Intervenciones para la prevención del SHEO en los ciclos de TRA: una revisión global de revisiones Cochrane
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012103.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 enero 2017see what's new
- Tipo:
-
- Overview
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SM drafted first versions of the protocol and overview manuscripts. All three overview authors (SM, JB, CF) contributed to preparation of the protocol, data extraction and analysis of reviews for this overview. JB and CF contributed to the definitive version of the manuscript.
Sources of support
Internal sources
-
Department of Obstetrics and Gynaecology, University of Auckland, New Zealand.
This department provided infrastructure support.
External sources
-
None, Other.
Declarations of interest
All three overview authors (SM, JB,CF) were co‐review authors on several of the included reviews. CF is a director/shareholder of a small day stay surgical unit and gynaecology clinic and undertakes private practice within these facilities. She has received travel/accommodation/meeting expenses from ESHRE or ASRM for attendance at scientific meetings. She does not receive any industry or commercial payments for research or travel. SM and JB report no conflicts of interest regarding industry.
Acknowledgements
Managing editor of the CGF Group Helen Nagels for helping with identification of registered titles and submitted protocols, and editor Jane Marjoribanks for reviewing and editing the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 23 | Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews | Review | Selma Mourad, Julie Brown, Cindy Farquhar | |
| 2016 Feb 26 | Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews | Protocol | Selma Mourad, Julie Brown, Cindy Farquhar | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Cabergoline;
- Ergolines [therapeutic use];
- Gonadotropin-Releasing Hormone [agonists, therapeutic use];
- Metformin [therapeutic use];
- Ovarian Hyperstimulation Syndrome [etiology, *prevention & control, therapy];
- Progesterone [therapeutic use];
- Reproductive Techniques, Assisted [*adverse effects];
- Review Literature as Topic;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
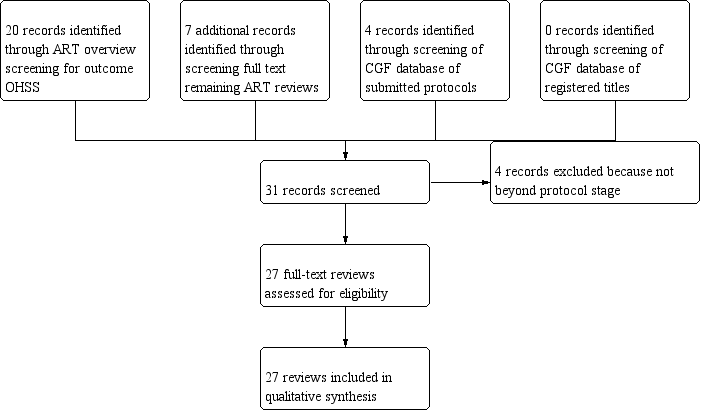
Flow diagram of included reviews.

Extent of effect of interventions on OHSS rate and live birth rate (when reported) or clinical pregnancy: OR and 95% CI.
| Review ID | Number of included trials | Population Definition of high risk for OHSS (where applicable) | Intervention | Comparison intervention/control | Primary outcomesa | Review limitations |
| ADA563 Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome | 4 RCTs | 340 women with PCOS downregulated by GnRHa, undergoing superovulation in IVF or ICSI cycles High risk: women with PCOS | Coasting when oestradiol levels were > 2500 pg/mL or > 9000 pmol/L | Early unilateral follicular aspiration No coasting or other interventions | OHSSa Live birtha Clinical pregnancy Number of oocytes retrieved Multiple pregnancy Miscarriage | Comparisons based on limited trial data Live birth reported in only 1 trial Trials lacked blinding, and half the trials lacked details on allocation concealment and incomplete outcome assessment |
| ADA561 Embryo freezing for preventing ovarian hyperstimulation syndrome | 2 RCTs | 151 women downregulated by GnRHa, undergoing superovulation in IVF or ICSI cycles. High risk: as defined by included studies | Cryopreservation | Fresh embryo transfer Intravenous albumin | OHSSa Clinical pregnancya Live birth Admissions | Evidence based on 2 trials, 1 for each comparison Live birth reported in only 1 trial Issues around methodological quality of both trials |
| TH1338 Dopamine agonists for preventing ovarian hyperstimulation syndrome | 16 RCTs | 2091 women at high risk of developing OHSS undergoing ART High risk: as defined by included studies | Cabergoline quinagolide, bromocriptine, cabergoline + albumin, cabergoline + HES | Placebo/no treatment/other treatment: Albumin alone HES Coasting Prednisolone | OHSSa Live birtha Clinical pregnancy Adverse effects Miscarriage Multiple pregnancy | Allocation concealment and blinding not adequately reported. One study used a co‐intervention of albumin IV and 1 of HES Different regimens of cabergoline administration between included studies Live birth rate reported in only 2 studies Incomplete reporting of multiple pregnancy rate, adverse effects and miscarriage rate |
| PMA481 Volume expanders for prevention of OHSS | 9 RCTs | 1660 (albumin) + 487 (HES) women at high risk of developing OHSS undergoing ART cycles High risk: determined as number of follicles or oestradiol levels on day of hCG, as defined by included studies | Human albumin Hydroxyethyl starch (HES) | Placebo/no treatment | OHSSa Clinical pregnancy Number of oocytes retrieved Multiple pregnancy Miscarriage Live birth | No reporting of live birth rate Limited by incomplete data reporting and lack of (details on) blinding |
| HA413 Recombinant vs urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles | 18 RCTs | 2952 women undergoing ART | Recombinant hCG Recombinant LH | Urinary hCG | OHSSa Clinical pregnancy Miscarriage Oocytes retrieved Tolerance Live birth | Review authors combined ongoing pregnancy and live births together Only 7 trials reported on live birth Trials lacked details on allocation concealment, randomisation and blinding |
| MM1690 GnRHa vs hCG for oocyte triggering in antagonist‐assisted reproductive technology | 17 RCTs | 1847 women undergoing ART | GnRH agonist | hCG | OHSSa Live birth ratea Ongoing pregnancy Clinical pregnancy Multiple pregnancy Miscarriage rate | Risk of bias in included studies. Limitations included premature termination, failure to clearly report methods and substantial heterogeneity Adverse events such as multiple pregnancy rate were not well reported |
| AWP1710 Long‐acting FSH vs daily FSH for women undergoing assisted reproduction | 6 RCTs | 3753 women with subfertility | Long‐acting FSH | Daily FSH | OHSSa Live birth ratea Ongoing pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Adverse events Satisfaction | Limited by risk of attrition bias in some primary studies and by serious imprecision |
| LDT120 Metformin treatment before and during IVF or ICSI in women with PCOS | 9 RCTs | 816 women with PCOS | Metformin | Placebo No treatment | OHSSa Live birtha Clinical pregnancya Miscarriage Adverse events Number of oocytes retrieved Total dose FSH (IU) Number of days gonadotrophin treatment Cycle cancellation rate Serum E2 level (nmol/L) | Half the trials were not blinded and lacked details on allocation concealment and randomisation |
| AM1335 Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing IVF | 14 RCTs, 12 for meta‐analysis | 2536 (12 trials) Subfertile women undergoing ART | Clomiphene citrate ± additional treatments | Alternative treatments for COH | OHSSa Live birth ratea Miscarriage rate Ectopic pregnancy Foetal abnormality Ongoing pregnancy rate Cancellation rate | Live birth reported in only 5 trials Most studies suffered from suboptimal methods and information on some outcomes was insufficient |
| TA1860 Natural cycle IVF for subfertile couples | 5 RCTs | 382 subfertile women and couples undertaking IVF treatment | Natural cycle IVF Modified natural cycle IVF | COH IVF | OHSSa Live birtha Pregnancy Ongoing pregnancy Number of oocytes retrieved Time to live birth Number of cycles required to conceive Cumulative pregnancy/live birth rate Multiple pregnancy Lack of embryos for cryopreservation Cycle cancellation Gestational abnormalities Cancellation of treatment Cost‐effectiveness | Few studies, live birth reported in only 1 very small trial Inclusion criteria differed |
| MV263 Luteal phase support for ART cycles | 94 RCTs | 26,198 women with any cause of subfertility undergoing ART | Progesterone hCG | Placebo or no treatment hCG Progesterone + oestrogen Progesterone + GnRHa | Live birtha Clinical pregnancy Ongoing pregnancy Miscarriage OHSS Multiple pregnancy | Poor reporting of study methods and imprecision due to small sample sizes |
| HA412 Gonadotrophin‐releasing hormone antagonists for ART | 73 RCTs | 12,212 women undergoing ART | GnRH antagonist | Long‐course GnRHa | OHSSa Live birtha Ongoing pregnancy Clinical pregnancy Miscarriage Cycle cancellation | Only 12 trials reported live birth Trial methods limited by lack of blinding Poor reporting of study methods for OHSS |
| AMY731 IVF vs tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation | No RCTs | NA | IVF | Tubal re‐anastomosis | Live birtha Clinical pregnancy Multiple pregnancy Other serious maternal morbidity, (incl OHSS) | Empty review with no trials No longer being updated |
| ZP672 IVF for unexplained subfertility | 6 RCTs | 733 couples with unexplained subfertility | IVF | Expectant management Intrauterine insemination Intrauterine insemination + ovarian stimulation Clomiphene citrate | Live birtha OHSS Clinical pregnancy Multiple pregnancy | Some evidence was based on a single trial Limitations included imprecision and heterogeneity for some outcomes |
| LA541 Depot vs daily administration of GnRHa protocols for pituitary desensitisation in assisted reproduction cycles | 16 RCTs, 12 for meta‐analysis | 1811 couples with any cause of subfertility undergoing IVF with COH with hFSH, hMG or rFSH | Pituitary downregulation with depot administration of GnRHa | Daily administration of GnRHa | OHSSa Live birtha Clinical pregnancya Miscarriage Multiple pregnancy | S tudy quality unclear due to poor reporting. O nly four stu dies reported live birth an d only five described adequate methods for allocation concealment . |
| IOK973 Recombinant vs urinary gonadotrophin for ovarian stimulation in ART cycles | 42 RCTs | 9606 normogonadotrophic women undergoing fresh and/or frozen thawed IVF or ICSI cycles | Recombinant FSH | Urinary FSH | OHSSa Live birtha Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects | No difference reported in moderate/severe OHSS |
| WPM1780 FSH replaced by low‐dose hCG in the late follicular phase vs continued FSH for ART | 5 RCTs | 351 women undergoing COH for ART | Low‐dose hCG instead of FSH in late follicular phase | Continued FSH in late follicular phase | OHSSa Live birtha Clinical pregnancy Miscarriage | Small studies and low event rate Total OHSS incidence reported |
| DHH752 Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing ART | 23 RCTs | 2596 women of any age with subfertility regardless of cause, undergoing ART | Pretreatment with combined oral contraceptive pills Pretreatment with progestogens | No pretreatment Placebo Progestogens Oestrogens | Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects | Only 3/23 studies reported on OHSS 2 of these 3 studies did not define how they diagnosed the condition |
| IOK972 Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) | 6 RCTs | 781 women undergoing COH in an IVF/ICSI cycle | Transvaginal ultrasonography + Oestradiol measurement | Transvaginal ultrasonography | Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects | Only total OHSS reported, including mild OHSS |
| CMB1261 Peri‐implantation glucocorticoid administration for ART cycles | 14 RCTs | 1879 subfertile patients undergoing IVF/ICSI, regardless of cause of infertility | Glucocorticoids in the peri‐implantation phase | No glucocorticoids in the peri‐implantation phase | Live birtha Multiple pregnancya OHSS Clinical pregnancy Miscarriage Adverse effects | Only 2 studies, pooled total OHSS |
| VJP951 Aspirin for IVF | 13 RCTs | 2653 women undergoing IVF/ICSI and their partners | Aspirin | No treatment Placebo | Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects | Only 1 of 13 studies reported on OHSS and without exact numbers or explanation for numerators/denominators |
| CS1400 In vitro maturation in subfertile women with PCOS undergoing assisted reproduction | None | 0 women with PCOS and subfertility | In vitro maturation + IVF/ICSI in women with PCOS | Conventional IVF/ICSI in women with PCOS | Live birtha OHSS Effectiveness Clinical pregnancy Miscarriage Adverse effects | Empty review |
| IRS911 Acupuncture and ART | 20 RCTs | 4544 women undergoing ART, any type of acupuncture at any time point before, after or during ART, intended to improve ART outcome | Acupuncture of men, women or both during COH Acupuncture + ART Acupuncture alone | No treatment Placebo Sham acupuncture Acupuncture + ART | Live birtha OHSS Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects | No trials reported on OHSS |
| MHM931 Recombinant luteinising hormone (rLH) for COH in assisted reproductive cycles | 14 RCTs | 2612 subfertile ovulatory women undergoing IVF or ICSI High risk: NA | Combination of rLH and rFSH for COH in IVF/ICSI followed by ET in GnRHa and GnRH antagonist protocols | rFSH alone for COH in IVF/ICSI followed by ET in GnRHa and GnRH antagonist protocols | OHSSa Live birtha Clinical pregnancy Miscarriage | Only 4/14 trials reported on OHSS Pooled OHSS No GRADE assessment in old version |
| KH291 Growth hormone for IVF | 10 RCTs | 440 women part of a subfertile couple undergoing IVF | Adjuvant growth hormone during conventional IVF | Conventional IVF | Live birtha OHSS Clinical pregnancy Adverse effects | Only 4 of 10 RCTs reported on adverse events (which could include OHSS) 1 study actually mentioned OHSS (however, no cases); pooled OHSS |
| SD265 GnRHa protocols for pituitary suppression in assisted reproduction | 37 RCTs | 3872 women/couples with all types of infertility undergoing ART and using GnRHa for pituitary downregulation | Long protocol Long luteal protocol Short protocol Dose continued Dose continued after hCG administration Pretreatment 2 weeks | Short protocol Ultrashort protocol Long follicular phase protocol Ultrashort protocol Dose stopped Dose reduced Dose discontinued after hCG administration Pretreatment 3 weeks | Live birtha OHSS Clinical pregnancy Adverse effects | Only 2 of 37 included RCTs reported on OHSS for 2 of 9 compared regimens |
| JC1630 Antioxidants for female subfertility | 28 RCTs | 3548 subfertile women referred to fertility clinic who might or might not undergo ART (IVF, ICSI or IUI) | Adjuvant antioxidants in females | No treatment Placebo Another antioxidant | Live birtha Clinical pregnancy Miscarriage Multiple pregnancy Adverse effects (incl OHSS) | Only 3 studies reported: 1 no data and 2 no cases |
| aPrimary review outcome. ART: artifical reproductive technology. COH: controlled ovarian hyperstimulation. ET: embryo transfer. FSH: follicle‐stimulating hormone. GnRHa: gonadotrophin‐releasing hormone agonist. hCG: human chorionic gonadotrophin. HES: hydroxyethyl starch. hFSH: human follicle‐stimulating hormone. hMG: human menopausal gonadotrophin. ICSI: intracytoplasmic sperm injection. IUI: intrauterine insemination. IVF: in vitro fertilisation. LH: luteinising hormone. NA: not applicable. OHSS: ovarian hyperstimulation syndrome. PCOS: polycystic ovary syndrome. RCT: randomised controlled trial. rFSH: recombinant follicle‐stimulating hormone. rLH: recombinant luteinising hormone. | ||||||
| Review no. | First review author | Review title | Date last assessed | < 3 years since last assessed up to date or deemed stable |
| ADA561 | Embryo freezing for preventing OHSS | 26/11/2010 | Stable | |
| ADA 563 | Coasting (withholding of gonadotrophins) for preventing OHSS | 19/07/2010 | X | |
| TH1338 | Dopamine agonists for preventing OHSS | 15/08/2016 | ✔ | |
| PMA481 | Volume expanders for prevention of OHSS | 21/09/2016 | ✔ | |
| HA413 | Recombinant vs urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles | 23/04/2015 | ✔ | |
| MM1690 | GnRHa vs hCG for oocyte triggering in antagonist‐assisted reproductive technology | 08/09/2014 | X | |
| LDT1201 | Metformin treatment before and during IVF or ICSI in women with PCOS | 15/10/2014 | ✔ | |
| AWP1710 | Long‐acting FSH vs daily FSH for women undergoing assisted reproduction | 8/06/2015 | ✔ | |
| AM1335 | Clomiphene citrate in combination with gonadotrophins for controlled ovarian stimulation in women undergoing IVF | 23/03/2012 | X | |
| TA1860 | Natural cycle IVF for subfertile couples | 5/03/2013 | ✔ | |
| MV263 | Luteal phase support for ART cycles | 25/11/2014 | ✔ | |
| HA412 | Gonadotrophin‐releasing hormone antagonists for ART | 28/04/2016 | ✔ | |
| AMY731 | IVF vs tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation | 15/05/2009 | Empty, stable | |
| ZP672 | IVF for unexplained subfertility | 4/05/2015 | ✔ | |
| LA541 | Depot vs daily administration of GnRHa protocols for pituitary desensitisation in assisted reproduction | 3/07/2012 | ✔ | |
| IOK973 | Recombinant vs urinary gonadotrophin for ovarian stimulation in ART cycles | 20/10/2010 | X | |
| WPM1780 | FSH replaced by low‐dose hCG in late follicular phase vs continued FSH for ART | 5/02/2013 | ✔ | |
| DHH752 | Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing ART | 16/11/2008 | X | |
| IOK972 | Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) | 30/05/2014 | ✔ | |
| CMB1261 | Peri‐implantation glucocorticoid administration for ART cycles | 20/09/2011 | X | |
| VJP951 | Aspirin for IVF | 9/05/2016 | ✔ | |
| CS1400 | In vitro maturation in subfertile women with PCOS undergoing assisted reproduction | 17/02/2011 | Empty | |
| IRS911 | Acupuncture and ART | 22/07/2013 | ✔ | |
| MHM931 | Recombinant luteinising hormone (rLH) for COH in assisted reproductive cycles | 25/01/2007 | X | |
| KH291 | Growth hormone for IVF | 20/07/2009 | X | |
| SD265 | GnRHa protocols for pituitary suppression in assisted reproduction | 23/04/2015 | ✔ | |
| JC1630 | Antioxidants for female subfertility | 15/04/2014 | ✔ | |
| ART: artifical reproductive technology. COH: controlled ovarian hyperstimulation. FSH: follicle‐stimulating hormone. GnRHa: gonadotrophin‐releasing hormone agonist. hCG: human chorionic gonadotrophin. ICSI: intracytoplasmic sperm injection. IUI: intrauterine insemination. IVF: in vitro fertilisation. OHSS: ovarian hyperstimulation syndrome. PCOS: polycystic ovary syndrome. rLH: recombinant luteinising hormone. ✔ under 3 years since last assessed as up to date X over 3 years since last assessed as up to date | ||||
| Review no. | First review author + year | Review title | AMSTAR criteria | |||||||||
| Prespecified question and inclusion criteria | Duplicate study selection and data extraction | Comprehensive literature search | Grey literature included | Lists included and excluded studies | Describes characteristics of included studies | Study quality assessed | Studies combined using appropriate methods | Likelihood of publication bias considered/tested | Potential for conflict of interest addressed | |||
| ADA561 | Embryo freezing for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ADA 563 | Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| TH1338 | Dopamine agonists for preventing ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| PMA481 | Volume expanders for the prevention of ovarian hyperstimulation syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| HA413 | Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| MM1690 | Gonadotropin‐releasing hormone agonist versus hCG for oocyte triggering in antagonist‐assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| LDT1201 | Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| AWP1710 | Long‐acting FSH versus daily FSH for women undergoing assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| AM1335 | Clomiphene citrate in combination with gonadotrophins for controlled ovarian stimulation in women undergoing in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| TA1860 | Natural cycle IVF for subfertile couples | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| MV263 | Luteal phase support for ART cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| HA412 | Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| AMY731 | In vitro fertilisation versus tubal re‐anastomosis (sterilisation reversal) for subfertility after tubal sterilisation | ✔ | ✔ | ✔ | ✔ | ✔ | NA | NA | NA | NA | ✔ | |
| ZP672 | In vitro fertilisation for unexplained subfertility | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| LA541 | Depot versus daily administration of gonadotrophin‐releasing hormone | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| IOK973 | Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| WPM1780 | FSH replaced by low‐dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| DHH752 | Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| IOK972 | Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| CMB1261 | Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| VJP951 | Aspirin for in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| CS1400 | In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | NA | NA | NA | NA | ✔ | |
| IRS911 | Acupuncture and assisted reproductive technology | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| MHM931 | Recombinant luteinising hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | X | ✔ | |
| KH291 | Growth hormone for in vitro fertilisation | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | X | ✔ | |
| SD265 | Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| JC1630 | Antioxidants for female subfertility | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Search date: 24/07/2016. ART: artifical reproductive technology. FSH: follicle‐stimulating hormone. hCG: human chorionic gonadotrophin. ICSI: intracytoplasmic sperm injection. IUI: intrauterine insemination. IVF: in vitro fertilisation. NA: not applicable. rLH: recombinant luteinising hormone. | ||||||||||||
| Review title and comparison intervention/control | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Embryo freezing for preventing ovarian hyperstimulation syndrome (Embryo freezing vs fresh transfer) | Overall OHSS: 60 per 1000 | Overall OHSS: 125 per 1000 (62 to 240) | OR 1.12 (0.01 to 2.29) | 125 (1 study) | Low | Imprecision, number of events < 300 Evidence based on a single open‐label study with insufficient methodological details provided | |
| Embryo freezing for preventing ovarian hyperstimulation syndrome (Embryo freezing vs intravenous albumin) | Moderate or severe OHSS: 77 per 1000 | Moderate or severe OHSS: 308 per 1000 (41 to 824) | OR 5.33 (0.51 to 56.24) | 26 (1 study) | Very low | Imprecision, number of events < 300 Evidence based on a single open‐label trial | |
| Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome | Moderate or severe OHSS: 265 per 1000 | Moderate or severe OHSS: 58 per 1000 (11 to 241) | OR 0.17 (0.03 to 0.88) | 68 (1 study) | Very low | Imprecision, number of events < 300 Evidence based on a single conference abstract | |
| Dopamine agonists for preventing ovarian hyperstimulation syndrome | Moderate or severe OHSS: 286 per 1000 | Moderate or severe OHSS: 97 per 1000 (71 to 135) | OR 0.27 (0.19 to 0.39) | 2091 (16 studies)) | Moderate | Imprecision, number of events < 300 Lack of details for allocation concealment and blinding, selective reporting | |
| Volume expanders for the prevention of ovarian hyperstimulation syndrome (human albumin vs placebo/no treatment) | Moderate or severe OHSS: 122 per 1000 | Moderate or severe OHSS: 85 per 1000 (61 to 177) | OR 0,67 (0.47 to 0.95) | 1452 (7 studies) | Very low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting | |
| Volume expanders for the prevention of ovarian hyperstimulation syndrome (HES vs placebo) | Moderate or severe OHSS: 164 per 1000 | Moderate or severe OHSS: 50 per 1000 (23 to 104) | OR 0.27 (0.12 to 0.59 | 272 (2 studies) | Very low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting | |
| Volume expanders for the prevention of ovarian hyperstimulation syndrome (mannitol vs placebo) | Moderate or severe OHSS: 517 per 1000 | Moderate or severe OHSS: 289 per 1000 (191 to 407) | OR 0.38 (0.22 to 0.64) | 226 (1 study) | Low | Imprecision, number of events < 300 Lack of details on allocation concealment and selective reporting | |
| Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles (r‐hCG vs u‐hCG) | Overall OHSS: 27 per 1000 | Overall OHSS:40 per 1000 (15 to 102) | OR 0.39 (0.25 to 0.61) | 374 (3 studies) | Moderate | Imprecision, number of events < 300 One of the trials lacked methodological details on randomisation, allocation concealment and blinding | |
| Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles (r‐LH vs u‐hCG) | Overall OHSS: 10 per 1000 | Overall OHSS: 17 per 1000 (11 to 84) | OR 1.76 (0.37 to 8.45) | 417 (3 studies) | Low | Imprecision, number of events < 300 One of the trials lacked adequate methodological details | |
| Gonadotropin‐releasing hormone agonist versus hCG for oocyte triggering in antagonist‐assisted reproductive technology | Overall OHSS: 5 per 1000 | Overall OHSS: 1 per 1000 (0 to 2) | OR 0.15 (0.05 to 0.47) | 989 (9 studies) | Moderate | Imprecision, number of events < 300 All studies at high risk of bias in 1 or more domains None clearly reported blinded outcome assessment | |
| Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. | Overall OHSS: 270 per 1000 | Overall OHSS: 97 per 1000 (62 to 153) | OR 0.29 (0.18 to 0.49) | 798 (8 studies) | Moderate | Imprecision, number of events < 300 | |
| Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (low dose) | Overall OHSS: 47 per 1000 | Overall OHSS: 57 per 1000 (26 to 125) | RR 1.22 (0.56 to 2.66) | 645 (3 studies) | Moderate | Imprecision, number of events < 300 | |
| Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (medium dose) | Overall OHSS: 63 per 1000 | Overall OHSS: 60 per 1000 (45 to 85) | RR 0.96 (0.68 to 1.35) | 3075 (5 studies) | Low | Imprecision, number of events < 300 Confidence intervals compatible with clinically meaningful benefit in either arm or with no effect, plus high risk of attrition bias in 2 studies | |
| Long‐acting FSH versus daily FSH for women undergoing assisted reproduction (high dose) | Overall OHSS: 0 per 1000 | Overall OHSS: 0 per 1000 (0 to 0) | RR 1.73 (0.09 to 32.75) | 33 (1 study) | Very low | Imprecision, number of events < 300 High risk of attrition bias | |
| Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation (clomiphene + gonadotropins vs gonadotropins) | Overall OHSS: 50 per 1000 | Overall OHSS:12 per 1000 (5 to 27) | OR 0.23 (0.1 to 0.52) | 1559 (5 studies) | Low | Imprecision, number of events < 300 Very wide 95% confidence interval crossing the threshold points of appreciable benefit or harm, which is 25% | |
| Natural cycle IVF for subfertile couples (natural cycle vs conventional IVF) | Overall OHSS: 67 per 1000 | Overall OHSS: 13 per 1000 (1 to 393) | OR 0.10 (0.01 to 4.06) | 60 (1 study) | Very low | Imprecision, number of events < 300 Only 1 study reporting on OHSS Allocation concealment method not reported | |
| Luteal phase support for ART cycles (hCG versus placebo/no treatment) | Overall OHSS: 41 per 1000 | Overall OHSS: 155 per 100 (76 to 292) | OR 4.28 (1.191 to 9.6) | 387 (1 study) | Low | Imprecision, number of events < 300 Poor reporting of study methods | |
| Luteal phase support for ART cycles (progesterone vs hCG regimens) | Overall OHSS: 126 per 1000 | Overall OHSS: 72 per 1000 (31 to 162) | OR 0.54 (0.22 to 1.34) | 615 (4 studies) | Low | Imprecision, number of events < 300 Poor reporting of study methods | |
| Luteal phase support for ART cycles (progesterone + GnRH agonist) | Overall OHSS: 50 per 1000 | Overall OHSS: 50 per 1000 (17 to 137) | OR 1.00 (0.33 to 3.01) | 300 (1 study) | Very low | Imprecision, number of events < 300 Poor reporting of study methods | |
| Luteal phase support for ART cycles (progesterone vs progesterone + oestrogens) | Overall OHSS: 39 per 1000 | Overall OHSS: 22 per 1000 (8 to 62) | OR 0.56 (0.2 to 1.63) | 461 (2 studies) | Low | Imprecision, number of events < 300 Poor reporting of study methods | |
| Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology (GnRH antagonist vs GnRH agonist) | Overall OHSS: 114 per 1000 | Overall OHSS: 73 per 1000 (62 to 85) | OR 0.61 | 7944 (36 studies) | Moderate | Methodological limitations including poor allocation concealment and lack of blinding | |
| In vitro fertilisation versus tubal reanastomosis (sterilisation reversal) for subfertility after tubal sterilisation (IVF vs tubal reanastomosis) | NA | NA | NA | NA | NA | Empty review | |
| In vitro fertilisation for unexplained subfertility (IVF vs IUI + gonadotropins/clomiphene citrate) | Overall OHSS: 58 per 1000 | Overall OHSS: 66 per 1000 (26 to 158) | OR 1.15 (0.43 to 3.06) | 324 (2 studies) | Low | Imprecision, number of events < 300 Only 2 studies on OHSS reported | |
| Depot versus daily administration of gonadotrophin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles (depot vs daily gonadotropins) | Overall OHSS: 3 per 100 | Overall OHSS: 2 per 100 | OR 0.84 | 570 (5 studies) | Low | Most studies were classified as at unclear risk of bias for all domains Imprecision, number of events < 300 | |
| Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs HMG/HMG‐HP) | Overall OHSS: 17 per 1000 | Overall OHSS: 17 per 1000 (10 to 28) | OR 1.00 (0.58 to 1.71) | 3197 (11 studies) | High | Imprecision, number of events < 300 | |
| Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs FSH‐P) | Overall OHSS: 28 per 1000 | Overall OHSS:49 per 1000 (25 to 95) | OR 1.79 (0.89 to 3.62) | 1490 (6 studies) | Higha | Imprecision, number of events < 300 | |
| Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rFSH vs FSH‐HP) | Overall OHSS: 28 per 1000 | Overall OHSS: 31 per 1000 (20 to 48) | OR 1.11 (0.70 vs 1.75) | 3053 (14 studies) | Higha | Two additional trials excluded in sensitivity analyses because it was unclear if data were reported according to ITT analysis (those were included for "Overall OHSS") | |
| Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles (rec‐hCG vs u‐hCG) | Overall OHSS: 19 per 1000 | Overall OHSS: 22 per 1000 (16 to 30) | OR 1.18 (0.86 vs 1.61) | 7740 (32 studies) | Higha | Imprecision number of events < 300 | |
| FSH replaced by low‐dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques (low‐dose hCG vs FSH in late follicular phase) | Overall OHSS: 3 per 100 | Overall OHSS: 1 per 100 (0 to 4) | OR 0.30 (0.06 to 1.59) | 351 (5 studies) | Very low | Imprecision, number of events < 300 Inconsistency, high risk of bias | |
| Oral contraceptive pill, progestogen or oestrogen pre‐ treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques (OAC plus antagonist vs antagonist) | Overall OHSS: 17 per 1000 | Overall OHSS: 25 per 1000 (5 to 133) | OR 1.5 (0.26 to 8.8) | 234 (1 study) | Very low | Single study reporting on OHSS Imprecision, number of events < 300 Wide confidence intervals that cross line of no effect High risk of attrition bias | |
| Oral contraceptive pill, progestogen or oestrogen pre‐ treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques (OAC plus antagonist vs agonist) | Overall OHSS: 55 per 1000 | Overall OHSS: 35 per 1000 (12 to 100) | OR 0.63 (0.21 to 1.92) | 290 (2 studies) | Very low | Imprecision, number of events < 300 One study at high risk of attrition bias | |
| Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) (transvaginal ultrasound + estradiol vs transvaginal ultrasound) | Overall OHSS: 36 per 1000 | Overall OHSS: 36 per 1000 (18 to 75) | OR 1.03 (0.48 to 2.20) | 781 (6 studies) | Low | Imprecision, number of events < 300 with wide confidence intervals Methods of randomisation inadequately described in 3 of 6 trials, allocation concealment inadequately described in all 6 trials and blinding inadequately described in 5 of 6 trials No definition of OHSS provided by authors of these 6 studies | |
| Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles (adjuvant glucocorticoids vs no glucocorticoids) | Overall OHSS: 194 per 1000 | Overall OHSS: 159 per 1000 (64 to 392) | OR 0.82 (0.33 to 2.02) | 151 (2 studies) | Low | Imprecision, number of events < 300 | |
| Aspirin for in vitro fertilisation (aspirin vs no treatment/placebo) | NA | NA | NA | NA | NA | Only 1 study reported on OHSS; no exact numbers or explanation of numerators/denominators given | |
| In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction (IVM vs conventional IVF) | NA | NA | NA | NA | NA | Empty review | |
| Acupuncture and assisted reproductive technology (acupuncture vs no acupuncture/sham acupuncture) | NA | NA | NA | NA | NA | No studies reported on OHSS | |
| Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles (combined rLH + FSH vs FSH ) | Overall OHSS: 20 per 1000 | Overall OHSS: 27 per 1000 (12 to 59) | OR 1.34 (0.58 to 3.09) | 986 (7 studies) | Low | Imprecision, number of events < 300 Some methodological details unclear | |
| Growth hormone for in vitro fertilization (growth hormone vs no treatment/placebo) | NA | NA | NA | NA | NA | Only 1 study reported on OHSS; however, no cases of OHSS were reported | |
| Gonadotropin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction (different protocols vs other protocol) | Overall OHSS: 20 per 1000 | Overall OHSS: 27 per 1000 (12 to 59) | OR 1.34 (0.58 to 3.09) | 986 (7 studies) | Low | Imprecision, number of events < 300 Some methodological details unclear | |
| Antioxidants for female subfertility (antioxidants vs no treatment/placebo/other antioxidant) | NA | NA | NA | NA | NA | Although 3 studies reported on OHSS, no numbers were given, so effect size could not be calculated | |
| aReview authors GRADED these outcomes as 'high quality'; however, the total event rate < 300 would justify downgrading for this to moderate‐quality evidence. ART: artifical reproductive technology. FSH: follicle‐stimulating hormone. FSH‐HP: highly purified FSH. hCG: human chorionic gonadotrophin. HES: hydroxyethyl starch. ICSI: intracytoplasmic sperm injection. IUI: intrauterine insemination. IVF: in vitro fertilisation. IVM: in vitro maturation. NA: not applicable. OAC: oral anticoagulant. OHSS: ovarian hyperstimulation syndrome. OR: odds ratio. rFSH: recombinant follicle‐stimulating hormone. r‐hCG: recombinant human chorionic gonadotrophin. rLH: recombinant luteinising hormone. Total: any grade of OHSS. u‐hCG: urinary human chorionic gonadotrophin. | |||||||

