安定期の慢性閉塞性肺疾患(COPD)に対する長時間作用型抗コリン薬(LAMA)+長時間作用型β刺激薬(LABA)対LABA+吸入ステロイド(ICS)
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: randomised, double‐blind, cross‐over, double‐dummy, placebo‐controlled 4‐period 4‐arm trial. Countries: 8 countries (mainly EU countries). Site: 29 centres. Study duration: 6 weeks × 4 time periods. Study start: October 2013. Run‐in period: unclear. | |
| Participants | Key inclusion criteria: %pred FEV1 30% to 80%, without recent exacerbation. Numbers of randomised and consequent‐treatment completed cases: 229 and 202. Age: 63.6 (SD 7.6) years. Male/female: 148/81. %pred FEV1: unclear | |
| Interventions | LAMA/LABA: tiotropium/olodaterol (2.5/5 μg) or tiotropium/olodaterol (5/5 μg). LABA/ICS: salmeterol/fluticasone (50/250 μg) twice daily or salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: change from baseline FEV1 AUC (0‐12 h) after 6 weeks of treatment. Tiotropium/olodaterol (2.5/5 μg): 0.295 (SE 0.014). Tiotropium/olodaterol (5/5 μg): 0.317 (SE 0.014). Salmeterol/fluticasone (50/250 μg): 0.192 (SE 0.015). Salmeterol/fluticasone (50/500 μg): 0.188 (SE 0.014). | |
| Notes | Registration: NCT01969721. COI: sponsored by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 202/229 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 6.9% with T+O 2.5/5 > T+O 5/5 > F+S 250/50 > F+S 500/50, 17.4% with T+O 5/5 > F+S 500/50 > T+O 2.5/5 > F+S 250/50, 10.0% with F+S 250/50 > T+O 2.5/5 > F+S 500/50 > T+O 5/5, and 11.5% with F+S 500/50 > F+S 250/50 > T+O 5/5 > T+O 2.5/5 Note: This was four‐arm crossover study. Each arm used four consecutive treatments. For example, patients in the first arm were treated by T+O2.5/5, then treated by T+O5/5, then treated by F+S 250/50, then F+S 500/50. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by Boehringer Ingelheim. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 7 countries (US and European countries). Site: 63 centres. Study duration: 12 weeks. Study start: March 2013. Run‐in period: 7 to 14 days. | |
| Participants | Key inclusion criteria: %pred FEV1 30% to 70%, mMRC ≥ 2, no recent exacerbation. Numbers of randomised and completed cases: 707 and 634. Age: 62.8 (SD 9.0) years. Male/female: 497/213. %pred FEV1: 49.4% (SD 10.9). | |
| Interventions | LAMA/LABA: umeclidinium/vilanterol (62.5/25 μg). LABA/ICS: salmeterol/fluticasone (50/250 μg) twice daily. | |
| Outcomes | Primary endpoint: change from baseline in 24‐h weighted‐mean serial FEV1 on day 84. Umeclidinium/vilanterol (62.5/25 μg): 0.165 (SE 0.0130). Salmeterol/fluticasone (50/250 μg) twice daily: 0.091 (SE 0.0131). | |
| Notes | Registration: NCT01817764, GSK‐DB2114930. COI: sponsored by GlaxoSmithKline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation schedule was generated using a validated computer system (RanAll, GSK). |
| Allocation concealment (selection bias) | Low risk | Centred randomisation prevented the foreknowledge of intervention assignments by neither researchers nor participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 634/707 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 9.6% in umeclidinium/vilanterol arm and 10.8% in salmeterol/fluticasone arms. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by GlaxoSmithKline. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 7 countries (US and European countries and Russia). Site: 71 centres. Study duration: 12 weeks. Study start: June 2013. Run‐in period: 7 to 14 days. | |
| Participants | Key inclusion criteria: %pred FEV1 30% to 70%, mMRC ≥ 2, no recent exacerbation. Numbers of randomised and completed cases: 700 and 638. Age: 63.6 (SD 8.9) years. Male/female: 528/169. %pred FEV1: 49.5% (SD 10.9). | |
| Interventions | LAMA/LABA: umeclidinium/vilanterol (62.5/25 μg). LABA/ICS: salmeterol/fluticasone (50/250 μg) twice daily. | |
| Outcomes | Primary endpoint: Change from baseline in 24‐h weighted‐mean serial FEV1 on treatment day 84. Umeclidinium/vilanterol (62.5/25 μg): 0.213 (SE 0.0137). Salmeterol/fluticasone (50/250 μg) twice daily: 0.112 (SE 0.0139). | |
| Notes | Registration: NCT01879410, GSK‐DB2114951. COI: sponsored by GlaxoSmithKline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation schedule was generated using a validated computer system (RanAll, GSK). |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation prevented the foreknowledge of intervention assignments by neither researchers nor participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 638/700 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 6.9% in umeclidinium/vilanterol arm and 10.9% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by GlaxoSmithKline. |
| Methods | Design: randomised, parallel‐group, open‐label trial. Countries: 1 country (Japan). Site: 1 centre. Study duration: 16 weeks. Study start: unclear. Run‐in period: 21 days. | |
| Participants | Key inclusion criteria: %pred FEV1 30% to 80%, without recent exacerbation. Numbers of randomised and completed cases: 46 and 43. Age: LAMA/LABA, 72 years (SD 7); LABA/ICS, 69 years (SD 6). Male/female: 36/7. %pred FEV1: LAMA/LABA, 61.9% (SD 16.3%); LABA/ICS, 60.8% (SD 16.4%). | |
| Interventions | LAMA/LABA: tiotropium/indacaterol (18/150 μg). LABA/ICS: salmeterol/fluticasone (50/250 μg) twice daily. | |
| Outcomes | Primary outcomes: Effects on airway dimensions. | |
| Notes | Registration: none. COI: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Performance bias was suspected due to open‐label study design. |
| Blinding of outcome assessment (detection bias) | High risk | Detection bias was suspected due to open‐label study design. |
| Incomplete outcome data (attrition bias) | Low risk | 43/46 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 8.3% in tiotropium/indacaterol arm and 4.5% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | High risk | Reporting bias could not be denied because this trial was not registered. |
| Other bias | Low risk | Authors found no risk of other bias. |
| Methods | Design: randomised, double‐blind, cross‐over, double‐dummy, placebo‐controlled trial. Countries: 7 countries. Site: 40 centres. Study duration: 8 weeks twice daily. Study start: September 2007. Run‐in period: 15 days. | |
| Participants | Key inclusion criteria: %pred FEV1 ≤ 65%, without recent exacerbation. Numbers of randomised and completed cases: 344 and 300. Age: 61.0 years (SD 7.6). Male/female: 247/97. %pred FEV1: 47% (SD 12%). | |
| Interventions | LAMA/LABA: tiotropium/salmeterol (18/50 μg) twice daily. LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Co‐primary endpoints 1: post‐dose thoracic gas volume (functional residual capacity) (after 8 weeks). Mean difference ‐0.087 (SE 0.044). Co‐primary endpoints 2: endurance time (after 8 weeks). Mean difference 3.0 (95% CI ‐9.5 to 27.5). | |
| Notes | Registration: NCT00530842. COI: sponsored by Boehringer Ingelheim and Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisations of 4 blocks stratified according to sites. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisations of 4 blocks stratified according to sites. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 300/344 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 13.4% in tiotropium/salmeterol > salmeterol/fluticasone arm and 12.2% in salmeterol/fluticasone > tiotropium/salmeterol arms. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. Authors found no risk of reporting bias. |
| Other bias | High risk | COI: sponsored by Boehringer Ingelheim and Pfizer. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 8 countries, mainly EU. Site: multi‐centre. Study duration: 6 weeks. Study start: November 2003. Run‐in period: 2 to 4 weeks. | |
| Participants | Key inclusion criteria: %pred FEV1 ≤ 65% without recent exacerbation. Numbers of randomised and completed cases: 605 and unclear. Age: 62 years (SD 9). Male/female: 414/191. %pred FEV1: 55% (SD 13%). | |
| Interventions | LAMA/LABA: tiotropium/formoterol (18 μg/24 μg) twice daily. LABA/ICS: salmeterol/fluticasone propionate (50 μg/500 μg) twice daily. | |
| Outcomes | Co‐primary endpoints 1: FEV1 AUC (0 to 12 h) after 6 weeks of treatment. Mean difference 78 mL (95% CI 34 to 122) higher in tiotropium/formoterol arm. Co‐primary endpoints 2: peak FEV1 measured after 6 weeks of treatment. Mean difference 103 mL (95% CI 55 to 150) higher in tiotropium/formoterol arm. | |
| Notes | Registration: NCT00239421. COI: sponsored by Boehringer and Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Peak FEV1 measured after 6 weeks of treatment. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 592/605 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 2.3% in tiotropium/formoterol arms and 2.0% in salmeterol/fluticasone propionate arms. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. Authors found no risk of reporting bias. |
| Other bias | High risk | COI: sponsored by Boehringer and Pfizer. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 8 countries (mainly EU). Site: 79 centres. Study duration: 12 weeks. Study start: April 2013. Run‐in period: 7 to 14 days. | |
| Participants | Key inclusion criteria: %pred FEV1 30% to 70%, mMRC ≥ 2, without recent exacerbation. Numbers of randomised and completed cases: 717 and 674. Age: 61.6 years (SD 8.0). Male/female: 515/201. %pred FEV1: 50.6% (SD 10.7%). | |
| Interventions | LAMA/LABA: umeclidinium/vilanterol (62.5/25 μg). LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: change from baseline in 0 to 24 h weighted mean serial FEV1 at day 84. Mean difference 0.080 L (95% CI 0.046 to 0.113) (P < 0.001). | |
| Notes | Registration: NCT01822899, GSK‐DB2116134. COI: sponsored by GlaxoSmithKline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Validated computer system (RandAll) was used to generate central randomisation. |
| Allocation concealment (selection bias) | Low risk | Validated computer system (RandAll) was used to generate central randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 674/717 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 6.7% in umeclidinium/vilanterol arm and 5.0% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. Authors found no risk of reporting bias. |
| Other bias | High risk | COI: sponsored by GlaxoSmithKline. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 10 countries (mainly EU). Site: 92 centres. Study duration: 26 weeks. Study start: March 2011. Run‐in period: 14 days. | |
| Participants | Key inclusion criteria: stage II/III, without recent exacerbation. Numbers of randomised and completed cases: 523 and 432. Age: LAMA/LABA, 63.2 years (SD 8.2); LABA/ICS, 63.4 years (SD 7.7). Male/female: LAMA/LABA, 181/77; LABA/ICS, 189/75. %pred FEV1: LAMA/LABA, 60.5% (SD 10.5%); LABA/ICS, 60.0% (SD 10.7%). | |
| Interventions | LAMA/LABA: indacaterol/glycopyrronium (110/50 μg). LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: FEV1 AUC (0 to 12 h). LAMA/LABA: 1.69 (SE 0.027). LABA/ICS: 1.56 (SE 0.026). | |
| Notes | Registration: NCT01315249. COI: sponsored by Novartis. Study name: ILLUMINATE. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used an automated, interactive response technology to assign randomisation numbers to participants. |
| Allocation concealment (selection bias) | Low risk | Investigators used an automated, interactive response technology to assign randomisation numbers to participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 432/523 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 17.0% in indacaterol/glycopyrronium arm and 17.0% in salmeterol/fluticasone arms. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by Novartis. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 14 countries (mainly EU). Site: 126 centres. Study duration: 24 weeks. Study start: September 2013. Run‐in period: unclear. | |
| Participants | Key inclusion criteria: %pred FEV1 < 80%, CAT ≥ 10, without recent exacerbation. Numbers of randomised and completed cases: 933 and 788. Age: 63.4 years (SD 7.8). Male/female: 607/326. | |
| Interventions | LAMA/LABA: aclidinium/formoterol (400/12 μg) twice daily. LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: peak FEV1 at week 24. LAMA/LABA: 1.655 (SE 0.011). LABA/ICS: 1.562 (SE 0.011). | |
| Notes | Registration: NCT01908140, EudraCT 2013‐000116‐14. COI: sponsored by AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 788/933 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 14.1% in aclidinium/formoterol arm and 17.0% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. Authors found no risk of reporting bias. |
| Other bias | High risk | COI: sponsored by AstraZeneca. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 43 countries. Site: 496 centres. Study duration: 52 weeks. Study start: July 2013. Run‐in period: 4 weeks. | |
| Participants | Key inclusion criteria: %pred FEV1 25% to 60%, mMRC ≥ 2, with recent exacerbation. Numbers of randomised and completed cases: 3362 and 2760. Age: 64.6 years (SD 7.8). Male/female: 2557/805. %pred FEV1: 44.1% (SD 9.5%). | |
| Interventions | LAMA/LABA: indacaterol/glycopyrronium (110/50 μg). LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: rate of COPD exacerbations per year. LAMA/LABA: 3.59 (95% CI 3.28 to 3.94). | |
| Notes | Registration: NCT01782326. COI: sponsored by Novartis. Study name: FLAME. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via Interactive Response Technology to 1 of the treatment arms. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised via Interactive Response Technology to 1 of the treatment arms. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 2760/3362 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 16.6% in indacaterol/glycopyrronium arm and 19.0% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by Novartis. |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 4 countries (recruited mainly in China). Site: 56 centres. Study duration: 26 weeks. Study start: November 2012. Run‐in period: 14 days. | |
| Participants | Key inclusion criteria: stage II/III mMRC ≥ 2, without recent exacerbation. Numbers of randomised and completed cases: 744 and 676. Age: LAMA/LABA 64.8 years (SD 7.8); LABA/ICS 65.3 years (SD 7.9). Male/female: 672/69. %pred FEV1: LAMA/LABA 51.6% (SD 12.8%), LABA/ICS 52.0% (SD 12.9%). | |
| Interventions | LAMA/LABA: indacaterol/glycopyrronium (110/50 μg). LABA/ICS: salmeterol/fluticasone (50/500 μg) twice daily. | |
| Outcomes | Primary outcome: trough FEV1 following 26 weeks of treatment to demonstrate the non‐inferiority of indacaterol/glycopyrronium to salmeterol/fluticasone. LAMA/LABA: 1.248 L (SE 0.0173). LABA/ICS: 1.176 L (SE 0.0172). | |
| Notes | Registration: NCT01709903. COI: sponsored by Novartis. Study name: LANTERN. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via Interactive Response Technology to 1 of the treatment arms. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised via Interactive Response Technology to 1 of the treatment arms. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blinded. Performance bias was not suspected. |
| Blinding of outcome assessment (detection bias) | Low risk | Study was double‐blinded. Detection bias was not suspected. |
| Incomplete outcome data (attrition bias) | Low risk | 676/744 participants completed the study. Considerable attrition bias was not suspected because attrition was < 20%. Attrition was 7.8% in indacaterol/glycopyrronium arm and 10.4% in salmeterol/fluticasone arm. |
| Selective reporting (reporting bias) | Low risk | Study was registered and the prespecified outcomes were appropriately described. |
| Other bias | High risk | COI: sponsored by Novartis. |
%pred FEV1: % predicted forced expiratory volume in one second; AUC: area under curve; CAT: chronic obstructive pulmonary disease assessment test; CI: confidence interval; COI: conflicts of interest; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; h: hour; ICS: inhaled corticosteroid; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale; SD: standard deviation; SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs LABA (salmeterol) vs ICS (fluticasone propionate).) | |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs LAMA (tiotropium).) | |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs ipratropium/albuterol. Ipratropium is not LAMA.) | |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs ipratropium/albuterol. Ipratropium is not LAMA.) | |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs ICS (fluticasone propionate).) | |
| Cost‐effectiveness analysis using previously published data. (Cost‐effectiveness of the LABA/LAMA (indacaterol/glycopyrronium.) | |
| Not comparing LAMA+LABA vs LABA+ICS. (Comparing LABA+ICS (salmeterol/fluticasone propionate) vs ipratropium/albuterol. Ipratropium is not LAMA.) |
ICS: inhaled corticosteroids; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Randomized, Double‐blind, Parallel‐Group, 24‐Week, Chronic‐Dosing, Multi‐center Study to Assess the Efficacy and Safety of PT010, PT003, and PT009 Compared with Symbicort® Turbuhaler® (Kronos). |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 2 countries (US and Canada). Site: 94 centres. Study duration: 24 weeks. Study start: July 2015. Run‐in period: unclear. |
| Participants | Key inclusion criteria: %pred FEV1 < 80%. Estimated enrolment: 1800. |
| Interventions | 4‐arm trial. Budesonide + glycopyrronium + formoterol fumarate inhalation aerosol (MDI, 320/14.4/9.6 μg, PT010). |
| Outcomes | Change from baseline in morning pre‐dose trough FEV1 after follow‐up. |
| Starting date | July 2015. |
| Contact information | Raul Lima, 862‐777‐8094. |
| Notes | Completion date: March 2017. Registration: NCT02497001. COI: Sponsored by Pearl Therapeutics, Inc. |
| Trial name or title | Assessment of Switching from Salmeterol/Fluticasone to Indacaterol/Glycopyrronium in a Symptomatic COPD Patient Cohort (FLASH). |
| Methods | Design: randomised, double‐blind, parallel‐group, double‐dummy, placebo‐controlled trial. Countries: 11 countries. Site: 55 centres. Study duration: 12 weeks. Study start: October 2015. Run‐in period: unclear. |
| Participants | %pred FEV1 30% to 80%, CAT ≥ 10, without recent exacerbation. Estimated enrolment: 492. |
| Interventions | Will investigate whether switching people with symptomatic COPD from a fixed‐dose combination of salmeterol/fluticasone 50/500 µg twice daily to a fixed‐dose combination of indacaterol/glycopyrronium 110/50 µg once daily. LAMA/LABA: switching from salmeterol/fluticasone (50/500 μg) twice daily to indacaterol/glycopyrronium (110/50 μg). LABA/ICS: continuing salmeterol/fluticasone (50/500 μg) twice daily. |
| Outcomes | Change from baseline in trough pre‐dose FEV1 in both arms after follow‐up. |
| Starting date | October 2015. |
| Contact information | Novartis Pharmaceuticals +41613241111. |
| Notes | Completion date: August 2016. Registration: NCT02516592. COI: Sponsored by Novartis. |
%pred FEV1: % predicted forced expiratory volume in one second; CAT: chronic obstructive pulmonary disease assessment test; COI: conflicts of interest; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; ICS: inhaled corticosteroids; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; MDI: metered dose inhaler.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation Show forest plot | 9 | 8922 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.96] |
| Analysis 1.1 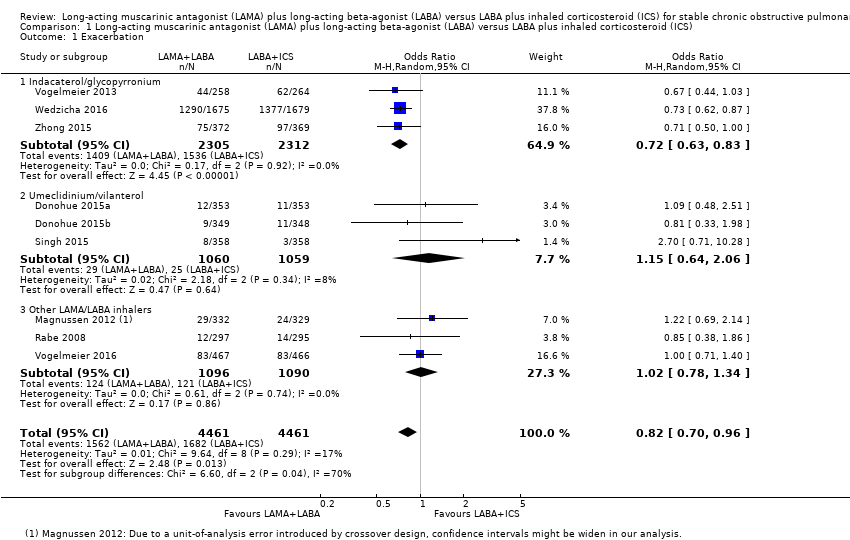 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 1 Exacerbation. | ||||
| 1.1 Indacaterol/glycopyrronium | 3 | 4617 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.63, 0.83] |
| 1.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.06] |
| 1.3 Other LAMA/LABA inhalers | 3 | 2186 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Serious adverse effect Show forest plot | 10 | 9793 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| Analysis 1.2  Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 2 Serious adverse effect. | ||||
| 2.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.10] |
| 2.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.43, 2.31] |
| 2.3 Other LAMA/LABA inhalers | 4 | 3053 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.57] |
| 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference) Show forest plot | 6 | 5858 | Mean Difference (Random, 95% CI) | ‐1.22 [‐2.52, 0.07] |
| Analysis 1.3 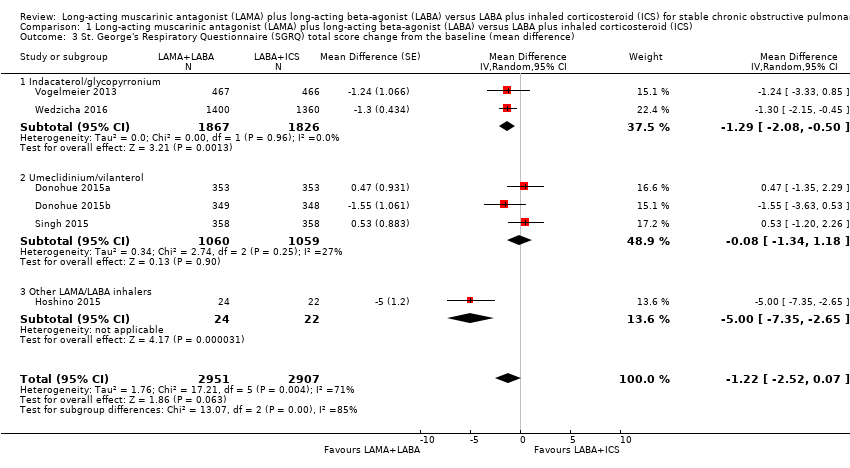 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference). | ||||
| 3.1 Indacaterol/glycopyrronium | 2 | 3693 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 3.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |
| 3.3 Other LAMA/LABA inhalers | 1 | 46 | Mean Difference (Random, 95% CI) | ‐5.0 [‐7.35, ‐2.65] |
| 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline Show forest plot | 6 | 7277 | Mean Difference (Random, 95% CI) | 0.08 [0.06, 0.09] |
| Analysis 1.4  Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline. | ||||
| 4.1 Indacaterol/glycopyrronium | 2 | 4291 | Mean Difference (Random, 95% CI) | 0.08 [0.04, 0.12] |
| 4.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | 0.09 [0.07, 0.11] |
| 4.3 Other LAMA/LABA inhalers | 1 | 867 | Mean Difference (Random, 95% CI) | 0.05 [0.02, 0.08] |
| 5 Pneumonia Show forest plot | 8 | 8540 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.79] |
| Analysis 1.5 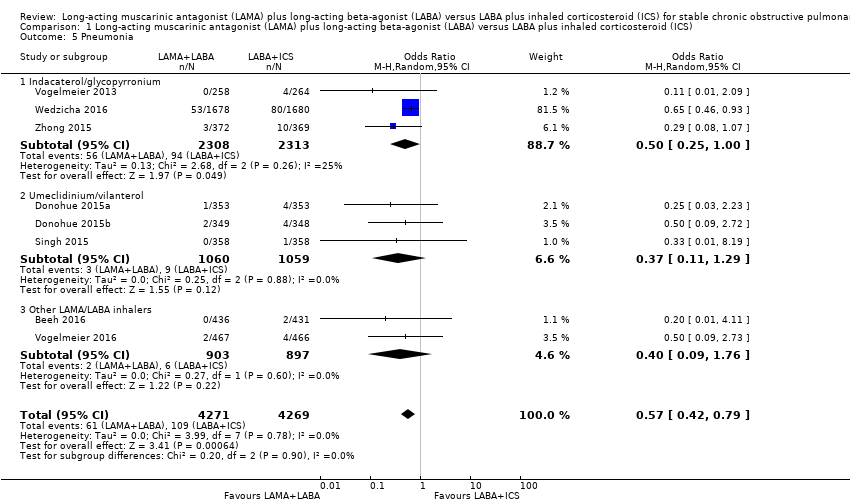 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 5 Pneumonia. | ||||
| 5.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
| 5.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.29] |
| 5.3 Other LAMA/LABA inhalers | 2 | 1800 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 6 All‐cause death Show forest plot | 8 | 8200 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.67] |
| Analysis 1.6 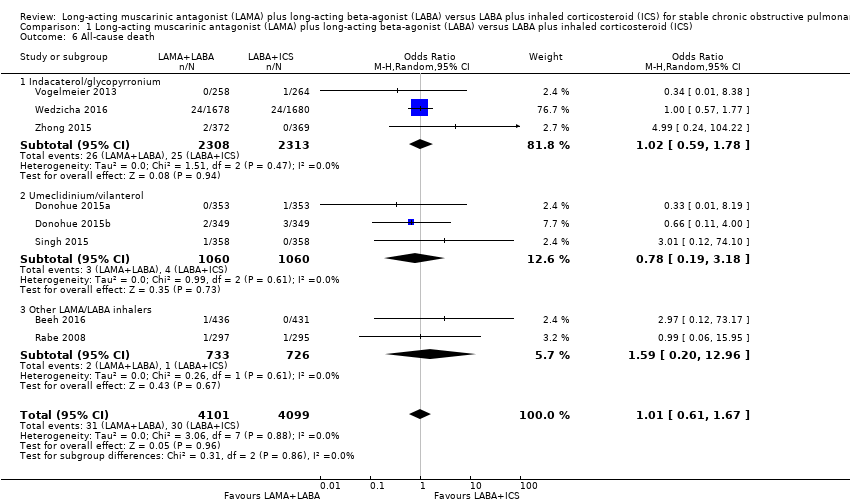 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 6 All‐cause death. | ||||
| 6.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.78] |
| 6.2 Umeclidinium/vilanterol | 3 | 2120 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.18] |
| 6.3 Other LAMA/LABA inhalers | 2 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.96] |
| 7 SGRQ total score improvement from the baseline (≥ 4 units) Show forest plot | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| Analysis 1.7 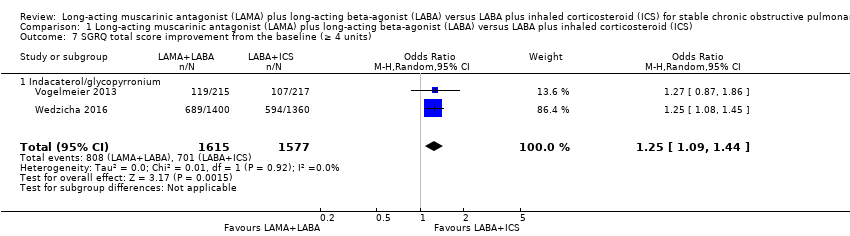 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 7 SGRQ total score improvement from the baseline (≥ 4 units). | ||||
| 7.1 Indacaterol/glycopyrronium | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 8 Total SGRQ change from the baseline (excluding unblinded studies) Show forest plot | 5 | 6360 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.58, 0.18] |
| Analysis 1.8 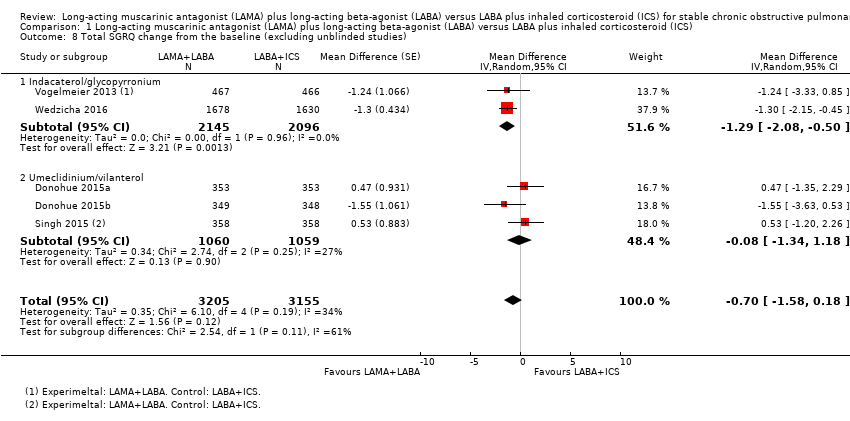 Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 8 Total SGRQ change from the baseline (excluding unblinded studies). | ||||
| 8.1 Indacaterol/glycopyrronium | 2 | 4241 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 8.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |

Study flow diagram.
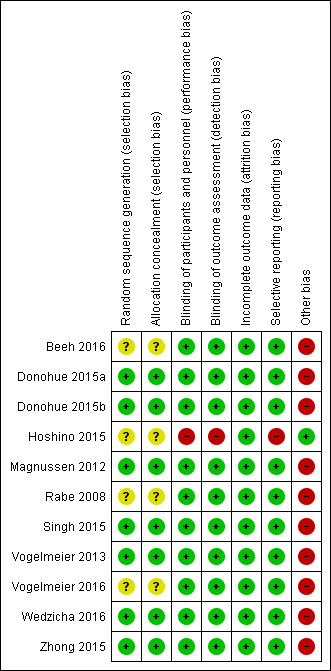
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.1 Exacerbation.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Funnel plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), outcome: 1.3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.4 Trough forced expiratory volume in one second (FEV1) change from the baseline.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 1 Exacerbation.
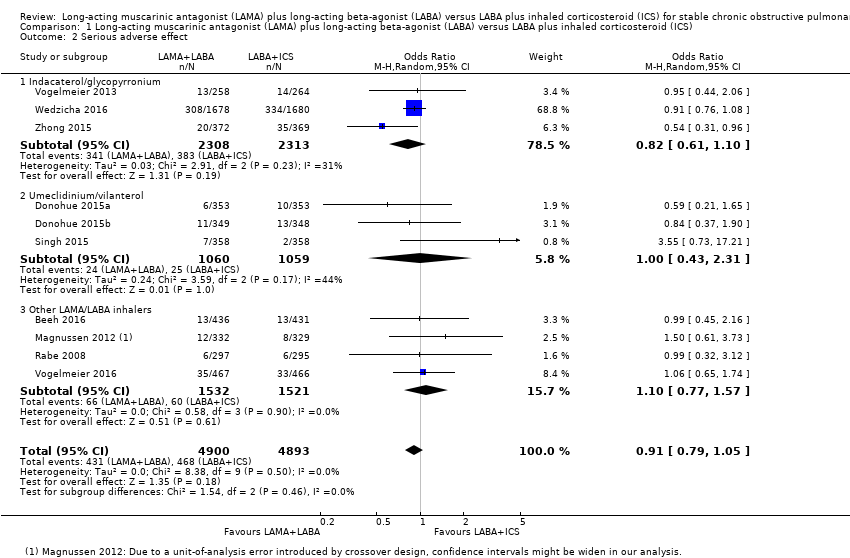
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 2 Serious adverse effect.
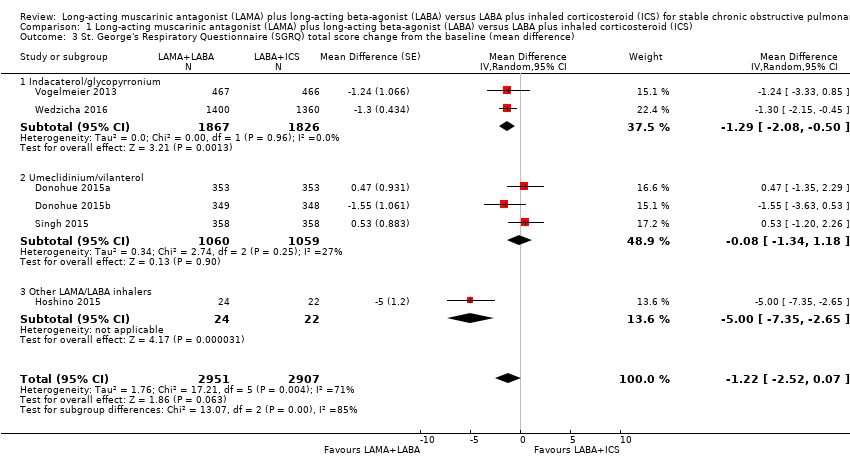
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).
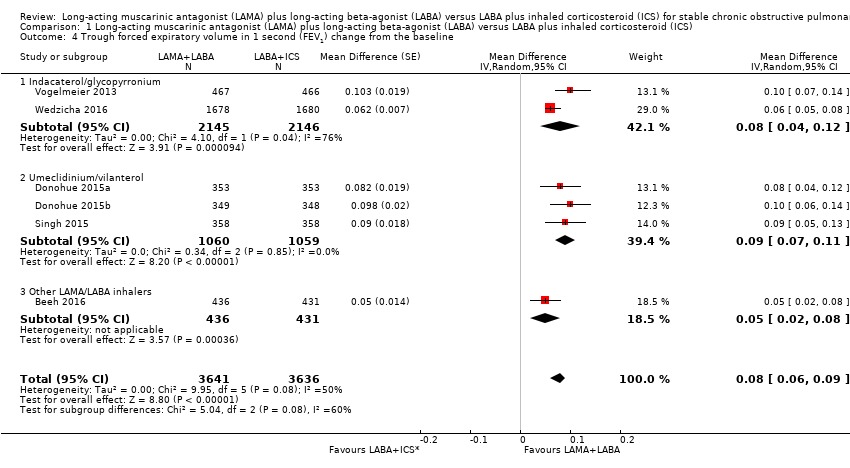
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline.
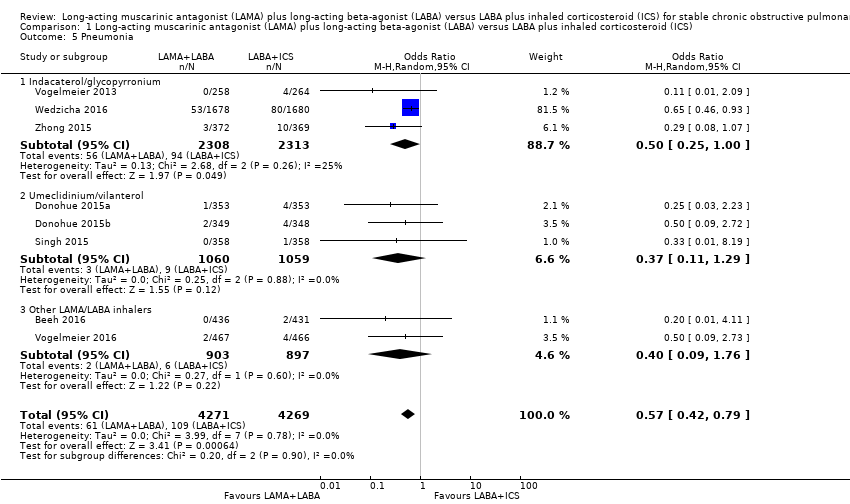
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 5 Pneumonia.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 6 All‐cause death.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 7 SGRQ total score improvement from the baseline (≥ 4 units).
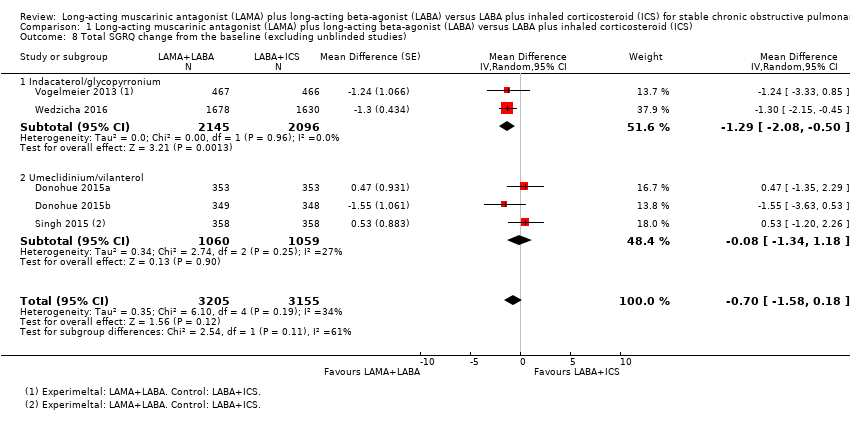
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 8 Total SGRQ change from the baseline (excluding unblinded studies).
| LAMA + LABA versus LABA + ICS for stable COPD | ||||||
| Population: stable COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effects | Number of participants | Quality of the evidence | Comments | |
| LABA+ICS | LAMA+LABA | |||||
| Exacerbations (number of people experiencing ≥ 1 exacerbations) Follow‐up: 12 to 52 weeks | 377 per 1000 | 332 per 1000 (298 to 368) | OR 0.82 | 8922 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| Serious adverse effects Follow‐up: 12 to 52 weeks | 96 per 1000 | 87 per 1000 | OR 0.91 | 9793 | ⊕⊕⊕⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (MD) Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD ‐1.22 (‐2.52 to 0.07) | 6055 (6 RCTs) | ⊕⊕⊝⊝ | Low MD means favourable outcome |
| Trough FEV1 change from the baseline Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD 0.08 L (0.06 to 0.09) | 6238 (6 RCTs) | ⊕⊕⊕⊝ | High MD means favourable outcome |
| Pneumonia Follow‐up: 12 to 52 weeks | 26 per 1000 | 15 per 1000 | OR 0.57 | 8540 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| All‐cause death Follow‐up: 12 to 52 weeks | 7 per 1000 | 7 per 1000 | OR 1.01 | 8200 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (≥ 4 points, MCID) Follow‐up: 24 to 52 weeks | 445 per 1000 | 500 per 1000 (466 to 535) | OR 1.25 (1.09 to 1.44) | 3192 (2 RCTs) | ⊕⊕⊕⊝ | High OR means favourable outcome |
| *The absolute risk (and its 95% CI) of LAMA+LABA group is based on the assumed risk in the LABA+ICS group and the OR of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect | ||||||
| 1 Every study had at least one domain of high risk of bias mostly due to conflicts of interest. 2 Indirectness due to definition of exacerbation. 3 There was a considerable heterogeneity, I2 = 71%. 4 Downgraded due to imprecision. | ||||||
| Study | LAMA+LABA | LABA+ICS | Key inclusion criteria | Follow‐up duration (weeks) | Mean/median age (years) | Number randomised |
| Tiotropium/olodaterol (2.5/5 μg) or tiotropium/olodaterol (5/5 μg) | Salmeterol/fluticasone (50/250 μg) twice daily or salmeterol/fluticasone (50/500 μg) twice daily. | %pred FEV1 30% to 80% Ex(‐) | 6 × 4 time periods (cross‐over) | 64 | 229 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 63 | 707 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 64 | 700 | |
| Tiotropium/indacaterol (18/150 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 80%, Ex(‐) | 16 | 71 | 46 | |
| Tiotropium/salmeterol (18/50 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 8 x 2 time periods (cross‐over) | 61 | 344 | |
| Tiotropium/formoterol (18/24 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 6 | 62 | 605 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 62 | 717 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, Ex(‐) | 26 | 63 | 523 | |
| Aclidinium/formoterol (400/12 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 < 80%, CAT ≥ 10, Ex(‐) | 24 | 63 | 933 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 25% to 60%, mMRC ≥ 2, Ex(+) | 52 | 65 | 3362 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, mMRC ≥ 2, Ex(‐) | 26 | 65 | 744 | |
| %pred FEV1: % predicted forced expiratory volume in one second; CAT: chronic obstructive pulmonary disease assessment test; Ex(‐): without recent exacerbation; Ex(+): with recent exacerbation; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale. | ||||||
| Sponsor | Record count | % of 1723 |
| GlaxoSmithKline | 134 | 7.78 |
| Novartis | 128 | 7.43 |
| AstraZeneca | 122 | 7.08 |
| Boehringer Ingelheim | 113 | 6.56 |
| Pfizer | 84 | 4.88 |
| Nycomed | 49 | 2.84 |
| GSK | 45 | 2.61 |
| Chiesi | 41 | 2.38 |
| Almirall | 36 | 2.09 |
| Merck | 30 | 1.74 |
| Web of Science Core Collection, advanced search for "TI=(COPD) AND TS=(inhal*)" without any restriction hit 1723 reports as of 13 June 2016. "Results analysis" > "Source Titles" output the table above. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation Show forest plot | 9 | 8922 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.96] |
| 1.1 Indacaterol/glycopyrronium | 3 | 4617 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.63, 0.83] |
| 1.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.06] |
| 1.3 Other LAMA/LABA inhalers | 3 | 2186 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Serious adverse effect Show forest plot | 10 | 9793 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.10] |
| 2.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.43, 2.31] |
| 2.3 Other LAMA/LABA inhalers | 4 | 3053 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.57] |
| 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference) Show forest plot | 6 | 5858 | Mean Difference (Random, 95% CI) | ‐1.22 [‐2.52, 0.07] |
| 3.1 Indacaterol/glycopyrronium | 2 | 3693 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 3.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |
| 3.3 Other LAMA/LABA inhalers | 1 | 46 | Mean Difference (Random, 95% CI) | ‐5.0 [‐7.35, ‐2.65] |
| 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline Show forest plot | 6 | 7277 | Mean Difference (Random, 95% CI) | 0.08 [0.06, 0.09] |
| 4.1 Indacaterol/glycopyrronium | 2 | 4291 | Mean Difference (Random, 95% CI) | 0.08 [0.04, 0.12] |
| 4.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | 0.09 [0.07, 0.11] |
| 4.3 Other LAMA/LABA inhalers | 1 | 867 | Mean Difference (Random, 95% CI) | 0.05 [0.02, 0.08] |
| 5 Pneumonia Show forest plot | 8 | 8540 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.79] |
| 5.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
| 5.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.29] |
| 5.3 Other LAMA/LABA inhalers | 2 | 1800 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 6 All‐cause death Show forest plot | 8 | 8200 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.67] |
| 6.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.78] |
| 6.2 Umeclidinium/vilanterol | 3 | 2120 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.18] |
| 6.3 Other LAMA/LABA inhalers | 2 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.96] |
| 7 SGRQ total score improvement from the baseline (≥ 4 units) Show forest plot | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 7.1 Indacaterol/glycopyrronium | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 8 Total SGRQ change from the baseline (excluding unblinded studies) Show forest plot | 5 | 6360 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.58, 0.18] |
| 8.1 Indacaterol/glycopyrronium | 2 | 4241 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 8.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |

