Использование внутрипузырной вакцины БЦЖ с интерфероном‐альфа и без него для лечения рака мочевого пузыря без инвазии в мышечный слой
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012112.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 marzo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Urología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors (Andrew RH Shepherd (ARHS), Emily Shepherd (ES), and Nicholas R Brook (NRB)) contributed to the design, development, and drafting of the protocol, and search strategy development.
ARHS and ES acquired trial reports and conducted trial selection and data extraction
NRB was consulted to resolve discrepancies or disagreements
ARHS led the data analysis, and ES and NRB assisted with data interpretation
All review authors contributed to review drafting, with ARHS in the leading role
All review authors will contribute to future review updates
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
ARHS: none known
ES: none known
NRB: none known
Acknowledgements
We thank the Cochrane Urology Group for their support, including the editors for their feedback on this review. We also thank the referees for their valuable feedback: Ashish Kamat, Jonathan Izawa, Bastian Keck, Maximilian Burger, and Stefanie Schmidt.
We would like to acknowledge Eduard Bercovich for his assistance in the procurement of manuscripts of two of the included studies, and Alexander Minich for the provision of the manuscript for one of the included studies. We thank Giorgio Maria Agazzi and Alexander Troelnikov for their kind assistance in the translation of included studies from Italian and Russian into English.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Mar 08 | Intravesical Bacillus Calmette‐Guérin with interferon‐alpha versus intravesical Bacillus Calmette‐Guérin for treating non‐muscle‐invasive bladder cancer | Review | Andrew RH Shepherd, Emily Shepherd, Nicholas R Brook | |
| 2016 Mar 05 | Intravesical Bacillus Calmette‐Guérin with interferon‐alpha versus intravesical Bacillus Calmette‐Guérin for treating non‐muscle‐invasive bladder cancer | Protocol | Andrew RH Shepherd, Emily Shepherd, Nicholas R Brook | |
Differences between protocol and review
This review was based on a published protocol (Shepherd 2016), with differences as described here.
We included a second comparison, 'intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone', which we believed was important and relevant to our review objective. We did not consider it appropriate for studies assessing alternating BCG and IFN‐α to be pooled with those assessing co‐administration of BCG and IFN‐α.
As for our first comparison, 'intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone', we were unable to obtain time‐to‐event information for the outcomes time‐to‐recurrence and time‐to‐progression, assessing the dichotomous outcomes recurrence and progression instead; we thus included these outcomes in the 'Summary of findings' table. For both comparisons, we were unable to obtain information for the outcome disease‐specific survival, assessing the outcome disease‐specific mortality instead; we thus included this outcome in the 'Summary of findings' tables.
Notes
Parts of the Methods section of this review were based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group that has been modified and adapted for use by the Cochrane Urology Group.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adjuvants, Immunologic [*administration & dosage, adverse effects];
- Administration, Intravesical;
- Antineoplastic Agents [*administration & dosage, adverse effects];
- BCG Vaccine;
- Carcinoma in Situ [mortality, pathology, *therapy];
- Combined Modality Therapy [adverse effects, methods];
- Disease Progression;
- Drug Administration Schedule;
- Interferon-alpha [*administration & dosage, adverse effects];
- Neoplasm Recurrence, Local;
- Randomized Controlled Trials as Topic;
- Time Factors;
- Urinary Bladder Neoplasms [mortality, pathology, *therapy];
- Withholding Treatment [statistics & numerical data];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram (searched 14 March 2016, updated 25 August 2016).
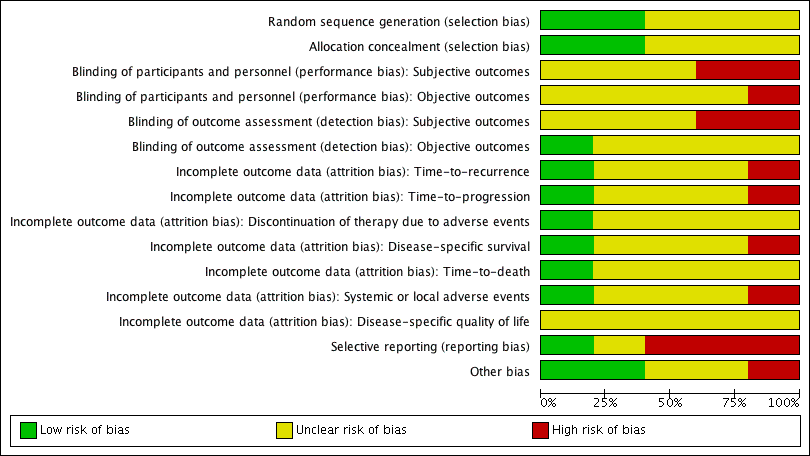
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
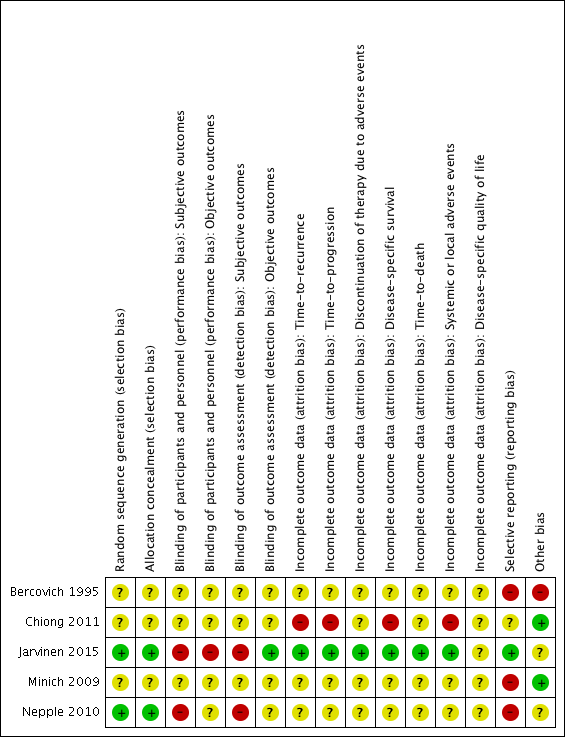
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence.

Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 2 Recurrence.
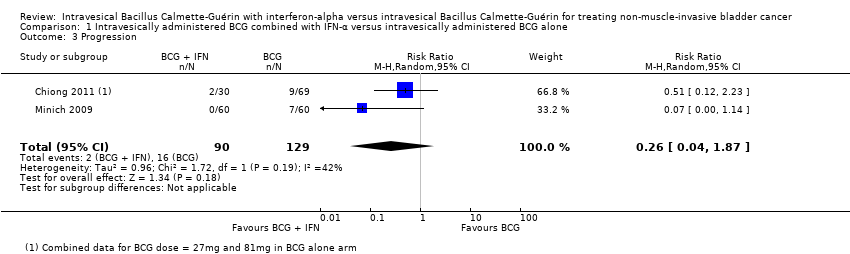
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 3 Progression.
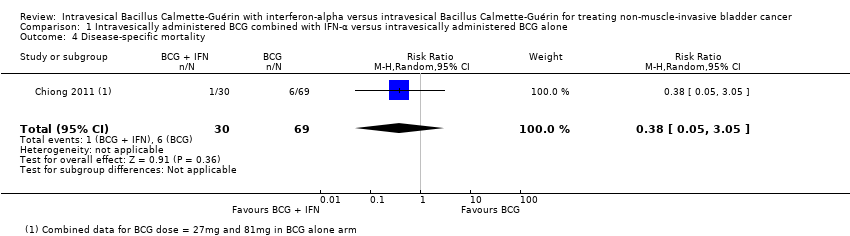
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality.
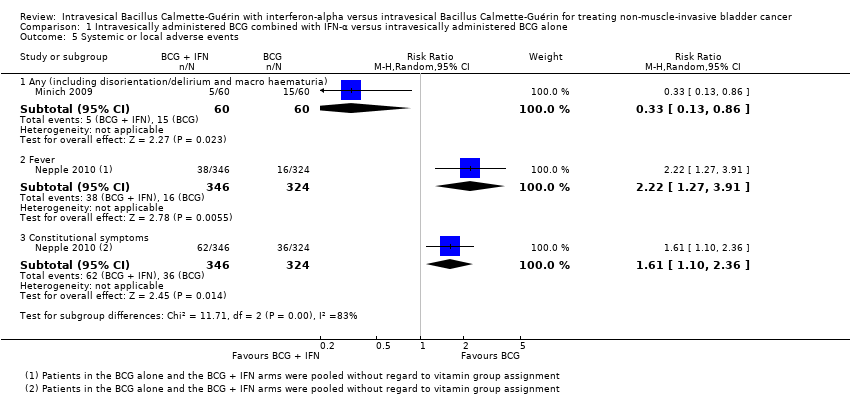
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 5 Systemic or local adverse events.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence.
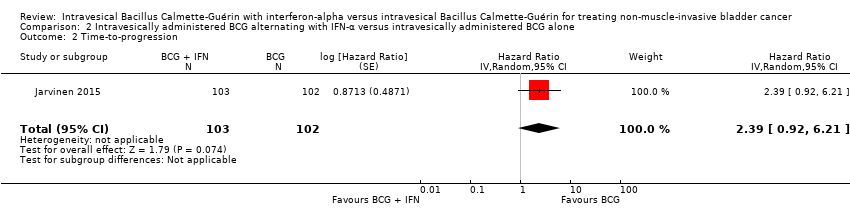
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 2 Time‐to‐progression.
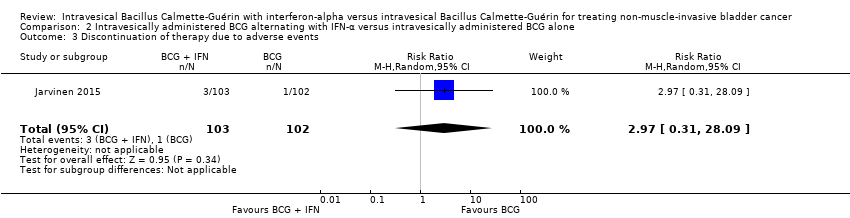
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 3 Discontinuation of therapy due to adverse events.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 5 Overall survival.
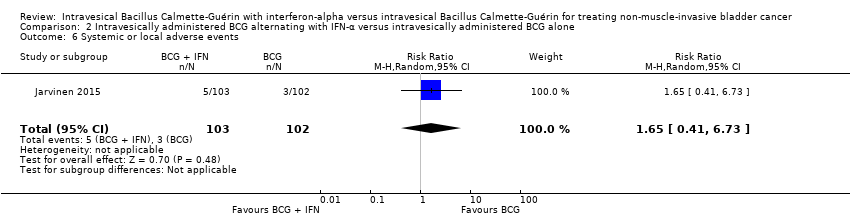
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 6 Systemic or local adverse events.
| Intravesically administered BCG combined with IFN‐α compared to intravesically administered BCG alone for treating non‐muscle‐invasive bladder cancer Patient or population: patients with non‐muscle invasive bladder cancer Intervention: BCG combined with IFN‐α Comparison: BCG alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with intravesically administered BCG alone | Risk difference with intravesically administered BCG combined with IFN‐α | ||||
| Recurrence Follow‐up: median 38.3 to 60 months | 925 | ⊕⊕⊝⊝ | RR 0.76 | Study population | |
| 342 per 1000 | 82 fewer per 1000 | ||||
| Progression Follow‐up: median 38.3 to 60 months | 219 | ⊕⊕⊝⊝ | RR 0.26 | Study population | |
| 124 per 1000 | 92 fewer per 1000 | ||||
| Discontinuation of therapy due to adverse events ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| Disease‐specific mortality Follow‐up: median 60 months | 99 | ⊕⊝⊝⊝ | RR 0.38 | Study population | |
| 87 per 1000 | 54 fewer per 1000 | ||||
| Disease‐specific quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; IFN‐α: interferon‐alpha; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel' (Nepple 2010); 'blinding of outcome assessment' (Nepple 2010); 'selective reporting' (Bercovich 1995; Minich 2009; Nepple 2010); 'other bias' (Bercovich 1995). | |||||
| Intravesically administered BCG alternating with IFN‐α compared to intravesically administered BCG alone for treating non‐muscle‐invasive bladder cancer Patient or population: patients with non‐muscle invasive bladder cancer Intervention: BCG alternating with IFN‐α Comparison: BCG alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with intravesically administered BCG alone | Risk difference with intravesically administered BCG alternating with IFN‐α | ||||
| Time‐to‐recurrence Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊕⊝ | HR 2.86 | Study population | |
| 431 per 1000 | 370 more per 1000 | ||||
| Time‐to‐progression Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | HR 2.39 | Study population | |
| 59 per 1000 | 76 more per 1000 | ||||
| Discontinuation of therapy due to adverse events Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | RR 2.97 | Study population | |
| 10 per 1000 | 19 more per 1000 | ||||
| Disease‐specific mortality Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | HR 2.74 | Study population | |
| 29 per 1000 | 49 more per 1000 | ||||
| Disease‐specific quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel', 'blinding of outcome assessment' (Jarvinen 2015). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 670 | Hazard Ratio (Random, 95% CI) | 1.11 [0.86, 1.43] |
| 2 Recurrence Show forest plot | 4 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| 2.1 IFN‐α higher dose (50 MU) weekly for 6 weeks | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.41] |
| 2.2 IFN‐α lower dose (6 to 10 MU) weekly for 6 weeks | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.36, 0.94] |
| 3 Progression Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.04, 1.87] |
| 4 Disease‐specific mortality Show forest plot | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 3.05] |
| 5 Systemic or local adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any (including disorientation/delirium and macro haematuria) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 5.2 Fever | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.27, 3.91] |
| 5.3 Constitutional symptoms | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.86 [1.98, 4.13] |
| 2 Time‐to‐progression Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.39 [0.92, 6.21] |
| 3 Discontinuation of therapy due to adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.31, 28.09] |
| 4 Disease‐specific mortality Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.74 [0.73, 10.28] |
| 5 Overall survival Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 1.0 [0.68, 1.47] |
| 6 Systemic or local adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.41, 6.73] |

