Ujian diagnostik yang tidak invasif untuk jangkitan Helicobacter pylori
Referencias
References to studies included in this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 73 | ||
| Index tests | Index test: urea breath test ‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 31 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 31 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 64 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 63 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 215 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 252 | ||
| Index tests | Index test: urea breath test ‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 95 | ||
| Index tests | Index test: urea breath test ‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 96 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 287 | ||
| Index tests | Index test 1a: serology Index test 1b: serology (serum) Index test 1c: serology (whole blood) | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 256 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 230 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 30 | ||
| Index tests | Index test 1: stool antigen test Index test 2: urea breath test‐14C Different criteria for positive diagnosis:
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 79 | ||
| Index tests | Index test 1: urea breath test‐13C Different criteria for positive diagnosis:
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 77 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 149 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 126 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 19 | ||
| Index tests | Index test 1: serology Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 55 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 126 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 106 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 84 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 500 | ||
| Index tests | Index test 1a: serology Index test 1b: serology Index test 1c: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 75 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 22 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 328 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 65 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 60 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 99 | ||
| Index tests | Index test: urea breath test ‐ unknown isotope | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test ‐ unknown isotope | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 122 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 112 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 33 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 209 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 35 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 103 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 55 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 107 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 20 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 109 | ||
| Index tests | Index test 1: urea breath test‐14C Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 130 | ||
| Index tests | Index test 1a: serology Index test 1b: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 27 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 71 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 50 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 28 | ||
| Index tests | Index test: urea breath test ‐ unknown isotope | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Urea breath test ‐ unknown isotope | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 56 for urea breath test, 100 for stool antigen test | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 240 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 182 | ||
| Index tests | Index test 1: urea breath test‐13C
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 125 | ||
| Index tests | Index test 1: urea breath test‐14C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 95 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 114 | ||
| Index tests | Index test 1a: serology (IgA) Index test 1b: serology (IgG) | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 179 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 104 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2a: serology Index test 2b: serology Index test 3: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 104 | ||
| Index tests | Index test 1: urea breath test‐14C First criterion: ≥ 1.6% excretion (time not stated) Second criterion: not stated | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 38 | ||
| Index tests | Index test 1: urea breath test‐13C
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 76 | ||
| Index tests | Index test 1: urea breath test‐14C
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 47 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 73 | ||
| Index tests | Index test 1a: urea breath test‐14C Index test 1b: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 145 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 200 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 72 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 96 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 94 | ||
| Index tests | Index test: urea breath test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 73 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 107 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology Index test 3: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 68 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 250 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 160 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 61 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 135 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 66 | ||
| Index tests | Index test: urea breath test‐13C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 651 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 100 | ||
| Index tests | Index test: serology Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 63 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 26 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 30 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 105 | ||
| Index tests | Index test 1a: stool antigen test Index test 1b: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 95 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 105 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 123 | ||
| Index tests | Index test: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 76 | ||
| Index tests | Index test: urea breath test ‐ C13 | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 95 | ||
| Index tests | Index test 1a: serology Index test 1b: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 31 | ||
| Index tests | Index test: stool antigen test | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 72 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: serology | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serology | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study | ||
| Patient characteristics and setting | Sample size: 88 | ||
| Index tests | Index test: urea breath test‐14C | ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Urea breath test‐14C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study | ||
| Patient characteristics and setting | Sample size: 32 | ||
| Index tests | Index test 1: urea breath test‐13C Index test 2: stool antigen test
| ||
| Target condition and reference standard(s) | Target condition: H pylori infection | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urea breath test‐13C | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Stool Antigen Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
CO2 ‐ carbon dioxide;
H & E stain ‐ haematoxylin and eosin stain;
HpSA ‐ H pylori stool antigen;
IgA ‐ immunoglobulin A;
IgG ‐ immunoglobulin G;
NMR ‐ nuclear magnetic resonance;
NSAID ‐ non‐steroidal anti‐inflammatory
WBC ‐ white blood cell
All other acronyms and abbreviations are the full title of either products or companies producing products included in the study.
Characteristics of studies awaiting classification [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test‐13C | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | salivary test, potential index tests of interest | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | serology | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test‐13C | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | serology | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | unclear | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | salivary test, potential index tests of interest | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test‐13C | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | serology | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | serology | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | |||
| Index tests | urea breath test‐13C | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Urea breath test‐13C Show forest plot | 34 | 3139 |
| Test 1 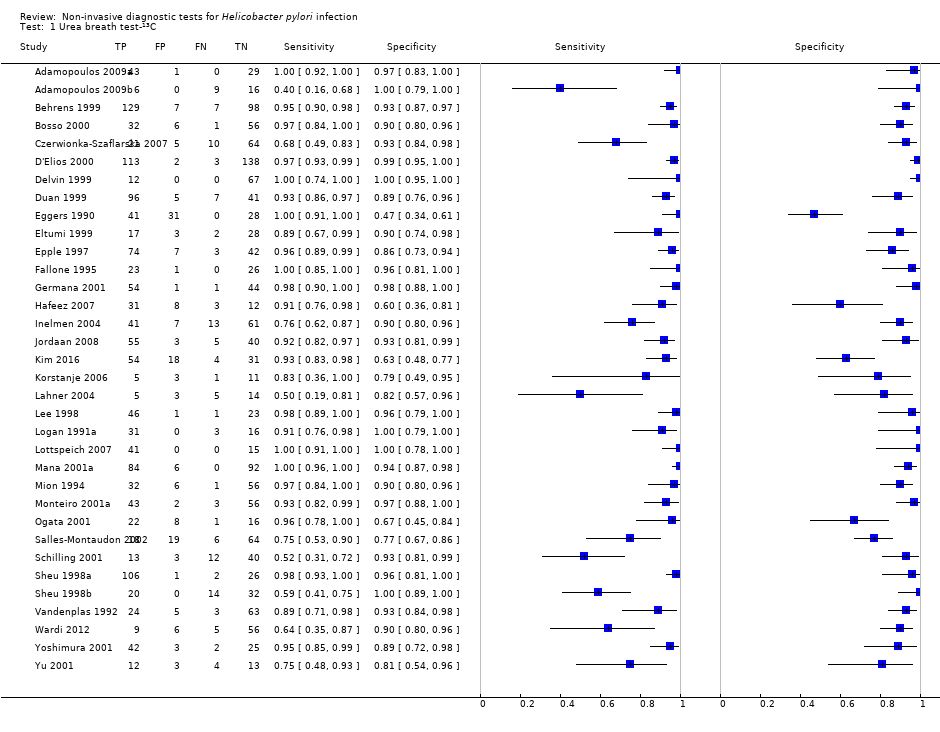 Urea breath test‐13C. | ||
| 2 Urea breath test‐14C Show forest plot | 21 | 1810 |
| Test 2  Urea breath test‐14C. | ||
| 3 Urea breath test ‐ Unknown isotope Show forest plot | 2 | 127 |
| Test 3  Urea breath test ‐ Unknown isotope. | ||
| 4 Serology Show forest plot | 34 | 4242 |
| Test 4 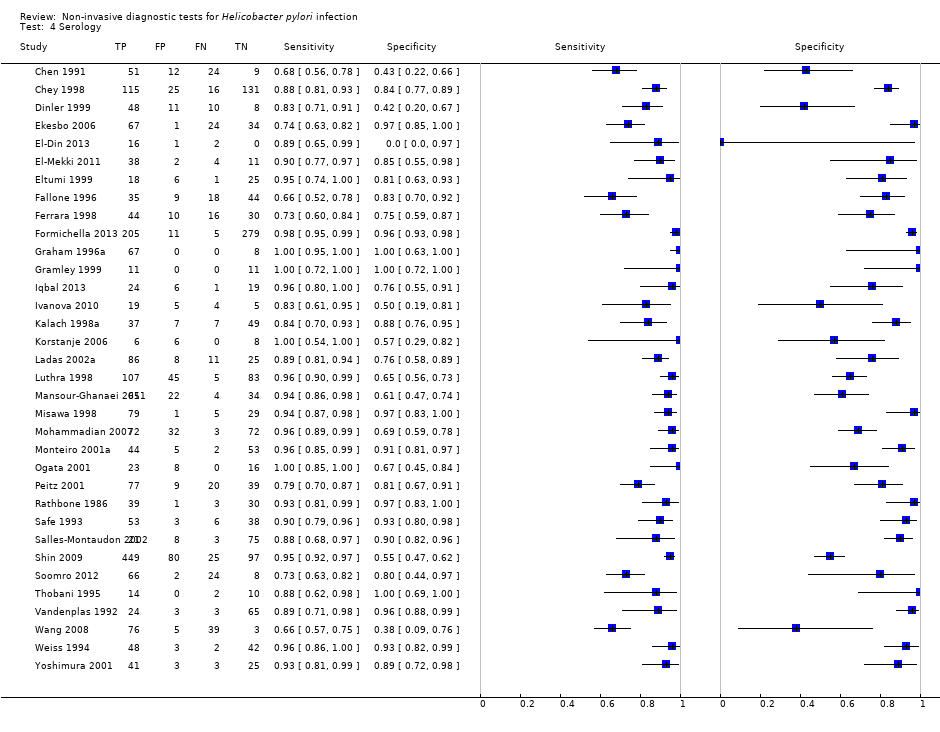 Serology. | ||
| 5 Stool antigen test Show forest plot | 29 | 2988 |
| Test 5  Stool antigen test. | ||
| 6 Urea breath test‐13C (delta over baseline > 3% (20 minutes)) Show forest plot | 2 | 254 |
| Test 6  Urea breath test‐13C (delta over baseline > 3% (20 minutes)). | ||
| 7 Urea breath test‐13C (delta over baseline > 3% (30 minutes)) Show forest plot | 3 | 333 |
| Test 7  Urea breath test‐13C (delta over baseline > 3% (30 minutes)). | ||
| 8 Urea breath test‐13C (delta over baseline > 3.5% (30 minutes)) Show forest plot | 3 | 368 |
| Test 8  Urea breath test‐13C (delta over baseline > 3.5% (30 minutes)). | ||
| 9 Urea breath test‐13C (delta over baseline > 4% (10 minutes)) Show forest plot | 2 | 236 |
| Test 9  Urea breath test‐13C (delta over baseline > 4% (10 minutes)). | ||
| 10 Urea breath test‐13C (delta over baseline > 4% (20 minutes)) Show forest plot | 2 | 236 |
| Test 10 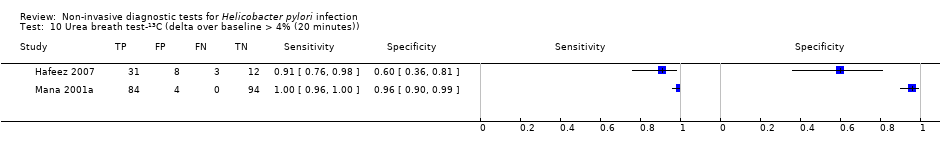 Urea breath test‐13C (delta over baseline > 4% (20 minutes)). | ||
| 11 Urea breath test‐13C (delta over baseline > 4% (30 minutes)) Show forest plot | 10 | 958 |
| Test 11  Urea breath test‐13C (delta over baseline > 4% (30 minutes)). | ||
| 12 Urea breath test‐13C (delta over baseline > 4.5% (30 minutes)) Show forest plot | 3 | 288 |
| Test 12  Urea breath test‐13C (delta over baseline > 4.5% (30 minutes)). | ||
| 13 Urea breath test‐13C (delta over baseline > 5% (30 minutes)) Show forest plot | 4 | 601 |
| Test 13  Urea breath test‐13C (delta over baseline > 5% (30 minutes)). | ||
| 14 Urea breath test‐14C (counts per minute > 50) Show forest plot | 6 | 471 |
| Test 14  Urea breath test‐14C (counts per minute > 50). | ||
| 15 Urea breath test‐14C (disintegrations per minute > 200) Show forest plot | 4 | 296 |
| Test 15 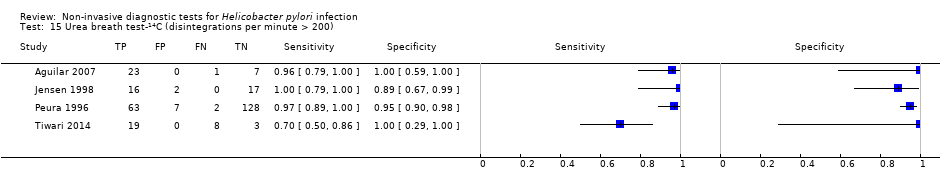 Urea breath test‐14C (disintegrations per minute > 200). | ||
| 16 Serology > 7 units/ml Show forest plot | 2 | 97 |
| Test 16 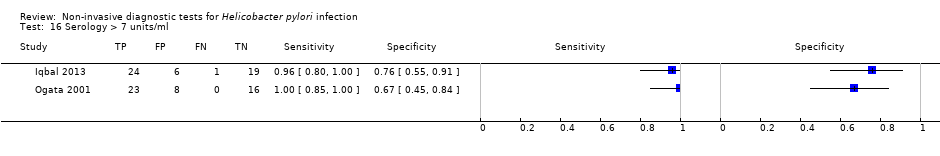 Serology > 7 units/ml. | ||
| 17 Serology ≥300 units Show forest plot | 2 | 234 |
| Test 17  Serology ≥300 units. | ||

Clinical pathway
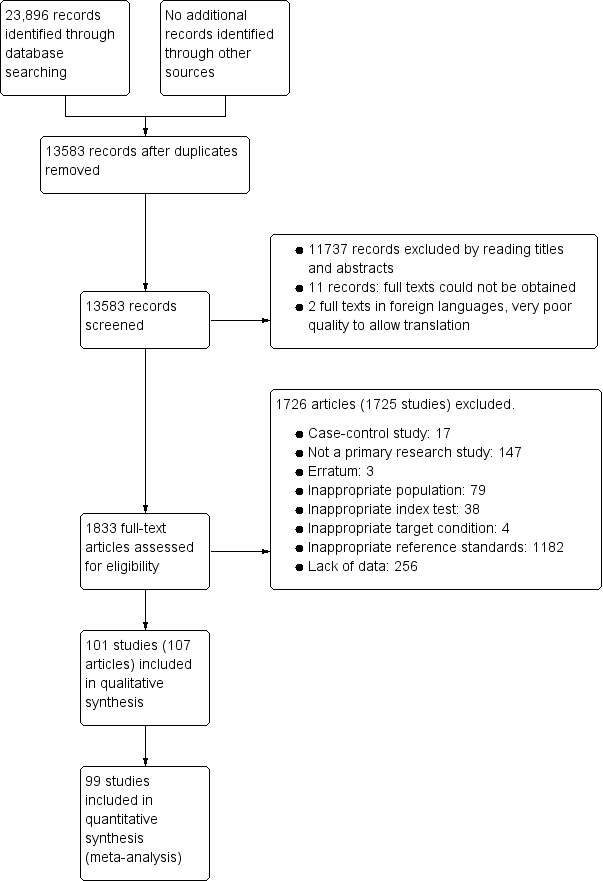
Study flow diagram.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies. For each domain, the numbers shown on the bar represent the number of studies that were scored as high, unclear or low in terms of risk of bias or applicability concern.
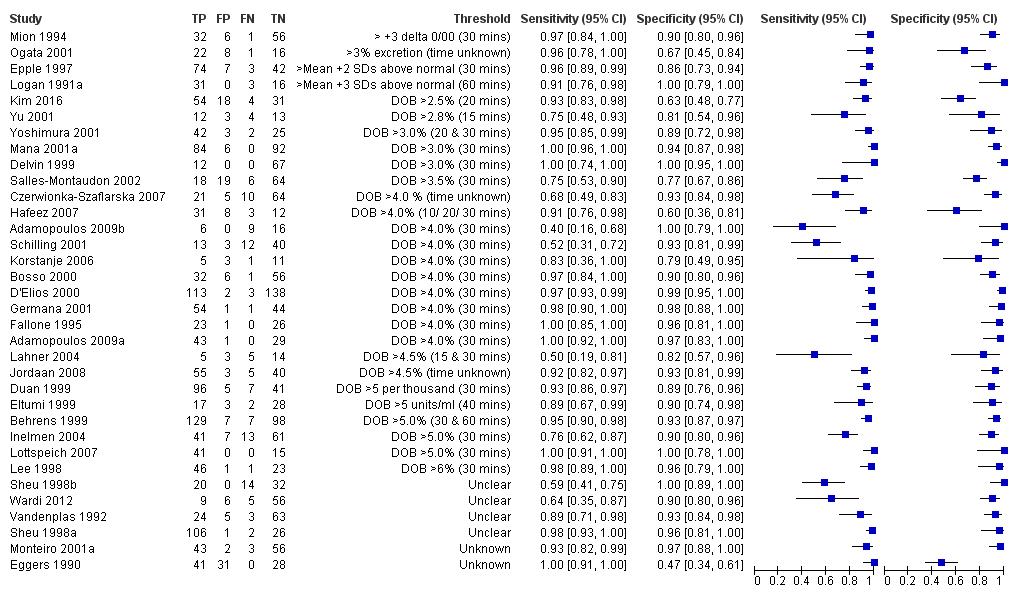
Forest plot of urea breath test‐13C.FN = false negative; FP = false positive; TN = true negative; TP = true positive. The forest plot shows an estimate of sensitivity and specificity from each study and the threshold used. Studies are sorted by threshold, sensitivity and specificity. For threshold, the number of minutes in brackets is the time after administration of urea.

Forest plot of urea breath test‐13C at commonly reported thresholds. FN = false negative; FP = false positive; TN = true negative; TP = true positive. Thresholds are shown in brackets and the number of minutes in brackets is the time after administration of urea.

Forest plot of urea breath test‐14C. FN = false negative; FP = false positive; TN = true negative; TP = true positive. The forest plot shows an estimate of sensitivity and specificity from each study and the threshold used. Studies are sorted by threshold, sensitivity and specificity. For threshold, the number of minutes in brackets is the time after administration of urea.

Forest plot of serology. FN = false negative; FP = false positive; SD = standard deviation; TN = true negative; TP = true positive. The forest plot shows an estimate of sensitivity and specificity from each study and the threshold used. Studies are sorted by threshold, sensitivity and specificity. Other threshold is staining of a 120kDa protein (CagA) gel band and/or at least two of five proteins between 28–33 kDa.
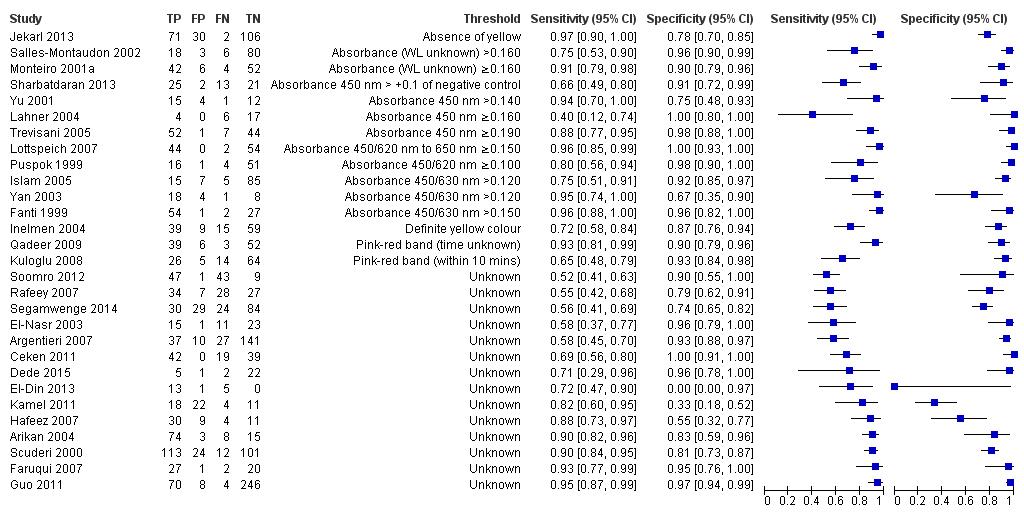
Forest plot of stool antigen test. FN = false negative; FP = false positive; TN = true negative; TP = true positive; WL = wavelength. The forest plot shows an estimate of sensitivity and specificity from each study and the threshold used. Studies are sorted by threshold, sensitivity and specificity.
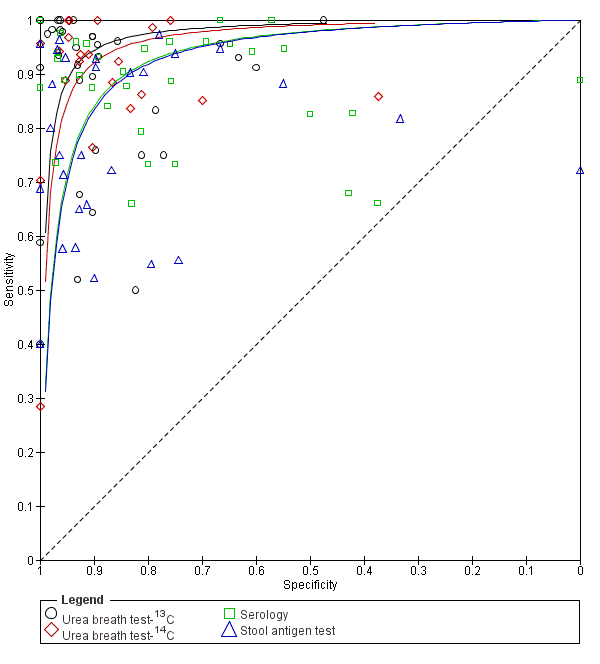
Summary ROC plot of non‐invasive tests for H pylori infection. The SROC curves for the four tests are parallel. The curve for each test is drawn within the range of estimates of specificity from the studies included for the test.

Summary ROC plot of direct comparisons of urea breath test‐13C and serology. Each summary curve was drawn restricted to the range of specificities for each test. The size of each symbol was scaled according to the precision of sensitivity and specificity in the study. A dotted line joins the pair of points for the two tests from each study.

Summary ROC plot of direct comparisons of urea breath test‐13C and stool antigen test. Each summary curve was drawn restricted to the range of specificities for each test. The size of each symbol was scaled according to the precision of sensitivity and specificity in the study. A dotted line joins the pair of points for the two tests from each study.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included for urea breath test‐13C

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included for urea breath test‐14C.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included for serology
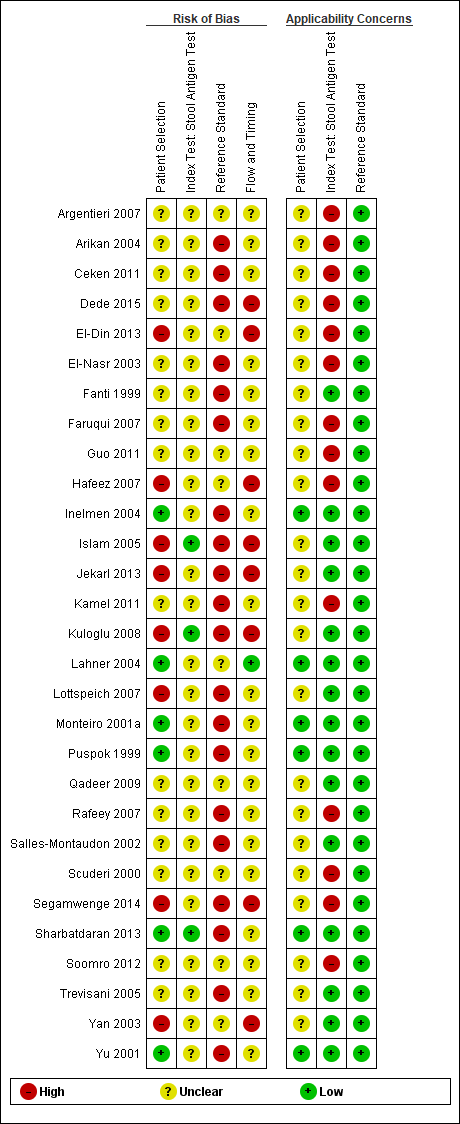
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included for the stool antigen test

Urea breath test‐13C.

Urea breath test‐14C.

Urea breath test ‐ Unknown isotope.
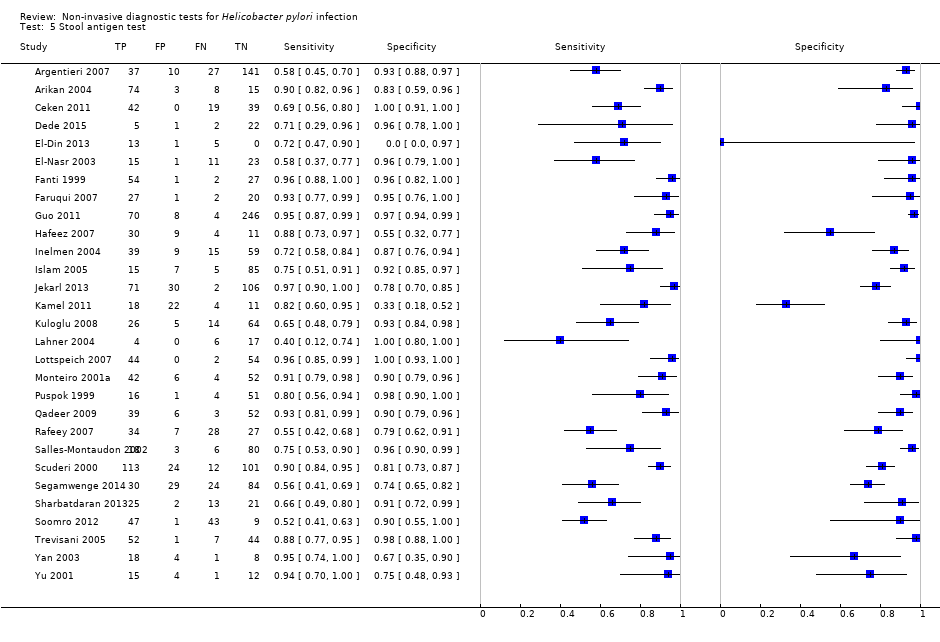
Stool antigen test.

Urea breath test‐13C (delta over baseline > 3% (20 minutes)).

Urea breath test‐13C (delta over baseline > 3% (30 minutes)).

Urea breath test‐13C (delta over baseline > 3.5% (30 minutes)).

Urea breath test‐13C (delta over baseline > 4% (10 minutes)).

Urea breath test‐13C (delta over baseline > 4% (20 minutes)).

Urea breath test‐13C (delta over baseline > 4% (30 minutes)).
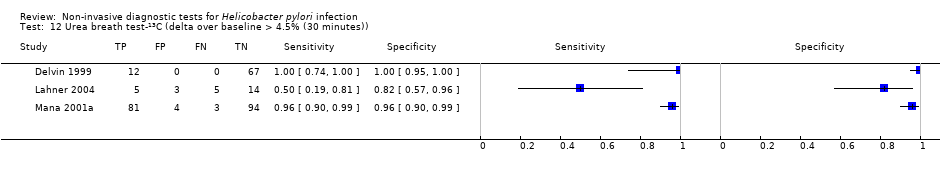
Urea breath test‐13C (delta over baseline > 4.5% (30 minutes)).
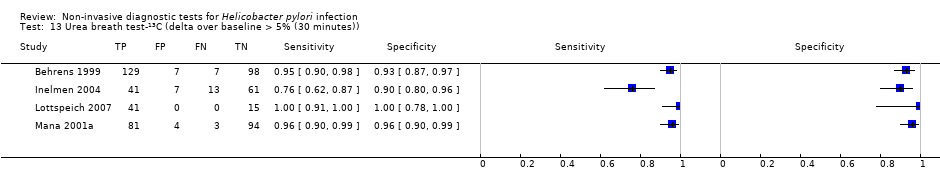
Urea breath test‐13C (delta over baseline > 5% (30 minutes)).

Urea breath test‐14C (counts per minute > 50).

Urea breath test‐14C (disintegrations per minute > 200).

Serology > 7 units/ml.
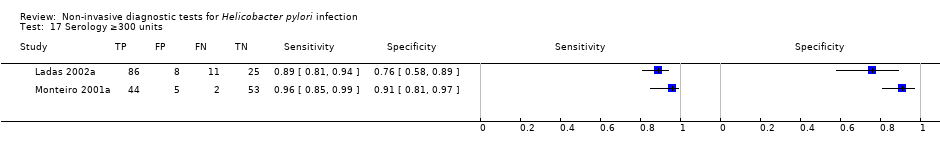
Serology ≥300 units.
| What is the best non‐invasive test for diagnosis of H pylori infection? | ||||
| Population | Children and adults with gastrointestinal symptoms | |||
| Setting | Primary care setting | |||
| Index tests | Urea breath test‐13C, Urea breath test‐14C, serology, and stool antigen test | |||
| Threshold | Various thresholds were used for each test | |||
| Role and purpose of test | Screening and diagnosis of H pylori | |||
| Reference standard | Endoscopic biopsy with Haemotoxylin & Eosin stain, special stains, or combination of Haemotoxylin & Eosin and special stains | |||
| Quality of evidence | Risk of bias was generally high or unclear with respect to the selection of participants, and the conduct and interpretation of the index tests and reference standard. Applicability concerns were also generally high or unclear with respect to selection of participants | |||
| Limitations | There was heterogeneity in thresholds and reference standards. Studies did not often prespecify or clearly report thresholds used | |||
| Pre‐test probability (prevalence of Helicobacter pylori) | Median (interquartile range) = 53.7% (42.0% to 66.5%) | |||
| Index test | Number of participants (studies) | Diagnostic odds ratio (95% CI) | Sensitivity (95% CI) at fixed specificity of 0.901 | Missed H pylori cases per 1000 people tested (95% CI)2 |
| Urea breath test‐13C | 3139 participants (34 studies) | 153 (95% CI 73.7 to 316) | 0.94 (0.89 to 0.97) | 30 (15 to 58) |
| Urea breath test‐14C | 1810 participants (21 studies) | 105 (95% CI 74.0 to 150) | 0.92 (0.89 to 0.94) | 42 (30 to 58) |
| Serology | 4242 participants (34 studies) | 47.4 (95% CI 25.5 to 88.1) | 0.84 (0.74 to 0.91) | 86 (50 to 140) |
| Stool antigen test | 2988 participants (29 studies) | 45.1 (95% CI 24.2 to 84.1) | 0.83 (0.73 to 0.90) | 89 (52 to 146) |
| Comparison of non‐invasive tests for H pylori infection Based on an indirect comparison of the four tests using all the studies, there was statistical evidence of a difference in diagnostic accuracy (P = 0.024). Direct comparisons were based on few head‐to‐head studies. The ratios of diagnostic odds ratios (95% CI; P value) were 0.68 (95% CI 0.12 to 3.70; P = 0.56) for urea breath test‐13C versus serology (seven studies), and 0.88 (95% CI 0.14 to 5.56; P = 0.84) for urea breath test‐13C versus stool antigen test (seven studies). The 95% confidence intervals of these estimates overlap with those of the ratios of diagnostic odds ratios from the indirect comparison. Data were limited or unavailable for meta‐analysis of other direct comparisons. | ||||
| Conclusions In people with no history of gastrectomy and those who have not recently had antibiotics or proton pump inhibitors, urea breath tests had high diagnostic accuracy while serology and stool antigen tests had lower accuracy to detect H pylori infection. Although susceptible to bias due to confounding, this conclusion is based on evidence from indirect test comparisons as evidence from direct comparisons was based on few studies or was unavailable. It should be noted that studies were generally of poor methodological quality. The thresholds used for the tests were highly variable and there is currently insufficient evidence to recommend specific thresholds for use in clinical practice. | ||||
| 1The sensitivities were estimated along the SROC curves at the median specificity across the studies included for the four tests. 2Based on the sensitivity estimated at the median specificity of 0.90, and the median prevalence of 53.7% from the included studies, the numbers of missed H pylori cases were calculated using a hypothetical cohort of 1000 people suspected of having H pylori infection. The 95% CI for the number of missed cases is from the 95% CI for sensitivity. For a specificity of 0.90 and prevalence of 53.7%, there will be 46 false positives. See Table 3 for results for other values of specificity and prevalence. | ||||
| Threshold | Studies | Number of participants (cases) | Sensitivity (95% CI) | Specificity (95% CI) |
| Urea breath test‐13C | ||||
| Delta over baseline > 3% (20 minutes) | 2 | 254 (128) | 0.98 (0.90 to 1.00) | 0.92 (0.82 to 0.97) |
| Delta over baseline > 3% (30 minutes) | 3 | 333 (140) | 0.99 (0.92 to 1.00) | 0.95 (0.90 to 0.98) |
| Delta over baseline > 3.5% (30 minutes) | 3 | 368 (120) | 0.75 to 1.00 | 0.77 to 1.00 |
| Delta over baseline > 4% (10 minutes) | 2 | 236 (118) | 0.91 to 1.00 | 0.60 to 0.95 |
| Delta over baseline > 4% (20 minutes) | 2 | 236 (118) | 0.91 to 1.00 | 0.60 to 0.96 |
| Delta over baseline > 4% (30 minutes) | 10 | 958 (423) | 0.95 (0.79 to 0.99) | 0.95 (0.87 to 0.98) |
| Delta over baseline > 4.5% (30 minutes) | 3 | 288 (106) | 0.50 to 0.96 | 0.82 to 0.96 |
| Delta over baseline > 5% (30 minutes) | 4 | 601 (315) | 0.95 (0.49 to 1.00) | 0.94 (0.84 to 0.98) |
| Urea breath test‐14C | ||||
| Counts per minute > 50 (10 minutes) | 6 | 471 (231) | 0.89 (0.55 to 0.98) | 0.91 (0.79 to 0.96) |
| Disintegrations per minute > 200 (10 minutes) | 4 | 296 (132) | 0.95 (0.33 to 1.00) | 0.95 (0.80 to 0.99) |
| Serology | ||||
| > 7 units/ml | 2 | 97 (48) | 0.98 (0.74 to 1.00) | 0.71 (0.51 to 0.86) |
| ≥ 300 unit | 2 | 234 (143) | 0.91 (0.82 to 0.96) | 0.86 (0.72 to 0.93) |
| Tests evaluated at the same threshold by more than one study are presented in the table. When there were two or three studies at the same threshold, and little or no heterogeneity was observed in ROC space, estimates of summary sensitivity and summary specificity were obtained by using univariate fixed‐effect logistic regression models to pool sensitivities and specificities separately. When there were two or three studies and we observed heterogeneity, we did not perform meta‐analysis but report the range of the sensitivities and specificities. | ||||
| Index tests | Studies; participants (H pyloripresent) | DOR (95% CI) | Ratio of diagnostic odds ratios (95% CI), P value | ||
| Urea breath test‐13C | Urea breath test‐14C | Serology | |||
| Urea breath test‐13C | 34; 3139 (1526) | 153 (73.7 to 316) | ‐ | ‐ | ‐ |
| Urea breath test‐14C | 21; 1810 (1018) | 105 (74.0 to 150) | 1.45 (0.65 to 3.26), P = 0.36 | ‐ | ‐ |
| Serology | 34; 4242 (2477) | 47.4 (25.5 to 88.1) | 3.22 (1.24 to 8.37), P = 0.017 | 2.22 (1.09 to 4.51), P = 0.028 | ‐ |
| Stool antigen test | 29; 2988 (1311) | 45.1 (24.2 to 84.1) | 3.39 (1.30 to 8.83), P = 0.013 | 2.33 (1.14 to 4.76), P = 0.020 | 1.05 (0.44 to 2.53), P = 0.91 |
| The indirect comparison included all studies that evaluated at least one of the four tests, i.e. all available data. The ratio of diagnostic odds ratios is the diagnostic odds ratio (DOR) of the test in the column divided by the DOR of the test in the row. If the ratio is greater than one, then the test in the column is more accurate than the test in the row; if the ratio is less than one, the test in the row is more accurate than the test in the column. | |||||
| Prevalence (%) | Specificity | False positives1 | Test | Sensitivity (95% CI) | Missed cases (95% CI) |
| 42.0 | 0.79 | 122 | Urea breath test‐13C | 0.98 (0.95 to 0.99) | 10 (5 to 20) |
| Urea breath test‐14C | 0.97 (0.95 to 0.98) | 15 (10 to 20) | |||
| Serology | 0.93 (0.87 to 0.96) | 31 (17 to 54) | |||
| Stool antigen test | 0.92 (0.87 to 0.96) | 32 (18 to 57) | |||
| 53.7 | 0.79 | 97 | Urea breath test‐13C | 0.98 (0.95 to 0.99) | 13 (6 to 26) |
| Urea breath test‐14C | 0.97 (0.95 to 0.98) | 19 (13 to 26) | |||
| Serology | 0.93 (0.87 to 0.96) | 39 (22 to 69) | |||
| Stool antigen test | 0.92 (0.87 to 0.96) | 41 (23 to 72) | |||
| 66.5 | 0.79 | 70 | Urea breath test‐13C | 0.98 (0.95 to 0.99) | 16 (8 to 32) |
| Urea breath test‐14C | 0.97 (0.95 to 0.98) | 23 (16 to 32) | |||
| Serology | 0.93 (0.87 to 0.96) | 49 (27 to 85) | |||
| Stool antigen test | 0.92 (0.87 to 0.96) | 51 (28 to 89) | |||
| 42.0 | 0.90 | 58 | Urea breath test‐13C | 0.94 (0.89 to 0.97) | 23 (12 to 46) |
| Urea breath test‐14C | 0.92 (0.89 to 0.94) | 33 (24 to 46) | |||
| Serology | 0.84 (0.74 to 0.91) | 67 (39 to 110) | |||
| Stool antigen test | 0.83 (0.73 to 0.90) | 70 (41 to 114) | |||
| 53.7 | 0.90 | 46 | Urea breath test‐13C | 0.94 (0.89 to 0.97) | 30 (15 to 58) |
| Urea breath test‐14C | 0.92 (0.89 to 0.94) | 42 (30 to 58) | |||
| Serology | 0.84 (0.74 to 0.91) | 86 (50 to 140) | |||
| Stool antigen test | 0.83 (0.73 to 0.90) | 89 (52 to 146) | |||
| 66.5 | 0.90 | 34 | Urea breath test‐13C | 0.94 (0.89 to 0.97) | 37 (18 to 72) |
| Urea breath test‐14C | 0.92 (0.89 to 0.94) | 53 (38 to 72) | |||
| Serology | 0.84 (0.74 to 0.91) | 106 (62 to 173) | |||
| Stool antigen test | 0.83 (0.73 to 0.90) | 111 (64 to 180) | |||
| 42.0 | 0.96 | 23 | Urea breath test‐13C | 0.86 (0.75 to 0.93) | 57 (30 to 103) |
| Urea breath test‐14C | 0.81 (0.76 to 0.86) | 78 (58 to 103) | |||
| Serology | 0.66 (0.52 to 0.79) | 141 (90 to 204) | |||
| Stool antigen test | 0.65 (0.50 to 0.78) | 146 (93 to 209) | |||
| 53.7 | 0.96 | 19 | Urea breath test‐13C | 0.86 (0.75 to 0.93) | 73 (38 to 132) |
| Urea breath test‐14C | 0.81 (0.76 to 0.86) | 100 (74 to 132) | |||
| Serology | 0.66 (0.52 to 0.79) | 181 (115 to 260) | |||
| Stool antigen test | 0.65 (0.50 to 0.78) | 187 (119 to 267) | |||
| 66.5 | 0.96 | 13 | Urea breath test‐13C | 0.86 (0.75 to 0.93) | 90 (47 to 163) |
| Urea breath test‐14C | 0.81 (0.76 to 0.86) | 124 (92 to 163) | |||
| Serology | 0.66 (0.52 to 0.79) | 224 (142 to 322) | |||
| Stool antigen test | 0.65 (0.50 to 0.78) | 231 (148 to 331) | |||
| 1Average number of participants who are diagnosed with H pylori infection but do not have the infection per 1000 tested. The sensitivities were estimated from the SROC curves at fixed values (lower quartile, median and upper quartile) of specificity from the included studies across all tests. Based on these sensitivities and specificities, and quartiles of prevalence from the included studies (across all tests), the numbers of missed H pylori cases and false positives (i.e. overdiagnosed people) were calculated using a hypothetical cohort of 1000 people suspected of having H pylori infection. | |||||
| Test | Urea breath test‐13C | Urea breath test‐14C | Serology |
| Urea breath test‐13C | ‐ | ‐ | ‐ |
| Urea breath test‐14C | N = 0 | ‐ | ‐ |
| Serology | N = 7 DOR (95% CI) of urea breath test‐13C = 74.8 (95% CI 17.8 to 314) DOR (95% CI) of serology = 111 (95% CI 41.2 to 297) RDORs (95% CI) of urea breath test‐13C versus serology, P value = 0.68 (95% CI 0.12 to 3.70), P = 0.56 | N = 1 | ‐ |
| Stool antigen test | N = 7 DOR (95% CI) of stool antigen test = 53.0 (95% CI 5.34 to 527) RDORs (95% CI) of urea breath test‐13C versus stool antigen test, P value = 0.88 (95% CI 0.14 to 5.56), P = 0.84 | N = 2 | N = 4 |
| DOR = diagnostic odds ratio; N = number of studies; RDORs = ratio of diagnostic odds ratios. Due to paucity of data and substantial heterogeneity observed in ROC space which precluded the use of simpler meta‐analytic models, meta‐analyses were not possible for two test comparisons that had more than one study. For the single study of urea breath test‐14C versus serology (Mansour‐Ghanaei 2011), both tests had similar sensitivity, but specificity was higher for urea breath test‐14C than for serology. The ratio of diagnostic odds ratios is the DOR of the test in the column divided by the DOR of the test in the row. If the ratio is greater than one, then the test in the column is more accurate than the test in the row; if the ratio is less than one, the test in the row is more accurate than the test in the column. | |||
| Test | No. of studies | No. of participants |
| 1 Urea breath test‐13C Show forest plot | 34 | 3139 |
| 2 Urea breath test‐14C Show forest plot | 21 | 1810 |
| 3 Urea breath test ‐ Unknown isotope Show forest plot | 2 | 127 |
| 4 Serology Show forest plot | 34 | 4242 |
| 5 Stool antigen test Show forest plot | 29 | 2988 |
| 6 Urea breath test‐13C (delta over baseline > 3% (20 minutes)) Show forest plot | 2 | 254 |
| 7 Urea breath test‐13C (delta over baseline > 3% (30 minutes)) Show forest plot | 3 | 333 |
| 8 Urea breath test‐13C (delta over baseline > 3.5% (30 minutes)) Show forest plot | 3 | 368 |
| 9 Urea breath test‐13C (delta over baseline > 4% (10 minutes)) Show forest plot | 2 | 236 |
| 10 Urea breath test‐13C (delta over baseline > 4% (20 minutes)) Show forest plot | 2 | 236 |
| 11 Urea breath test‐13C (delta over baseline > 4% (30 minutes)) Show forest plot | 10 | 958 |
| 12 Urea breath test‐13C (delta over baseline > 4.5% (30 minutes)) Show forest plot | 3 | 288 |
| 13 Urea breath test‐13C (delta over baseline > 5% (30 minutes)) Show forest plot | 4 | 601 |
| 14 Urea breath test‐14C (counts per minute > 50) Show forest plot | 6 | 471 |
| 15 Urea breath test‐14C (disintegrations per minute > 200) Show forest plot | 4 | 296 |
| 16 Serology > 7 units/ml Show forest plot | 2 | 97 |
| 17 Serology ≥300 units Show forest plot | 2 | 234 |


