author were unsuccessful, and we eventually excluded the study. See Characteristics of excluded studies .
Risk of bias in included studies
The risks of bias across studies and for the three included studies (which also contributed to the meta‐analysis) are presented in a 'Risk of bias' table in Figure 2. We rated the risk of bias as high for all three included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The choice of intervention for the control group had elements of randomisation in the three trials. However, two trials used a non‐random sequence generation (Neto 1990; Turck 1962) and were considered at high risk of bias. In the Fernandez 1993 study, random sequence generation was achieved through using a random‐number table (low risk of bias) ‐ however, the sequence could easily have been broken by the head midwife who conducted the randomisation, and the random‐number table was located in the labour ward (Fernandez 1993).
The risk of selection bias due to poor allocation concealment was high for all three included trials. Preservation of allocation concealment could have been broken due to an inadequate sequence of randomisation in the two trials using non‐random sequence generation (participant's hospital registration number (Turck 1962), and even/odd protocol number (Neto 1990)). Selection bias may have been introduced by participants or investigators who may have foreseen assignments. In the Fernandez 1993 study, the choice of treatment was known before inclusion.
Blinding
Performance bias
We rated blinding in terms of performance bias as low risk for one study (Turck 1962), where the control group received an identical‐appearing placebo in prepackaged pill boxes, and distributed in double‐blind fashion. We judged the risk of performance bias to be high for the other two studies (Fernandez 1993; Neto 1990), with no treatment in the control groups.
Detection bias
In terms of detection bias, the risk was high for two studies (Fernandez 1993; Turck 1962). In the Fernandez 1993 study there was was no blinding of the women nor the investigators. In the Turck 1962 study the clinical diagnosis of puerperal urinary tract infection was made by the attending physicians, who were unaware whether the woman was receiving a drug or the placebo. However, blinding could have been broken if the hospital registration number and sequence generation were known by the attending physician. We rated the third study (Neto 1990) as having an unclear risk of bias for this domain as there was insufficient information provided in the trial report.
Incomplete outcome data
The risk of attrition bias was low in one study (Turck 1962), as data were provided for all participants included. We assessed risk of attrition bias to be high risk for the other two studies (Fernandez 1993; Neto 1990). In Neto 1990 information was not available for all 80 women randomised. Six women in the intervention group and one in the control group were missing from follow‐up at 10 days postpartum when outcomes were assessed. However, there were no differences reported in baseline characteristics among those followed up. In Fernandez 1993, 1373 women were conscripted with an intention to treat. Eighty‐two of these women were excluded after randomisation from the treated group. Among the 1291 eventually analysed, 91 unknowns (it was unknown whether they had infection or not) were reported.
Selective reporting
Two studies (Fernandez 1993; Neto 1990) reported on all prespecified outcomes and we therefore considered them to be at a low risk of reporting bias. We assessed the Turck 1962 study as being at a high risk of reporting bias because the trial report did not provide details on the frequency of some outcomes including adverse effects and hospital stay.
Other potential sources of bias
We found no other important risks of bias for the three included trials (Fernandez 1993; Neto 1990; Turck 1962).
Effects of interventions
See summary of findings Table for the main comparison table for results of the main comparison: use of prophylactic antibiotics, compared to placebo or no treatment, after normal vaginal birth.
Routine antibiotic prophylaxis versus no treatment/placebo
Primary outcomes
Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal intensive care unit (ICU) admission, or organ failure) as reported by trial authors
The incidence of severe maternal infectious morbidity was not addressed by any of the included studies.
Endometritis (with or without myometritis and with or without salpingitis causing maternal febrile morbidity), as reported by the trial authors.
Prophylactic antibiotics after uncomplicated vaginal birth may reduce the risk of endometritis by 72% (risk ratio (RR) 0.28, 95% confidence interval (CI) 0.09 to 0.83, two trials, 1364 women, very low‐quality evidence, see Analysis 1.1). However, one trial was of very small size (73 women) and reported zero events for this outcome (Neto 1990).
Urinary tract infection (fever or dysuria and positive urine culture)
There was substantial heterogeneity (I2 = 67%, P = 0.08) for the risk of urinary tract infection between the two trials contributing data. Pooled estimates from a random‐effects model showed that there was little or no difference in the risk of urinary tract infection (RR 0.25, 95% CI 0.05 to 1.19, T2 = 0.84 , two trials, 1706 women, low‐quality evidence, see Analysis 1.2). We deemed a pooled estimate to be appropriate, as the effect sizes of both trials were in the same direction and the characteristics of the women were considered similar; both studies were conducted in high‐income countries and most of the women had in‐out catheterisation. It is noteworthy that the 16 women who were excluded from Turck 1962 had the highest incidence of urinary tract infection. Four of 16 women not given either antibiotics or placebo had symptoms of urinary infection early in the puerperium. This analysis also presents our subgroup analysis according to the duration of prophylactic antibiotics and the risk of urinary tract infections, as the two trials used short‐duration (single dose) (Fernandez 1993) or long‐duration (3 to 5 days) (Turck 1962) regimens. Applying a fixed‐effect model produced, as expected, a narrower CI and suggests a reduction in the risk of urinary tract infection (RR 0.25, 95% CI 0.11 to 0.57). However, this analysis should be interpreted with caution, as the two included trials tested different antibiotic regimens, used different comparison groups (placebo or no treatment) and Turck 1962 had a quasi‐randomised design.
Secondary outcomes
There were few or no differences between the treated and control groups for the risk of episiotomy wound infection, reported as wound dehiscence, in the included trials (RR 0.78, 95% CI 0.31 to 1.96, T2 = 0.15, I2 = 33%, P = 0.22, two trials, 1364 women, very low‐quality evidence, see Analysis 1.3). There is substantial heterogeneity in the analysis of risk of episiotomy infection. Although the P value is not significant and the CI of the only two studies included in this analysis overlap greatly, we considered the characteristics of women in both trials to be different. Indeed, one trial included only low socioeconomic‐class women from one public hospital in one middle‐income country, while the other trial was conducted in a high‐income country. This baseline difference in risk could explain the greater effect of antibiotics use to prevent infection after episiotomy in Neto 1990. A random‐effects model also equalised the weighting of the contributing studies. A fixed‐effect model showed a very similar estimate (RR 0.78, 95% CI 0.38 to 1.61) without changes in the overall effect.
There were few or no differences between treated and control groups for reported adverse effects (RR 3.03, 95% CI 0.32 to 28.95, two trials, 1706 women, very low‐quality evidence, see Analysis 1.4). One woman in each study recorded skin rash related to antibiotic intake, which resolved with cessation of the drug (Turck 1962) and within a few hours in the other study (Fernandez 1993). In the former, one of the 217 women treated with sulphamethoxypyridazine developed a maculopapular rash and had an untoward reaction of sufficient severity to warrant discontinuation of the drug. Turck 1962 also reported that "the incidence of unexplained pyrexia, headache or dizziness" was similar in both groups. No other maternal or neonatal adverse effects were reported.
There were few or no differences between treated and control groups in the length of maternal hospital stay in days (mean difference ‐0.15, 95% CI ‐0.31 to 0.01, one trial (Fernandez 1993), 1291 women, very low‐quality evidence, see Analysis 1.5). No data were provided for comparisons between groups in the other included studies. However, Turck 1962 reported that the length of hospital stay was slightly prolonged in women with symptomatic illness, but that in general the length of hospital stay was similar between the two groups.
A 2½‐fold increase in costs of care in the control group was reported in Fernandez 1993, compared to antibiotic prophylaxis (USD 3600: USD 9000, one trial, 1291 women, see Analysis 1.6). These costs included prophylactic antibiotics, treatment of endometritis and hospitalisations. These estimates did not include expenditures related to prolonged hospitalisation of the newborn or subsequent management of possible secondary sterility.
The incidence of antimicrobial resistance and women's satisfaction with care were not addressed by any of the included studies.
Discussion
Summary of main results
We found a marked reduction in the risk of puerperal endometritis in women who received routine prophylactic antibiotics compared to those who received either placebo or no treatment. There was little or no difference between the groups for the risk of urinary tract infection, wound infection and length of hospital stay. Adverse effects due to antibiotic intake were reported in one woman each in two studies. No data were provided on the incidence of severe maternal infectious morbidity, antimicrobial resistance and women's satisfaction with care.
The antibiotic prophylaxis regimens used in the included studies vary widely in terms of the class of antibiotic used and duration of the course. Only one trial used a regimen in line with the current understanding of antibiotic prophylaxis as a short‐term course of less than 24 hours. However, our subgroup analysis according to the duration of prophylaxis was limited to the urinary infections outcome only, as not enough data were available for the other two primary outcomes in this review. It is also notable that women enrolled in all three trials underwent some type of intervention during normal vaginal birth, including episiotomy and in‐out bladder catheterisation, which may have increased their baseline risk of infection.
Administration of prophylactic antibiotics after normal vaginal births may be successful in preventing puerperal endometritis. However, the nature of the studies and the low numbers of participants and low frequency of events (with one trial reporting no events) limit firm conclusions on the benefits of systematic antibiotic prescriptions, more so in women who gave birth without complications.
The cost of treatment and potential for prevention of endometritis propose a possible benefit for routine antibiotic prophylaxis. However, the low incidence of endometritis in the included studies suggests that many women may have to be treated to avoid few case of endometritis. The treatment of a large number of women who are not at risk of developing endometritis poses the problem of exposing mothers and their babies to unnecessary treatments and threats to increasing antimicrobial resistance patterns. A separate Cochrane Review looks at different antibiotic regimens for treatment of postpartum endometritis (Mackeen 2015).
The incidence of urinary tract infections was also relatively low. We found significant heterogeneity between studies for the incidence of urinary tract infection, but a pooled estimate was deemed appropriate as the effect sizes in both trials were in the same direction and showed some clinical overlap. However, this analysis should be interpreted with caution, as it only included two trials which tested different antibiotic regimens (drug class and duration of treatment), used different comparison groups (placebo or no treatment) and follow‐up periods, and one trial had a quasi‐randomised design. The differences between studies may have also been influenced by catheterisation (although single), which may have affected the relatively high incidence of infection, as seen in both the control group and excluded women in one study.
The incidence of wound infection and length of stay were similar in both treated and untreated women, but the low number of participants in the studies limits conclusions on the benefits of antibiotic intake. The reporting of adverse effects from antibiotics in two studies is of concern, although as expected they were very low.
Overall completeness and applicability of evidence
The frequency of routine antibiotic prophylaxis is high in low‐ and middle‐income countries. Eligible studies provided data from two high‐income countries (France and the USA) and one low‐income country (Brazil) in the 1960s and 1990s. The risks of bias ranged from high to low across several domains.
We found substantial heterogeneity in the incidence of urinary tract infection between the two studies reporting this outcome. This analysis also allowed for exploration of the planned subgroup analysis for difference in duration of treatment, suggesting differences between groups. This observation could also be interpreted in terms of differences in the method of comparison or drug class between studies. However, the effect was similar and in the same direction for both subgroups, as evidenced by the overlap of confidence intervals. However, the paucity of studies and frequencies of participants limited further interpretation.
Finally, the two quasi‐RCTs included in this review used antibiotics (chloramphenicol, sulphamethoxypyridazine) with limited use in current clinical practice, raising questions about any applicability of the results of these trials to current obstetric practice.
Quality of the evidence
We adjusted the outcome 'wound infection' to include episiotomy dehiscence as reported by authors as a sign of episiotomy infection. The assessments of risk of bias ranged from low to high, although most domains were at high risk of bias for the included studies, given inadequate concealment of allocations and blinding.
The quality of evidence based on GRADE assessment ranged from low to very low across all reported outcomes (summary of findings Table for the main comparison), because of very serious design limitations, small sample sizes, few events and wide confidence intervals.
Potential biases in the review process
We adhered to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also conducted an exhaustive search of several trial registries (both English and non‐English) and evaluated results for eligibility, thus minimising potential biases.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other systematic reviews or studies related to the use of routine antibiotic prophylaxis for the prevention of maternal morbidity or mortality in women who had normal vaginal births.
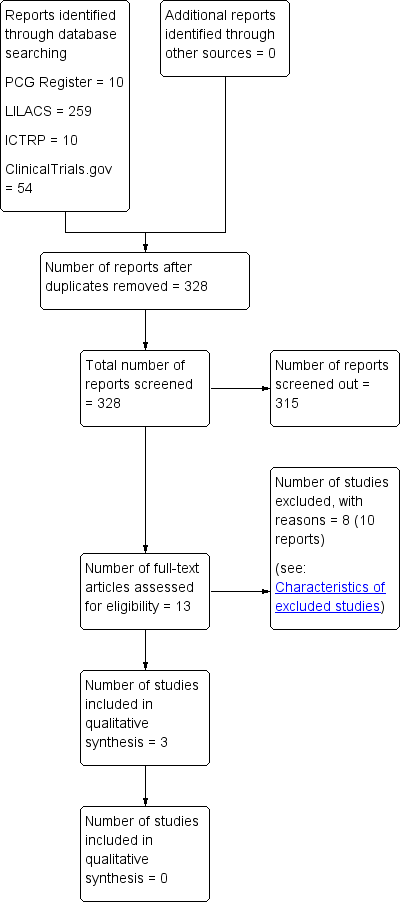
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 1 Endometritis.
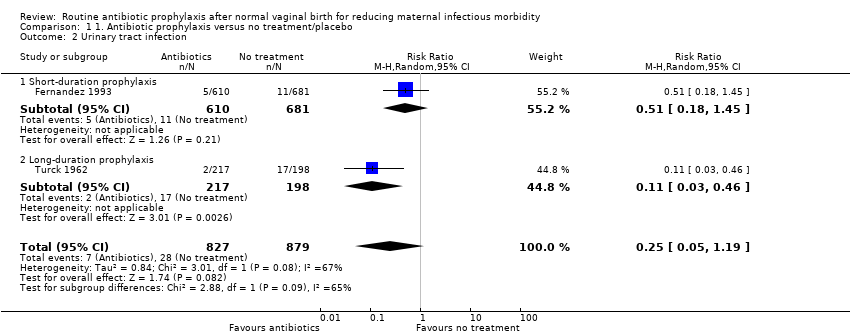
Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 2 Urinary tract infection.

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 3 Wound infection (episiotomy dehiscence).

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 4 Adverse effects of antibiotics (skin rash).

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 5 Length of maternal hospital stay ‐ days.
| Study | Experimental | Control |
| Fernandez 1993 | 3600 USD | 9000 USD |
Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 6 Cost of care (cost of antibiotic prophylaxis, prolonged hospitalisation and treatment of endometritis).
| Routine antibiotic prophylaxis compared to no treatment/placebo for preventing infections in women who had normal vaginal births | ||||||
| Patient or population: Women who had normal vaginal births | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment/placebo | Risk with routine antibiotic prophylaxis | |||||
| Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal intensive care unit admission, or organ failure) | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| Endometritis | Study population | RR 0.28 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 23 per 1000 | 7 per 1000 | |||||
| Urinary tract infection | Study population | RR 0.25 | 1706 | ⊕⊕⊝⊝ | ‐ | |
| 32 per 1000 | 8 per 1000 | |||||
| Wound infection (episiotomy dehiscence) | Study population | RR 0.78 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 7 per 1000 | 10 per 1000 | |||||
| Adverse effects of antibiotics (skin rash) | Study population | RR 3.03 | 1706 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Length of maternal hospital stay | The mean length of maternal hospital stay (including maternal re‐admission to hospital) was 5.12 days | MD 0.15 lower | not estimable | 1291 | ⊕⊝⊝⊝ | ‐ |
| Individual antimicrobial resistance | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Two studies with very serious design limitations (‐2). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 2 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.09, 0.83] |
| 2 Urinary tract infection Show forest plot | 2 | 1706 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.19] |
| 2.1 Short‐duration prophylaxis | 1 | 1291 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.45] |
| 2.2 Long‐duration prophylaxis | 1 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.03, 0.46] |
| 3 Wound infection (episiotomy dehiscence) Show forest plot | 2 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.96] |
| 4 Adverse effects of antibiotics (skin rash) Show forest plot | 2 | 1706 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.32, 28.95] |
| 5 Length of maternal hospital stay ‐ days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Cost of care (cost of antibiotic prophylaxis, prolonged hospitalisation and treatment of endometritis) Show forest plot | Other data | No numeric data | ||

