Antagonista muscarínico de acción prolongada (AMAP) más agonista beta de acción prolongada (ABAP) versus ABAP más corticosteroide inhalado (CSI) para la enfermedad pulmonar obstructiva crónica (EPOC) estable
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012066.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
NH conducted the study search, data extraction, analysis, study quality assessment, and drafting as a principal investigator.
AG provided statistical advice.
YS conducted the study search, study quality assessment, and data extraction.
EO managed editing of the full review as a Cochrane methodologist.
KN, KN, and TK contributed to the conception, study design, interpretation of the data, and critical revision as pulmonologists.
Sources of support
Internal sources
-
Grant from the National Center for Child Health and Development 26A‐5, Japan.
External sources
-
Japan Agency for Medical Research and Development, Japan.
AMED No. 27300101
Declarations of interest
TK: received grants or lecture fees (or both) from GlaxoSmithKline, Boehringer Ingelheim, Pfizer, Meiji, AstraZeneca, and Novartis from 2013 to 2016. This review was not funded by any pharmaceutical companies.
HN, GA, SY, OE, NK, and NK: none known.
Acknowledgements
We would like to thank Ms Emma Barber from the National Center for Child Health and Development for editorial assistance.
We also would like to thank Mrs Elizabeth Stovold from the Cochrane Airways Group for constructing the search formula.
Dr Ian Yang was the Editor for the protocol and commented critically upon it.
The 'Background' and 'Methods' sections of this review were based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health System or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Jun 05 | Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease | Review | Nobuhiko Fukuda, Nobuyuki Horita, Ayami Kaneko, Atsushi Goto, Takeshi Kaneko, Erika Ota, Kayleigh M Kew | |
| 2017 Feb 10 | Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD) | Review | Nobuyuki Horita, Atsushi Goto, Yuji Shibata, Erika Ota, Kentaro Nakashima, Kenjiro Nagai, Takeshi Kaneko | |
| 2016 Feb 02 | Long‐acting muscarinic antagonist plus long‐acting beta agonist versus long‐acting beta agonist plus inhaled corticosteroid for stable chronic obstructive pulmonary disease | Protocol | Nobuyuki Horita, Atsushi Goto, Erika Ota, Kentaro Nakashima, Kenjiro Nagai, Takeshi Kaneko | |
Differences between protocol and review
Yuji Shibata was added as the third review author.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Inhalation;
- Adrenal Cortex Hormones [*administration & dosage];
- Adrenergic beta-2 Receptor Agonists [adverse effects, *therapeutic use];
- Cause of Death;
- Disease Progression;
- Drug Therapy, Combination [adverse effects];
- Forced Expiratory Volume;
- Muscarinic Antagonists [adverse effects, *therapeutic use];
- Pneumonia [etiology];
- Pulmonary Disease, Chronic Obstructive [*drug therapy];
- Quality of Life;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
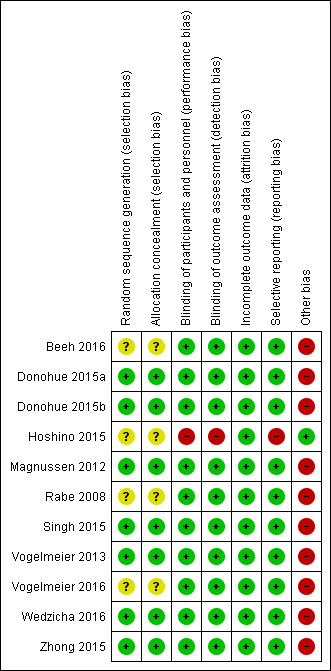
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.1 Exacerbation.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Funnel plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), outcome: 1.3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.4 Trough forced expiratory volume in one second (FEV1) change from the baseline.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 1 Exacerbation.
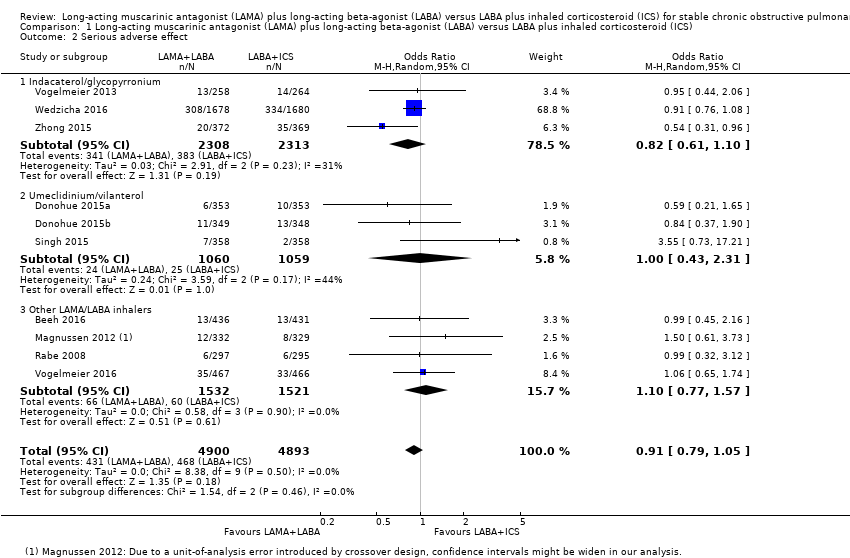
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 2 Serious adverse effect.
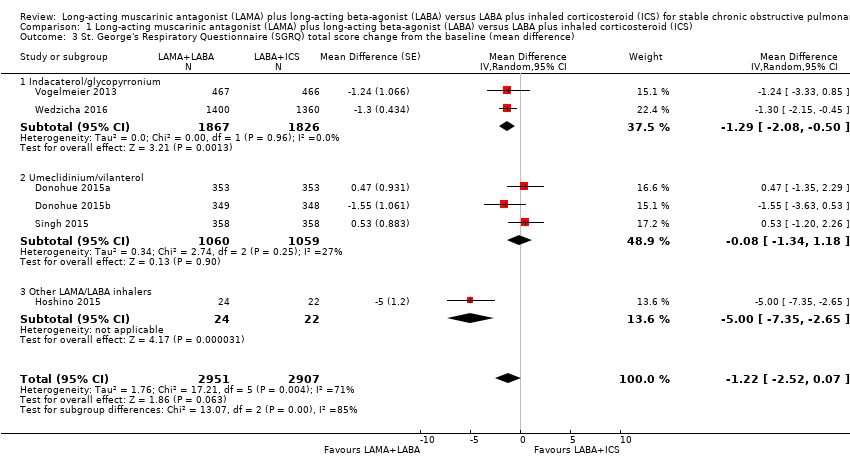
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).
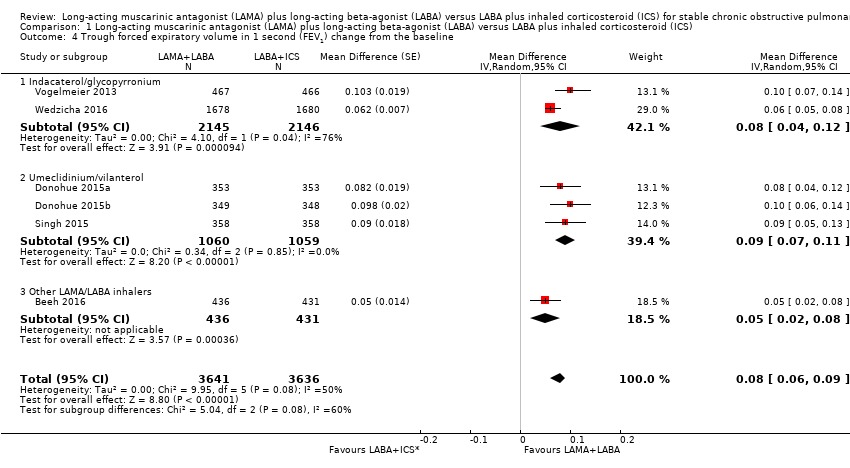
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline.
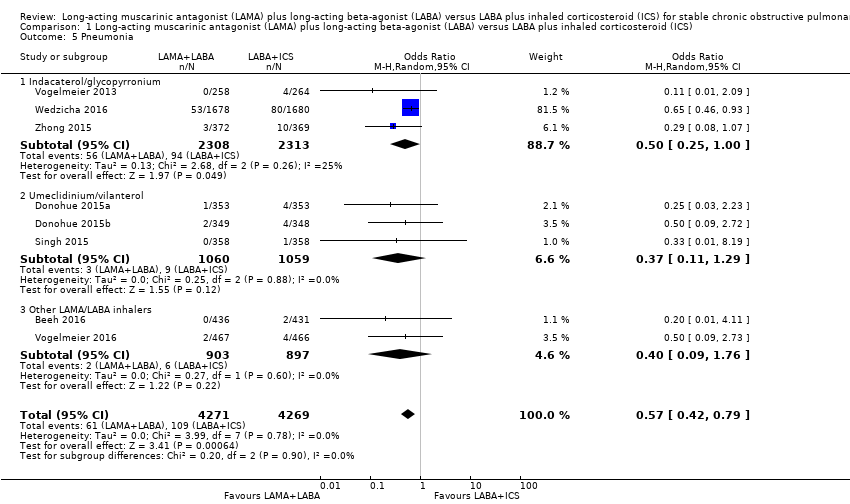
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 5 Pneumonia.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 6 All‐cause death.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 7 SGRQ total score improvement from the baseline (≥ 4 units).
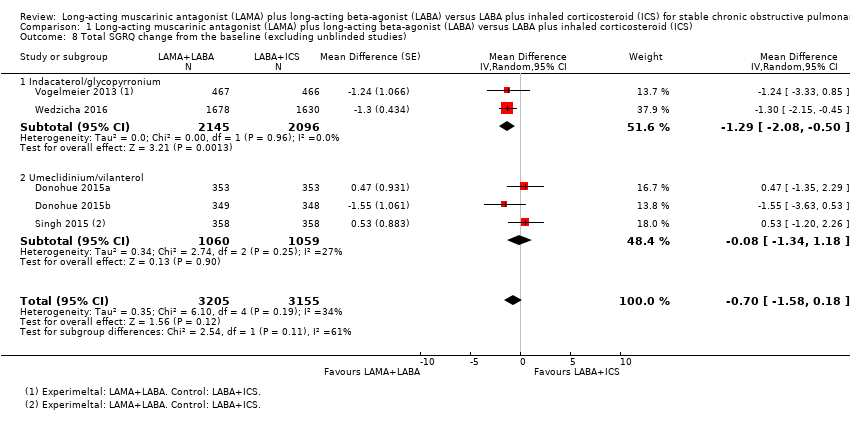
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 8 Total SGRQ change from the baseline (excluding unblinded studies).
| LAMA + LABA versus LABA + ICS for stable COPD | ||||||
| Population: stable COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effects | Number of participants | Quality of the evidence | Comments | |
| LABA+ICS | LAMA+LABA | |||||
| Exacerbations (number of people experiencing ≥ 1 exacerbations) Follow‐up: 12 to 52 weeks | 377 per 1000 | 332 per 1000 (298 to 368) | OR 0.82 | 8922 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| Serious adverse effects Follow‐up: 12 to 52 weeks | 96 per 1000 | 87 per 1000 | OR 0.91 | 9793 | ⊕⊕⊕⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (MD) Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD ‐1.22 (‐2.52 to 0.07) | 6055 (6 RCTs) | ⊕⊕⊝⊝ | Low MD means favourable outcome |
| Trough FEV1 change from the baseline Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD 0.08 L (0.06 to 0.09) | 6238 (6 RCTs) | ⊕⊕⊕⊝ | High MD means favourable outcome |
| Pneumonia Follow‐up: 12 to 52 weeks | 26 per 1000 | 15 per 1000 | OR 0.57 | 8540 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| All‐cause death Follow‐up: 12 to 52 weeks | 7 per 1000 | 7 per 1000 | OR 1.01 | 8200 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (≥ 4 points, MCID) Follow‐up: 24 to 52 weeks | 445 per 1000 | 500 per 1000 (466 to 535) | OR 1.25 (1.09 to 1.44) | 3192 (2 RCTs) | ⊕⊕⊕⊝ | High OR means favourable outcome |
| *The absolute risk (and its 95% CI) of LAMA+LABA group is based on the assumed risk in the LABA+ICS group and the OR of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect | ||||||
| 1 Every study had at least one domain of high risk of bias mostly due to conflicts of interest. 2 Indirectness due to definition of exacerbation. 3 There was a considerable heterogeneity, I2 = 71%. 4 Downgraded due to imprecision. | ||||||
| Study | LAMA+LABA | LABA+ICS | Key inclusion criteria | Follow‐up duration (weeks) | Mean/median age (years) | Number randomised |
| Tiotropium/olodaterol (2.5/5 μg) or tiotropium/olodaterol (5/5 μg) | Salmeterol/fluticasone (50/250 μg) twice daily or salmeterol/fluticasone (50/500 μg) twice daily. | %pred FEV1 30% to 80% Ex(‐) | 6 × 4 time periods (cross‐over) | 64 | 229 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 63 | 707 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 64 | 700 | |
| Tiotropium/indacaterol (18/150 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 80%, Ex(‐) | 16 | 71 | 46 | |
| Tiotropium/salmeterol (18/50 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 8 x 2 time periods (cross‐over) | 61 | 344 | |
| Tiotropium/formoterol (18/24 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 6 | 62 | 605 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 62 | 717 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, Ex(‐) | 26 | 63 | 523 | |
| Aclidinium/formoterol (400/12 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 < 80%, CAT ≥ 10, Ex(‐) | 24 | 63 | 933 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 25% to 60%, mMRC ≥ 2, Ex(+) | 52 | 65 | 3362 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, mMRC ≥ 2, Ex(‐) | 26 | 65 | 744 | |
| %pred FEV1: % predicted forced expiratory volume in one second; CAT: chronic obstructive pulmonary disease assessment test; Ex(‐): without recent exacerbation; Ex(+): with recent exacerbation; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale. | ||||||
| Sponsor | Record count | % of 1723 |
| GlaxoSmithKline | 134 | 7.78 |
| Novartis | 128 | 7.43 |
| AstraZeneca | 122 | 7.08 |
| Boehringer Ingelheim | 113 | 6.56 |
| Pfizer | 84 | 4.88 |
| Nycomed | 49 | 2.84 |
| GSK | 45 | 2.61 |
| Chiesi | 41 | 2.38 |
| Almirall | 36 | 2.09 |
| Merck | 30 | 1.74 |
| Web of Science Core Collection, advanced search for "TI=(COPD) AND TS=(inhal*)" without any restriction hit 1723 reports as of 13 June 2016. "Results analysis" > "Source Titles" output the table above. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation Show forest plot | 9 | 8922 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.96] |
| 1.1 Indacaterol/glycopyrronium | 3 | 4617 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.63, 0.83] |
| 1.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.06] |
| 1.3 Other LAMA/LABA inhalers | 3 | 2186 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Serious adverse effect Show forest plot | 10 | 9793 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.10] |
| 2.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.43, 2.31] |
| 2.3 Other LAMA/LABA inhalers | 4 | 3053 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.57] |
| 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference) Show forest plot | 6 | 5858 | Mean Difference (Random, 95% CI) | ‐1.22 [‐2.52, 0.07] |
| 3.1 Indacaterol/glycopyrronium | 2 | 3693 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 3.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |
| 3.3 Other LAMA/LABA inhalers | 1 | 46 | Mean Difference (Random, 95% CI) | ‐5.0 [‐7.35, ‐2.65] |
| 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline Show forest plot | 6 | 7277 | Mean Difference (Random, 95% CI) | 0.08 [0.06, 0.09] |
| 4.1 Indacaterol/glycopyrronium | 2 | 4291 | Mean Difference (Random, 95% CI) | 0.08 [0.04, 0.12] |
| 4.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | 0.09 [0.07, 0.11] |
| 4.3 Other LAMA/LABA inhalers | 1 | 867 | Mean Difference (Random, 95% CI) | 0.05 [0.02, 0.08] |
| 5 Pneumonia Show forest plot | 8 | 8540 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.79] |
| 5.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
| 5.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.29] |
| 5.3 Other LAMA/LABA inhalers | 2 | 1800 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 6 All‐cause death Show forest plot | 8 | 8200 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.67] |
| 6.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.78] |
| 6.2 Umeclidinium/vilanterol | 3 | 2120 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.18] |
| 6.3 Other LAMA/LABA inhalers | 2 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.96] |
| 7 SGRQ total score improvement from the baseline (≥ 4 units) Show forest plot | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 7.1 Indacaterol/glycopyrronium | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 8 Total SGRQ change from the baseline (excluding unblinded studies) Show forest plot | 5 | 6360 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.58, 0.18] |
| 8.1 Indacaterol/glycopyrronium | 2 | 4241 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 8.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |

