非類固醇抗發炎藥(NSAIDs)對慢性下背痛的治療
Appendices
Appendix 1. CENTRAL search strategy
Last searched 24 June 2015. Line 34 is added and line 42 is revised.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 lumbar next pain or coccyx or coccydynia or spondylosis
#5 MeSH descriptor: [Spine] explode all trees
#6 MeSH descriptor: [Spinal Diseases] explode all trees
#7 lumbago and discitis and disc near herniation
#8 spinal fusion
#9 spinal neoplasms
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 nsaid*
#29 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees
#30 MeSH descriptor: [Cyclooxygenase Inhibitors] explode all trees
#31 MeSH descriptor: [Cyclooxygenase 2 Inhibitors] explode all trees
#32 non‐steroidal anti inflammat*
#33 non‐steroidal anti‐inflammat*
#34 (cyclooxygenase or cyclo‐oxygenase) next/3 inhibitor*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin or indomethacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 parecoxib
#67 vioxx
#68 celebrex
#69 bextra
#70 prexige
#71 arcoxia
#72 etodolac
#73 floctafenine
#74 meclofenam*
#75 meloxicam
#76 oxaprozin
#77 piroxicam
#78 tenoxicam
#79 tolmetin
#80 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79
#81 #27 and #80
#82 #81 in Trials
May 2012 strategy. In 2015, Line 77 and 66 are removed (duplicate with line 52 and 59), disc degeneration and prolapse are removed from line 8 (captured in line 20 and 23), and sciatica is removed from line 5 (captured in line 25).
#1 MeSH descriptor Back Pain explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor Low Back Pain explode all trees
#5 (lumbar next pain) or (coccyx) or (coccydynia) or (sciatica) or (spondylosis)
#6 MeSH descriptor Spine explode all trees
#7 MeSH descriptor Spinal Diseases explode all trees
#8 (lumbago) or (discitis) or (disc near degeneration) or (disc near prolapse) or (disc near herniation)
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor Intervertebral Disk explode all trees
#13 postlaminectomy
#14 arachnoiditis 36
#15 failed near back
#16 MeSH descriptor Cauda Equina explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor Sciatic Neuropathy explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 nsaid*
#30 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees
#31 MeSH descriptor Cyclooxygenase Inhibitors explode all trees
#32 MeSH descriptor Cyclooxygenase 2 Inhibitors explode all trees
#33 non‐steroidal anti inflammat*
#34 non‐steroidal anti‐inflammat*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 etoricoxib
#67 parecoxib
#68 vioxx
#69 celebrex
#70 bextra
#71 prexige
#72 arcoxia
#73 etodolac
#74 floctafenine
#75 meclofenam*
#76 meloxicam
#77 naproxen
#78 oxaprozin
#79 piroxicam
#80 tenoxicam
#81 tolmetin
#82 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81)
#83 (#28 AND #82), from 2007 to 2012
Appendix 2. MEDLINE search strategy
Last searched 24 June 2015. Line 3 and 61 are added and line 6, 22, 29, and 39 are revised.
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
pragmatic clinical trial.pt.
-
comparative study.pt.
-
clinical trial.pt.
-
randomi#ed.ab.
-
placebo.ab,ti.
-
drug therapy.fs.
-
randomly.ab,ti.
-
trial.ab,ti.
-
groups.ab,ti.
-
or/1‐11
-
(animals not (humans and animals)).sh.
-
12 not 13
-
dorsalgia.ti,ab.
-
exp Back Pain/
-
backache.ti,ab.
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
exp sciatic neuropathy/
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
back disorder$.ti,ab.
-
or/15‐25
-
exp Anti‐Inflammatory Agents, Non‐Steroidal/
-
nsaids.mp.
-
non‐steroidal antiinflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
aspirin.mp. or exp Aspirin/
-
acetylsalicyl$.mp.
-
exp Salicylic Acid/
-
carbasalate calcium.mp.
-
diflunisal.mp. or exp Diflunisal/
-
aceclofenac.mp.
-
alclofenac.mp.
-
diclofenac.mp. or exp Diclofenac/
-
(indometacin or indomethacin).mp. or exp Indomethacin/
-
sulindac.mp. or exp Sulindac/
-
meloxicam.mp.
-
piroxicam.mp. or exp Piroxicam/
-
dexibuprofen.mp.
-
dexketoprofen.mp.
-
fenoprofen.mp. or exp Fenoprofen/
-
flurbiprofen.mp. or exp Flurbiprofen/
-
ibuprofen.mp. or exp Ibuprofen/
-
ketoprofen.mp. or exp Ketoprofen/
-
naproxen.mp. or exp Naproxen/
-
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
metamizol.mp. or exp Dipyrone/
-
phenylbutazone.mp. or exp Phenylbutazone/
-
phenazone.mp. or exp Antipyrine/
-
propyphenazone.mp.
-
celecoxib.mp.
-
etoricoxib.mp.
-
nabumeton.mp.
-
parecoxib.mp.
-
or/27‐58
-
exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
-
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
-
rofecoxib.mp.
-
celecoxib.mp.
-
valdecoxib.mp.
-
lumiracoxib.mp.
-
etoricoxib.mp.
-
parecoxib.mp.
-
vioxx.mp.
-
celebrex.mp.
-
bextra.mp.
-
prexige.mp.
-
arcoxia.mp.
-
etodolac.mp. or exp Etodolac/
-
floctafenine.mp.
-
exp Meclofenamic Acid/
-
meclofenamate.mp.
-
meloxicam.mp.
-
oxaprozin.mp.
-
piroxicam.mp. or exp Piroxicam/
-
tenoxicam.mp.
-
tolmetin.mp. or exp Tolmetin/
-
or/60‐81
-
59 or 82
-
14 and 26 and 83
-
limit 84 to yr=2014‐2015
-
limit 84 to ed=20140410‐20150624
-
85 or 86
May 2012 strategy. Line 77 is removed in 2015 (duplicate with line 49).
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
comparative study.pt.
-
clinical trial.pt.
-
randomized.ab.
-
placebo.ab,ti.
-
drug therapy.fs.
-
randomly.ab,ti.
-
trial.ab,ti.
-
groups.ab,ti.
-
or/1‐10
-
(animals not (humans and animals)).sh.
-
11 not 12
-
dorsalgia.ti,ab.
-
exp Back Pain/
-
backache.ti,ab.
-
exp Low Back Pain/
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
sciatic neuropathy/
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
back disorder$.ti,ab.
-
or/14‐25 33294
-
exp Anti‐Inflammatory Agents, Non‐Steroidal/
-
nsaids.mp.
-
non‐steroidal anti inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
aspirin.mp. or exp Aspirin/
-
acetylsalicyl$.mp.
-
exp Salicylic Acid/
-
carbasalate calcium.mp.
-
diflunisal.mp. or exp Diflunisal/
-
aceclofenac.mp.
-
alclofenac.mp.
-
diclofenac.mp. or exp Diclofenac/
-
indometacin.mp. or exp Indomethacin/
-
sulindac.mp. or exp Sulindac/
-
meloxicam.mp.
-
piroxicam.mp. or exp Piroxicam/
-
dexibuprofen.mp.
-
dexketoprofen.mp.
-
fenoprofen.mp. or exp Fenoprofen/
-
flurbiprofen.mp. or exp Flurbiprofen/
-
ibuprofen.mp. or exp Ibuprofen/
-
ketoprofen.mp. or exp Ketoprofen/
-
naproxen.mp. or exp Naproxen/
-
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
metamizol.mp. or exp Dipyrone/
-
phenylbutazone.mp. or exp Phenylbutazone/
-
phenazone.mp. or exp Antipyrine/
-
propyphenazone.mp.
-
celecoxib.mp.
-
etoricoxib.mp.
-
nabumeton.mp.
-
parecoxib.mp.
-
or/27‐58
-
exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
-
rofecoxib.mp.
-
celecoxib.mp.
-
valdecoxib.mp.
-
lumiracoxib.mp.
-
etoricoxib.mp.
-
parecoxib.mp.
-
vioxx.mp.
-
celebrex.mp.
-
bextra.mp.
-
prexige.mp.
-
arcoxia.mp.
-
etodolac.mp. or exp Etodolac/
-
floctafenine.mp.
-
exp Meclofenamic Acid/
-
meclofenamate.mp.
-
meloxicam.mp.
-
naproxen.mp. or exp Naproxen/
-
oxaprozin.mp.
-
piroxicam.mp. or exp Piroxicam/
-
tenoxicam.mp.
-
tolmetin.mp. or exp Tolmetin/
-
or/60‐81
-
59 or 82
-
13 and 26 and 83
-
limit 84 to yr="2007 ‐ 2012"
-
limit 84 to ed=20070601‐20120524
-
85 or 86
Appendix 3. MEDLINE In‐Process & Other Non‐Indexed Citations
Last searched 24 June 2015. Line 3 is added, line 6, 27, 37, and 58 are revised.
-
randomized controlled trial.ti,ab.
-
controlled clinical trial.ti,ab.
-
pragmatic.ti,ab.
-
comparative study.ti,ab.
-
clinical trial.ti,ab.
-
randomi#ed.ab.
-
placebo.ab,ti.
-
drug therapy.fs.
-
randomly.ab,ti.
-
trial.ab,ti.
-
groups.ab,ti.
-
or/1‐11
-
dorsalgia.ti,ab.
-
Back Pain.ti,ab.
-
backache.ti,ab.
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
sciatic neuropathy.ti,ab.
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
back disorder$.ti,ab.
-
or/13‐23
-
Anti‐Inflammatory Agents, Non‐Steroidal.mp.
-
nsaids.mp.
-
non‐steroidal antiinflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
aspirin.mp.
-
acetylsalicyl$.mp.
-
Salicylic Acid.mp.
-
carbasalate calcium.mp.
-
diflunisal.mp.
-
aceclofenac.mp.
-
alclofenac.mp.
-
diclofenac.mp.
-
(indomethacin or indometacin).mp.
-
sulindac.mp.
-
meloxicam.mp.
-
piroxicam.mp.
-
dexibuprofen.mp.
-
dexketoprofen.mp.
-
fenoprofen.mp.
-
flurbiprofen.mp.
-
ibuprofen.mp.
-
ketoprofen.mp.
-
naproxen.mp.
-
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
metamizol.mp.
-
phenylbutazone.mp.
-
phenazone.mp.
-
propyphenazone.mp.
-
celecoxib.mp.
-
etoricoxib.mp.
-
nabumeton.mp.
-
parecoxib.mp.
-
or/25‐56
-
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
-
rofecoxib.mp.
-
celecoxib.mp.
-
valdecoxib.mp.
-
lumiracoxib.mp.
-
etoricoxib.mp.
-
parecoxib.mp.
-
vioxx.mp.
-
celebrex.mp.
-
bextra.mp.
-
prexige.mp.
-
arcoxia.mp.
-
etodolac.mp.
-
floctafenine.mp.
-
Meclofenamic Acid.mp.
-
meclofenamate.mp.
-
meloxicam.mp.
-
oxaprozin.mp.
-
piroxicam.mp.
-
tenoxicam.mp.
-
tolmetin.mp.
-
or/58‐78
-
57 or 79
-
12 and 24 and 80
-
limit 81 to yr=2014‐2015
-
limit 81 to ed=20140410‐20150624
-
82 or 83
April 2014 search strategy
-
randomized controlled trial.ti,ab.
-
controlled clinical trial.ti,ab.
-
comparative study.ti,ab.
-
clinical trial.ti,ab.
-
randomized.ab.
-
placebo.ab,ti.
-
drug therapy.fs.
-
randomly.ab,ti.
-
trial.ab,ti.
-
groups.ab,ti.
-
or/1‐10
-
dorsalgia.ti,ab.
-
Back Pain.ti,ab.
-
backache.ti,ab.
-
(lumbar adj pain).ti,ab.
-
coccyx.ti,ab.
-
coccydynia.ti,ab.
-
sciatica.ti,ab.
-
sciatic neuropathy.ti,ab.
-
spondylosis.ti,ab.
-
lumbago.ti,ab.
-
back disorder$.ti,ab.
-
or/12‐22
-
Anti‐Inflammatory Agents, Non‐Steroidal.mp.
-
nsaids.mp.
-
non‐steroidal anti inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
aspirin.mp.
-
acetylsalicyl$.mp.
-
Salicylic Acid.mp.
-
carbasalate calcium.mp.
-
diflunisal.mp.
-
aceclofenac.mp.
-
alclofenac.mp.
-
diclofenac.mp.
-
indomethacin.mp.
-
sulindac.mp.
-
meloxicam.mp.
-
piroxicam.mp.
-
dexibuprofen.mp.
-
dexketoprofen.mp.
-
fenoprofen.mp.
-
flurbiprofen.mp.
-
ibuprofen.mp.
-
ketoprofen.mp.
-
naproxen.mp.
-
tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
metamizol.mp.
-
phenylbutazone.mp.
-
phenazone.mp.
-
propyphenazone.mp.
-
celecoxib.mp.
-
etoricoxib.mp.
-
nabumeton.mp.
-
parecoxib.mp.
-
or/24‐55
-
(cyclooxygenase inhibitors or cyclooxygenase 2 inhibitors).mp.
-
rofecoxib.mp.
-
celecoxib.mp.
-
valdecoxib.mp.
-
lumiracoxib.mp.
-
etoricoxib.mp.
-
parecoxib.mp.
-
vioxx.mp.
-
celebrex.mp.
-
bextra.mp.
-
prexige.mp.
-
arcoxia.mp.
-
etodolac.mp.
-
floctafenine.mp.
-
Meclofenamic Acid.mp.
-
meclofenamate.mp.
-
meloxicam.mp.
-
oxaprozin.mp.
-
piroxicam.mp.
-
tenoxicam.mp.
-
tolmetin.mp.
-
or/57‐77
-
56 or 78
-
11 and 23 and 79
Appendix 4. EMBASE search strategy
Last searched 24 June 2015. The study design filter, line 38, and line 46 are revised and line 68 is added.
-
Randomized Controlled Trial/ (374656)
-
exp Controlled Clinical Trial/ (511712)
-
Controlled Study/ (4627382)
-
Double Blind Procedure/ (121249)
-
Single Blind Procedure/ (20436)
-
crossover procedure/ (43275)
-
placebo/ (258120)
-
allocat$.mp. (105697)
-
assign$.mp. (262956)
-
blind$.mp. (343130)
-
((control$ or compar$ or prospectiv$ or clinical) adj25 (trial or study)).mp. (7800092)
-
(crossover or cross‐over).mp. (81850)
-
factorial$.mp. (50965)
-
(followup or follow‐up).mp. (1253262)
-
placebo$.mp. (339829)
-
random$.mp. (1133643)
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp. (222737)
-
volunteer$.mp. (196350)
-
or/1‐18 (8994276)
-
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ (21299526)
-
human/ or normal human/ or human cell/ (15984909)
-
20 and 21 (15952556)
-
20 not 22 (5346970)
-
19 not 23 (6914940)
-
dorsalgia.mp. (102)
-
back pain.mp. (59723)
-
exp BACKACHE/ (73517)
-
(lumbar adj pain).mp. (1626)
-
coccyx.mp. (800)
-
coccydynia.mp. (120)
-
sciatica.mp. (4597)
-
exp ISCHIALGIA/ (5449)
-
spondylosis.mp. (7198)
-
lumbago.mp. (1454)
-
or/25‐34 (93822)
-
exp Nonsteroid Antiinflammatory Agent/ (444580)
-
nsaids.mp. (24138)
-
non‐steroidal anti‐inflammator$.mp. (16629)
-
exp Acetylsalicylic Acid/ (161086)
-
acetylsalicyl$.mp. (163662)
-
carbasalate calcium.mp. or exp Carbasalate Calcium/ (242)
-
diflunisal.mp. or exp DIFLUNISAL/ (2399)
-
aceclofenac.mp. or exp ACECLOFENAC/ (1287)
-
alclofenac.mp. or exp ALCLOFENAC/ (355)
-
diclofenac.mp. or exp DICLOFENAC/ (32204)
-
exp INDOMETACIN/ or (indometacin or indomethacin).mp. (70465)
-
sulindac.mp. or exp SULINDAC/ (6849)
-
meloxicam.mp. or exp MELOXICAM/ (4723)
-
exp PIROXICAM/ or piroxicam.mp. (10561)
-
dexibuprofen.mp. or exp DEXIBUPROFEN/ (212)
-
dexketoprofen.mp. or exp DEXKETOPROFEN/ (463)
-
exp FENOPROFEN/ or fenoprofen.mp. (2484)
-
flurbiprofen.mp. or exp FLURBIPROFEN/ (6927)
-
ibuprofen.mp. or exp IBUPROFEN/ (39286)
-
ketoprofen.mp. or exp KETOPROFEN/ (10969)
-
naproxen.mp. or exp NAPROXEN/ (22293)
-
tiapro$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1330)
-
metamizol.mp. or exp Dipyrone/ (6416)
-
phenylbutazone.mp. or exp PHENYLBUTAZONE/ (11876)
-
phenazone.mp. or exp PHENAZONE/ (5587)
-
exp PROPYPHENAZONE/ or propyphenazone.mp. (829)
-
celecoxib.mp. or exp CELECOXIB/ (17414)
-
etoricoxib.mp. or exp ETORICOXIB/ (2236)
-
exp Nabumetone/ or nabumeton.mp. (1837)
-
parecoxib.mp. or exp PARECOXIB/ (1501)
-
or/36‐65 (464519)
-
exp Cyclooxygenase 2 Inhibitor/ (41240)
-
((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp. (27816)
-
rofecoxib.mp. or exp ROFECOXIB/ (9957)
-
valdecoxib.mp. or exp VALDECOXIB/ (2464)
-
lumiracoxib.mp. or exp LUMIRACOXIB/ (1046)
-
etoricoxib.mp. or exp ETORICOXIB/ (2236)
-
parecoxib.mp. or exp PARECOXIB/ (1501)
-
vioxx.mp. (2888)
-
celebrex.mp. (2353)
-
bextra.mp. (569)
-
prexige.mp. (174)
-
arcoxia.mp. (276)
-
etodolac.mp. or exp ETODOLAC/ (2403)
-
floctafenine.mp. or exp FLOCTAFENINE/ (216)
-
exp Meclofenamic Acid/ (2319)
-
meclofenam$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (2769)
-
oxaprozin.mp. or exp OXAPROZIN/ (658)
-
exp PIROXICAM/ or piroxicam.mp. (10561)
-
tenoxicam.mp. or exp TENOXICAM/ (1889)
-
tolmetin.mp. or exp TOLMETIN/ (2406)
-
or/67‐86 (62118)
-
66 or 87 (469269)
-
24 and 35 and 88 (3792)
-
limit 89 to yr="2014 ‐ 2015" (394)
-
limit 89 to em=201414‐201525 (396)
-
90 or 91 (453)
Study design and animal filter used in the April 2014 search. The animal filter is revised in 2013 and line 31 is revised in 2014.
1 Clinical Article/
2 exp Clinical Study/
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 or 30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or normal human/ or human cell/
34 32 and 33
35 32 not 34
36 31 not 35
May 2012 search strategy
-
Clinical Article/
-
exp Clinical Study/
-
Clinical Trial/
-
Controlled Study/
-
Randomized Controlled Trial/
-
Major Clinical Study/
-
Double Blind Procedure/
-
Multicenter Study/
-
Single Blind Procedure/
-
Phase 3 Clinical Trial/
-
Phase 4 Clinical Trial/
-
crossover procedure/
-
placebo/
-
or/1‐13
-
allocat$.mp.
-
assign$.mp.
-
blind$.mp.
-
(clinic$ adj25 (study or trial)).mp.
-
compar$.mp.
-
control$.mp.
-
cross?over.mp.
-
factorial$.mp.
-
follow?up.mp.
-
placebo$.mp.
-
prospectiv$.mp.
-
random$.mp.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
-
trial.mp.
-
(versus or vs).mp.
-
or/15‐29
-
14 and 30
-
human/
-
Nonhuman/
-
exp ANIMAL/
-
Animal Experiment/
-
33 or 34 or 35
-
32 not 36
-
31 not 36
-
37 and 38
-
38 or 39
-
dorsalgia.mp.
-
back pain.mp.
-
exp BACKACHE/
-
(lumbar adj pain).mp.
-
coccyx.mp.
-
coccydynia.mp.
-
sciatica.mp.
-
exp ISCHIALGIA/
-
spondylosis.mp.
-
lumbago.mp.
-
exp Low Back Pain/
-
or/41‐51
-
exp Nonsteroid Antiinflammatory Agent/
-
nsaids.mp.
-
non‐steroidal anti‐inflammatory.mp.
-
exp Acetylsalicylic Acid/
-
acetylsalicyl$.mp.
-
carbasalate calcium.mp. or exp Carbasalate Calcium/
-
diflunisal.mp. or exp DIFLUNISAL/
-
aceclofenac.mp. or exp ACECLOFENAC/
-
alclofenac.mp. or exp ALCLOFENAC/
-
diclofenac.mp. or exp DICLOFENAC/
-
exp INDOMETACIN/ or indometacin.mp.
-
sulindac.mp. or exp SULINDAC/
-
meloxicam.mp. or exp MELOXICAM/
-
exp PIROXICAM/ or piroxicam.mp.
-
dexibuprofen.mp. or exp DEXIBUPROFEN/
-
dexketoprofen.mp. or exp DEXKETOPROFEN/
-
exp FENOPROFEN/ or fenoprofen.mp.
-
flurbiprofen.mp. or exp FLURBIPROFEN/
-
ibuprofen.mp. or exp IBUPROFEN/
-
ketoprofen.mp. or exp KETOPROFEN/
-
naproxen.mp. or exp NAPROXEN/
-
tiapro$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
metamizol.mp. or exp Dipyrone/
-
phenylbutazone.mp. or exp PHENYLBUTAZONE/
-
phenazone.mp. or exp PHENAZONE/
-
exp PROPYPHENAZONE/ or propyphenazone.mp.
-
celecoxib.mp. or exp CELECOXIB/
-
etoricoxib.mp. or exp ETORICOXIB/
-
exp Nabumetone/ or nabumeton.mp.
-
parecoxib.mp. or exp PARECOXIB/
-
or/53‐82
-
exp Cyclooxygenase 2 Inhibitor/
-
rofecoxib.mp. or exp ROFECOXIB/
-
valdecoxib.mp. or exp VALDECOXIB/
-
lumiracoxib.mp. or exp LUMIRACOXIB/
-
etoricoxib.mp. or exp ETORICOXIB/
-
parecoxib.mp. or exp PARECOXIB/
-
vioxx.mp.
-
celebrex.mp.
-
bextra.mp.
-
prexige.mp.
-
arcoxia.mp.
-
etodolac.mp. or exp ETODOLAC/
-
floctafenine.mp. or exp FLOCTAFENINE/
-
exp Meclofenamic Acid/
-
meclofenam$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
-
oxaprozin.mp. or exp OXAPROZIN/
-
exp PIROXICAM/ or piroxicam.mp.
-
tenoxicam.mp. or exp TENOXICAM/
-
tolmetin.mp. or exp TOLMETIN/
-
or/84‐102
-
83 or 103
-
40 and 52 and 104
-
limit 105 to yr="2007 ‐ 2012"
-
limit 105 to em=200712‐201220 1071
-
106 or 107
Appendix 5. Search strategies for clinical trials registries and PubMed
ClinicalTrials.gov
Last searched 24 June 2015.
Basic search: “back pain” and NSAIDS, received from 10 April 2014 to 24 June 2015.
May 2012 search strategy.
Condition: back pain AND Intervention: NSAID
WHO ICTRP
Last searched 24 June 2015.
Basic search: back pain and NSAIDS; we reviewed results from 2014 to 2015.
May 2012 search strategy.
Condition: back pain AND Intervention: NSAID
PubMed
Searched 24 June 2015.
((nsaids OR non‐steroidal anti‐inflammator* OR non‐steroidal antiinflammator* OR aspirin OR acetylsalicyl* OR salicylic acid OR carbasalate calcium OR diflunisal OR aceclofenac OR alclofenac OR diclofenac OR indomethacin OR indometacin OR sulindac OR meloxicam OR piroxicam OR dexibuprofen OR dexketoprofen OR fenoprofen OR flurbiprofen OR ibuprofen OR ketoprofen OR naproxen OR tiapro* OR metamizol OR phenylbutazone OR phenazone OR propyphenazone OR celecoxib OR etoricoxib OR nabumeton OR parecoxib OR cyclooxygenase inhibitor* OR cyclo‐oxygenase inhibitor* OR rofecoxib OR celecoxib OR valdecoxib OR lumiracoxib OR etoricoxib OR parecoxib OR vioxx OR celebrex OR bextra OR prexige OR arcoxia OR etodolac OR floctafenine OR Meclofenamic Acid OR meclofenamate OR meloxicam OR oxaprozin OR piroxicam OR tenoxicam OR tolmetin) AND (back pain OR sciatica OR lumbar pain OR lumbago OR dorsalgia OR backache OR back disorder*) AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
Appendix 6. Criteria for assessing risk of bias for internal validity
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because investigators used one of the following, or an equivalent method, to conceal allocation: central allocation (including telephone, internet‐based and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if trial investigators ensured blinding of participants and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is unlikely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the trial
There is a low risk of performance bias if trial investigators ensured blinding of personnel and it was unlikely that blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if trial investigators ensured the blinding of the outcome assessment and it was unlikely that blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
-
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005)
-
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005)
-
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is unavailable but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomized patients were reported/analysed in the group to which they were allocated by randomization.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).

study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials.

Funnel plot of comparison: 1 NSAIDs versus placebo, outcome: 1.1 Change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 12 weeks.

Funnel plot of comparison: 1 NSAIDs versus placebo, outcome: 1.2 Change in disability from baseline.

Funnel plot of comparison: 1 NSAIDs versus placebo, outcome: 1.3 Proportion of patients experiencing adverse events. Follow‐up ≤ 16 weeks.
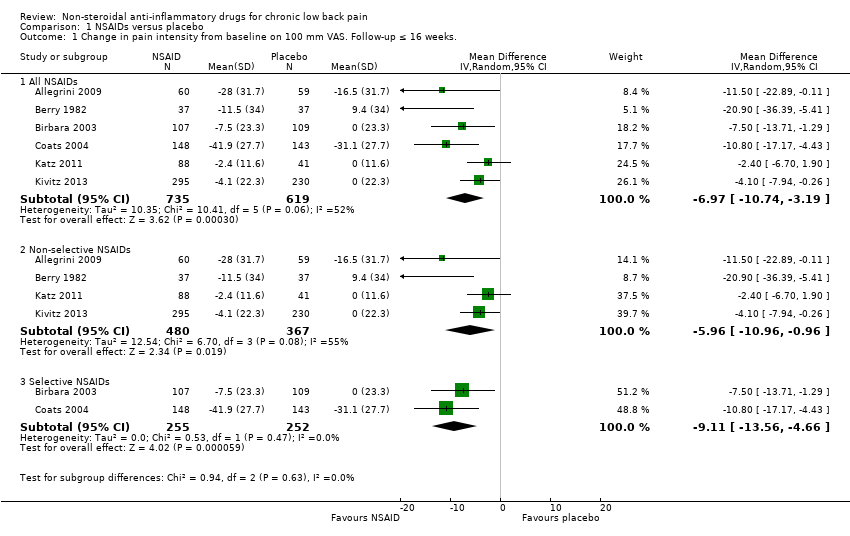
Comparison 1 NSAIDs versus placebo, Outcome 1 Change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 16 weeks..

Comparison 1 NSAIDs versus placebo, Outcome 2 Change in disability from baseline.

Comparison 1 NSAIDs versus placebo, Outcome 3 Proportion of patients experiencing adverse events. Follow‐up ≤ 16 weeks..
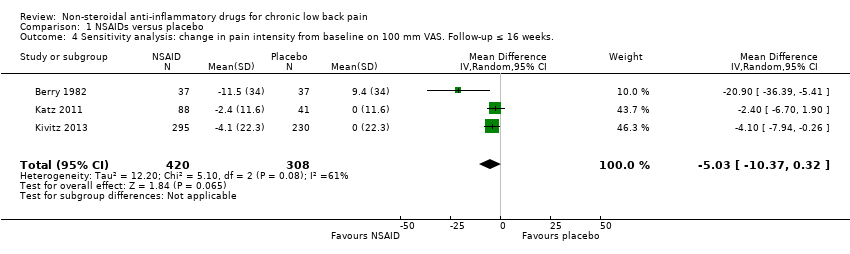
Comparison 1 NSAIDs versus placebo, Outcome 4 Sensitivity analysis: change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 16 weeks..

Comparison 1 NSAIDs versus placebo, Outcome 5 Sensitivity analysis: change in disability from baseline.
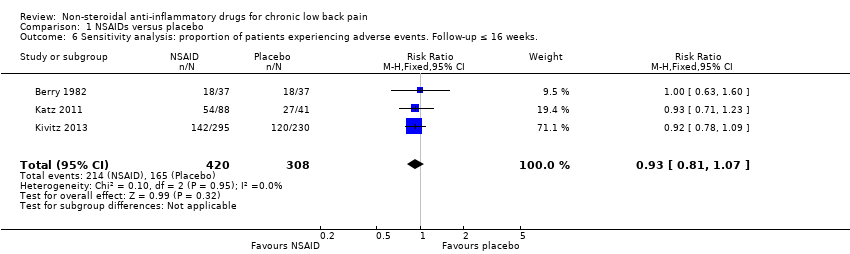
Comparison 1 NSAIDs versus placebo, Outcome 6 Sensitivity analysis: proportion of patients experiencing adverse events. Follow‐up ≤ 16 weeks..
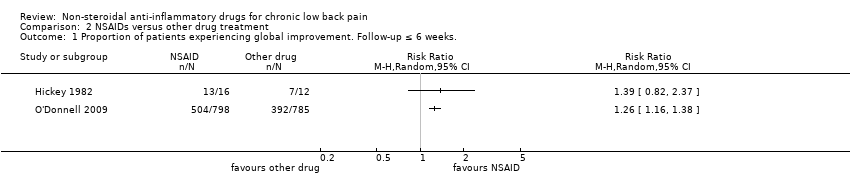
Comparison 2 NSAIDs versus other drug treatment, Outcome 1 Proportion of patients experiencing global improvement. Follow‐up ≤ 6 weeks..

Comparison 2 NSAIDs versus other drug treatment, Outcome 2 Proportion of patients experiencing adverse events. Follow‐up ≤ 6 weeks..
| NSAIDs for people with chronic low back pain compared to placebo | |||||
| Participant or population: people with chronic low back pain | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | NSAIDs | ||||
| Change in pain intensity from baseline | Not estimable | The mean change in pain intensity from baseline in the intervention groups was | ‐ | 1354 | ⊕⊕⊝⊝ |
| Change in disability from baseline | Not estimable | The mean change in disability from baseline in the intervention groups was | ‐ | 1161 | ⊕⊕⊝⊝ |
| Proportion of participants experiencing adverse events | Study population | RR 1.04 | 1354 | ⊕⊕⊝⊝ | |
| 410 per 1000 | 427 per 1000 | ||||
| Moderate | |||||
| 477 per 1000 | 496 per 1000 | ||||
| Sensitivity analysis: change in pain intensity from baseline | Not estimable | The mean sensitivity analysis change in pain intensity from baseline. in the intervention groups was | ‐ | 728 | ⊕⊕⊕⊝ |
| Sensitivity analysis: change in disability from baseline | Not estimable | The mean sensitivity analysis change in disability from baseline in the intervention groups was | ‐ | 654 | ⊕⊕⊕⊝ |
| Sensitivity analysis: proportion of participants experiencing adverse events. Follow‐up ≤ 16 weeks | Study population | RR 0.93 | 728 | ⊕⊕⊕⊝ | |
| 536 per 1000 | 498 per 1000 | ||||
| Moderate | |||||
| 522 per 1000 | 485 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Allocation concealment was uncertain in most included trials, and randomization was uncertain in half of the included trials, therefore selection bias is likely. Five out of six trials had high drop‐out rates, so attrition bias is likely, one level downgrade. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 16 weeks. Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All NSAIDs | 6 | 1354 | Mean Difference (IV, Random, 95% CI) | ‐6.97 [‐10.74, ‐3.19] |
| 1.2 Non‐selective NSAIDs | 4 | 847 | Mean Difference (IV, Random, 95% CI) | ‐5.96 [‐10.96, ‐0.96] |
| 1.3 Selective NSAIDs | 2 | 507 | Mean Difference (IV, Random, 95% CI) | ‐9.11 [‐13.56, ‐4.66] |
| 2 Change in disability from baseline Show forest plot | 4 | 1161 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.30, ‐0.40] |
| 3 Proportion of patients experiencing adverse events. Follow‐up ≤ 16 weeks. Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 All NSAIDs | 6 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 3.2 Non‐selective NSAIDs | 4 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 3.3 Selective NSAIDs | 2 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.00, 1.56] |
| 4 Sensitivity analysis: change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 16 weeks. Show forest plot | 3 | 728 | Mean Difference (IV, Random, 95% CI) | ‐5.03 [‐10.37, 0.32] |
| 5 Sensitivity analysis: change in disability from baseline Show forest plot | 2 | 654 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.04, 0.23] |
| 6 Sensitivity analysis: proportion of patients experiencing adverse events. Follow‐up ≤ 16 weeks. Show forest plot | 3 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients experiencing global improvement. Follow‐up ≤ 6 weeks. Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Proportion of patients experiencing adverse events. Follow‐up ≤ 6 weeks. Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

