Desmopresina para el tratamiento de la nicturia en hombres
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: RCT, parallel, single‐blind. Randomisation ratio: 1:1. Setting: outpatient/Egypt. Dates when study was conducted: November 2011 to February 2014. | |
| Participants | Inclusion criteria: men with LUTS/BPH, ages ≥ 50 years (nocturia ≥ 2 voids/night), nocturnal polyuria (nocturnal urine volume > 30% of 24‐hour urine volume) on voiding diary. Exclusion criteria: men on, or previously treated (within 3 months prior to enrolment) with, desmopressin or BPH medical therapy; uncontrolled diabetes mellitus or hypertension; previous surgery for bladder neck obstruction or BPH; renal, cardiovascular, and neurologic diseases; diuretic use; voiding dysfunctions; BPH; IPSS ≤ 8, Qmax < 5 mL/s; abnormal digital rectal examination; elevated PSA (≥ 10 ng/dL); urinary tract infection; hyponatraemia (serum sodium < 135 mEq/L); lower urinary tract lithiasis, urothelial malignancy, and significant high PVR (≥ 250 mL). Total number of participants randomly assigned: 248. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin (Melt: 60 μg) with tamsulosin (oral controlled absorption system: 0.4 mg) daily. Control: tamsulosin (oral controlled absorption system: 0.4 mg) tablet daily. Duration: 3 months. | |
| Outcomes | Primary endpoint:
Secondary endpoints:
| |
| Funding sources | Not reported. | |
| Declarations of interest | No conflict of interest. | |
| Notes | Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized into two groups using computer randomization program (http://random‐allocation‐software.software.informer.com/2.0/). Randomization in blocks of two was used, and each center had its own list to keep the groups closely balanced." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described/unclear |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Group I, received daily oral dose of tamsulosin OCAS 0.4‐mg tablet and desmopressin (MELT) 60 mcg, and Group II, received only tamsulosin (OCAS) 0.4‐mg tablet daily." "prospective, randomized, single‐blind, comparative study." Comment: "single blinding" undertaken. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "prospective, randomized, single‐blind, comparative study." Comment: "single blinding" undertaken, but it was unclear who was blinded and how this was achieved. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 16/139 (11.5%) participants in desmopressin group and 9/134 (6.7%) participants in tamsulosin group not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 16/139 (11.5%) participants in desmopressin group and 9/134 (6.7%) participants in tamsulosin group not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 16/139 (11.5%) participants in desmopressin group and 9/134 (6.7%) participants in tamsulosin group not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 16/139 (11.5%) participants in desmopressin group and 9/134 (6.7%) participants in tamsulosin group not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: "time to first void" not described, and protocol not published. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: RCT, cross‐over. Setting: Bristol, UK. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria: men aged > 50 years with nocturnal polyuria (using 48 h of inpatient monitoring or 1‐week frequency volume chart). Exclusion criteria: men with nocturnal enuresis or incontinence; significant cardiovascular, renal, or hepatic disease; diabetes; urinary tract infection;or concomitantmedication active on the lower urinary tract. Total number of participants randomly assigned: cross‐over study.
| |
| Interventions | Run‐in period: 1‐week placebo run‐in. Extension period: desmopressin 40 µg for all participants for final 2 weeks after 4 weeks' cross‐over. Experimental: desmopressin (nasal spray: 20 µg) before going to bed each evening. Control: placebo (nasal spray) before going to bed each evening. Duration: 4 weeks (8 weeks (cross‐over study design)). | |
| Outcomes |
| |
| Funding sources | Not reported. | |
| Declarations of interest | Acknowledgements: support of Ferring Pharmaceuticals Ltd. | |
| Notes | Review authors used data before cross‐over due to unit of analysis error. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but not clear who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but not clear who was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2/20 (10.0%) participants excluded from analysis, and insufficient data were given for analysis due to cross‐over design. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2/20 (10.0%) participants excluded from analysis, and insufficient data were given for analysis due to cross‐over design. |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | Unclear risk | Comment: active run‐in period |
| Methods | Design: RCT, parallel, preliminary (pilot) study. Randomisation ratio: 1:1. Setting: outpatient/Ankara, Turkey. Dates when study was conducted: January 2011 to June 2011. | |
| Participants | Inclusion criteria: men with advanced age, BPH with complaints of LUTS and nocturia ≥ 3 times/night. Exclusion criteria: men with pathologically diagnosed prostate cancer; positive urine culture; prior surgery of the bladder, prostate, urethra; and additional urological pathology. Total number of participants randomly assigned: 31. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no Experimental: desmopressin 20 μg intranasal before bedtime. Control: doxazosin orally 2 mg orally for 2 weeks followed by 4 mg for 6 weeks before bedtime. Duration: 2 months. | |
| Outcomes |
| |
| Funding sources | Not reported. | |
| Declarations of interest | No conflict of interest. | |
| Notes | Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: appearance of large proportion of participants (51/80) excluded postrandomisation |
| Incomplete outcome data (attrition bias) | High risk | Comment: appearance of large proportion of participants (51/80) excluded postrandomisation |
| Incomplete outcome data (attrition bias) | High risk | Comment: appearance of large proportion of participants (51/80) excluded postrandomisation |
| Incomplete outcome data (attrition bias) | High risk | Comment: appearance of large proportion of participants (51/80) excluded postrandomisation |
| Incomplete outcome data (attrition bias) | High risk | Comment: appearance of large proportion of participants (51/80) excluded postrandomisation |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: randomised, double‐blind, placebo‐controlled study. Randomization ratio: 1:1. Setting: multicentre (8)/South Korea. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria: men aged 40 to 65 years with LUTS, IPSS ≥ 13, persistent nocturia (≥ 2 episodes/night), nocturia index score ≥ 1 despite use of alpha‐blocker treatment for ≥ 8 weeks, and nocturnal polyuria defined as nocturnal polyuria index > 33%. Exclusion criteria: men with total daily urine volume of ≥ 3000 mL, diabetes insipidus, history of prostate surgery in the past 6 months, cardiac failure, serum sodium < 135 mmol/L, clinically significant abnormalities of serum potassium or creatinine, current treatment for insomnia, desmopressin treatment in previous month, uncontrolled hypertension, urgency urinary incontinence, significant anatomical abnormalities in the urinary tract, use of other drugs that could influence desmopressin (diuretics, tricyclic antidepressants, indomethacin, carbamazepine, chlorpropamide, or a combination), or use of anticholinergics or 5‐alpha reductase inhibitor in the past 3 months excluded from analysis. Total number of participants randomly assigned: 109. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 0.2 mg oral + alpha‐blocker at bedtime. Control: placebo + alpha‐blocker at bedtime. Duration: 8 weeks. Alpha‐blocker: type and dose were not reported. | |
| Outcomes | Primary endpoint:
Secondary endpoints:
Safety assessment:
| |
| Funding sources | None. | |
| Declarations of interest | Not reported. | |
| Notes | Short‐term primary and secondary outcome, information related to risk of bias obtainedfrom author. Protocol: KCT0000271. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author reply: computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Author reply: sequentially numbered, opaque, sealed envelopes used. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Enrolled patients received oral desmopressin 0.2 mg or matching placebo at bedtime for 8 weeks." Comment: participants and investigator blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: placebo‐controlled study, participants and investigator blinded. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10/57 (17.6%) participants in intervention group and 13/52 (25.0%) participants in control group not included in analysis. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10/57 (17.6%) participants in intervention group and 13/52 (25.0%) participants in control group not included in analysis. Information on QoL obtained from author. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10/57 (17.6%) participants in intervention group and 13/52 (25.0%) participants in control group not included in analysis. Information on QoL obtained from author. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10/57 (17.6%) participants in intervention group and 13/52 (25.0%) participants in control group not included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol (KCT0000271) published, and data obtained through contact with author. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: RCT, parallel. Randomisation ratio: 1:1. Setting: outpatient/Istanbul, Turkey. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria: men with LUTS and nocturia (≥ 2/night) aged between 50 and 70 years. Exclusion criteria: history of congestive heart failure, neurological diseases, and diabetes mellitus who are taking diuretics; BPH because of medical treatments; prostate cancer; and urethral strictures. Total number of participants randomly assigned: 49 (data from only 45 participants who competed the study were shown). Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin (Minirin) 0.2 mg with alfuzosin 10 mg tablets. Control: alfuzosin 10 mg tablets. Duration: 3 months. | |
| Outcomes |
| |
| Funding sources | Not reported. | |
| Declarations of interest | No conflict of interest. | |
| Notes | Language of article: Turkish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/49 (8.1%) participants not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/49 (8.1%) participants not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/49 (8.1%) participants not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: RCT, double‐blind, placebo‐controlled, parallel‐group, phase III study. Randomisation ratio: 1:1. Setting: multicentre/UK, Sweden, the Netherlands, and the USA. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria: men aged 18 years with nocturia (mean of ≥ 2 voids/night) and nocturnal urine production greater than their maximum functional bladder capacity (nocturia index scores of > 1). Exclusion criteria: men with nocturia arising from other well‐defined causes of increased urinary frequency, e.g. diagnosed or suspected diabetes insipidus, primary polydipsia (40 mL/kg/24 h) or multiple sclerosis, urge incontinence or recently commenced medical or surgical treatment for benign prostatic obstruction; conditions characterised by fluid or electrolyte imbalance (or both) where antidiuresis was inappropriate (e.g. cardiac failure, use of diuretics); serum sodium levels below normal range, and uncontrolled hypertension. Total number of participants randomly assigned: 151. Experimental group:
Control group:
| |
| Interventions | Run‐in period: desmopressin dose titration (1 to 3 weeks, followed by a 1‐week 'washout'). Experimental: desmopressin oral: from 0.1 mg to 0.4 mg after dose titration 1 h before bedtime. Control: placebo 1 h before bedtime. Duration: 3‐week double‐blind treatment period. | |
| Outcomes | Primary efficacy endpoint:
Secondary efficacy endpoints:
| |
| Funding sources | Not reported. | |
| Declarations of interest | No conflict of interest. | |
| Notes | Only participants who obtained ≥ 20% reduction in nocturnal diuresis during dose titration period were randomised to either placebo or active treatment. Participants who experienced < 20% decrease in nocturnal diuresis at all doses during dose titration were classified as not responding and did not continue in study. If treatment‐related adverse events were experienced during dose titration, participants were allocated to maximum tolerated dose that showed the best pharmacodynamic response. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double‐blind, placebo‐controlled, parallel‐group, multinational, phase III study evaluated." Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but not clear who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but not clear who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/85 (5.8%) participants in desmopressin group and 3/65 (4.6%) participants in placebo group not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/85 (5.8%) participants in desmopressin group and 3/65 (4.6%) participants in placebo group not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/85 (5.8%) participants in desmopressin group and 3/65 (4.6%) participants in placebo group not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | High risk | Comment: active run‐in period with exclusion of participants who did not respond |
| Methods | Design: RCT, parallel, double‐blind, placebo controlled. Randomisation ratio: 1:1. Setting: outpatient/single centre/Tehran, Iran. Dates when study was conducted: 2008 to 2009. | |
| Participants | Inclusion criteria: men with voiding ≥ 2/night. Exclusion criteria: uncontrolled disease such as diabetes and cardiac disease, use of diuretics, hypertension, diabetes insipidus, diseases that influence medulla of kidney such as medullary cystic of kidney diseases, multiple sclerosis, urge incontinence and recent surgical treatment for BPH, known functional disease in urinary system, e.g. neurogenic bladder. Total number of participants randomly assigned: 60. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 0.1 mg 1 h before bedtime. Control: placebo 1 h before bedtime. Duration: 8 weeks. | |
| Outcomes |
| |
| Funding sources | Acknowledgement: thanks to Ferring drug company. | |
| Declarations of interest | No conflict of interest. | |
| Notes | Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided into 2 study groups." Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind placebo‐controlled study." Comment: placebo‐controlled study, described as double‐blind but unclear who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo‐controlled study, described as double‐blind but unclear who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all 60 participants included in final analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all 60 participants included in final analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all 60 participants included in final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: incorporated report of 4 randomised, double‐blind, placebo‐controlled, parallel‐arm, phase III trials. Randomisation ratio: 1:1:1:1 (placebo, 0.75 μg, 1.0 μg, or 1.5 μg desmopressin). Setting: multicentre/USA and Canada. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria:
Exclusion criteria: nocturnal enuresis, diabetes insipidus, unstable diabetes mellitus, congestive heart failure (New York Heart Association Class II‐IV), polydipsia or thirst disorders, uncontrolled hypertension, unstable angina, urinary retention (PVR > 150 mL) by medical history, hepatic or renal impairment, syndrome of inappropriate secretion of antidiuretic hormone, nephrotic syndrome, > 2+ pretibial oedema on physical exam, urinary bladder surgery or radiation therapy within the last 24 months prior to enrolment, severe daytime LUTS secondary to BPH, overactive bladder or severe stress urinary incontinence, daytime urinary frequency > 8 episodes/day by medical history or by 24‐hour urine frequency/volume chart during screening, women with unexplained pelvic masses or > stage II pelvic prolapse, current or past malignancy (except cured basal cell carcinoma or squamous cell carcinoma of the skin) unless in remission for ≥ 5 years and with approval of the medical monitor, urinary bladder dysfunction of neurologic aetiology that in the judgement of the investigator would interfere with study assessments, neurogenic detrusor overactivity, obstructive sleep apnoea, hyperkinetic limb disorders, work or lifestyle activities that interfere with nighttime sleep, alcohol or substance abuse within 12 months of enrolment. Total number of participants randomly assigned: 1556 (latest 2 phase III trials). Experimental group:
Control group:
| |
| Interventions | Run‐in period: double‐blind, 2‐week, placebo lead‐in period. Experimental: desmopressin 0.75 μg, 1.0 μg, or 1.5 μg in nasal spray at nighttime. Control: placebo at nighttime. Duration: 12 weeks. | |
| Outcomes | Coprimary efficacy endpoints:
Secondary efficacy endpoints:
Safety:
| |
| Funding sources | Serenity Pharmaceuticals. | |
| Declarations of interest | Serenity Pharmaceuticals. | |
| Notes | FDA briefing document based on 4 phase III studies (efficacy analysis: latest 2 trials, safety analysis: all 4 phase III trials). Protocol: NCT00937859, NCT00937378, NCT01357356, NCT01900704. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind placebo‐controlled study." Comment: participants and investigator were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind placebo‐controlled study." Comment: placebo‐controlled study, participants and investigator were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1080/1098 (98.3%) participants in desmopressin group and 446/458 (97.3%) participants in placebo group were included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1080/1098 (98.3%) participants in desmopressin group and 446/458 (97.3%) participants in placebo group were included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis. |
| Selective reporting (reporting bias) | High risk | Comment: published protocols included a part of reported outcomes in FDA document. |
| Other bias | Unclear risk | Quote: "double blind placebo lead‐in." Comment: no stratification based on gender |
| Methods | Design: RCT, cross‐over. Randomisation ratio: 1:1. Setting: South Korea. Dates when study was conducted: July 2010 to April 2013. | |
| Participants | Inclusion criteria: men age ≥ 50 years diagnosed with LUTS due to bladder outlet obstruction, with a maximum urinary flow rate ≤ 15 mL/s, nocturia (≥ 1 void/night), and total IPSS ≥ 14 (voiding subscore ≥ 8 and storage subscore ≥ 6). Exclusion criteria: neurogenic bladder dysfunction, hyponatraemia, uncontrolled hypertension, congestive heart failure, history of prostate surgery, interstitial cystitis, elevated PSA, or previously treated with anticholinergic drugs or diuretics. Total number of participants randomly assigned: 427. Experimental group:
Control group:
| |
| Interventions | Run‐in period: 4 weeks of alpha‐blocker treatment. Experimental: desmopressin 0.2 mg oral with tamsulosin 0.2 mg oral at bedtime. Control: solifenacin 5 mg oral with tamsulosin 0.2 mg oral at bedtime. Duration: 4 weeks (8 weeks (cross‐over study design)). | |
| Outcomes |
| |
| Funding sources | Acknowledgements: supported by grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea. | |
| Declarations of interest | No conflicts of interest. | |
| Notes |
Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups at the time of enrollment." Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not published, but data obtained through contact with author. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: RCT, parallel, double‐blind, placebo controlled. Randomisation ratio: 1:1. Setting: single centre/Taiwan. Dates when study was conducted: October 2007 to December 2009. | |
| Participants | Inclusion criteria: men with BPH age > 65 years, nocturia (mean ≥ 2), nocturnal polyuria (nocturnal urine volume > 30% of total daily urine volume). Exclusion criteria: men with urge incontinence, another voiding dysfunction, or urinary tract infection; received treatment with drugs known or suspected to interact with desmopressin (e.g. diuretics, tricyclic antidepressants, indomethacin, carbamazepine, or chlorpropamide); uncontrolled hypertension and diabetes mellitus; or had evidence of clinically relevant cardiac failure. Total number of participants randomly assigned: 115. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 0.1 mg oral at bedtime. Control: placebo oral at bedtime. Duration: 12 months. | |
| Outcomes | Primary efficacy endpoint:
Secondary efficacy endpoints:
| |
| Funding sources | Not reported. | |
| Declarations of interest | Not reported. | |
| Notes | Language of article: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were prospectively randomized into placebo and desmopressin groups using random numbers tables." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but unclear who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo‐controlled study, double‐blind but unclear who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 126 patients were enrolled into the treatment and randomized to both groups. In the placebo group 1 patient and in the desmopressin group 2 withdrew consent. During treatment 2 patients in the placebo group and 1 in the desmopressin group did not comply with followup protocol. In addition, 1 patient in the placebo group had a stroke and 1 had consciousness disturbance due to hyponatremia (116 mmol/l) so they could not complete the protocol. Thus, a total of 115 patients were enrolled into the study." Comment: 9/126 (7.1%) participants not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 9/126 (7.1%) participants not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 9/126 (7.1%) participants not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 9/126 (7.1%) participants not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: review outcomes insufficiently described, and protocol not published. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: RCT. Randomisation ratio: 1:1. Setting: outpatient/single centre/China. Dates when study was conducted: 2009 to 2010. | |
| Participants | Inclusion criteria: older men with ≥ 2 voids/night. Exclusion criteria: not reported. Total number of participants randomly assigned: 60. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 0.1 mg at bedtime. Control: liquid restriction during nighttime. Duration: 8 weeks. | |
| Outcomes |
| |
| Funding sources | Not reported. | |
| Declarations of interest | Not reported. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients were evaluated by nocturia cure rate, mean number of nocturia, mean duration of the first period and sleep quality." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients were evaluated by nocturia cure rate, mean number of nocturia, mean duration of the first period and sleep quality." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients were evaluated by nocturia cure rate, mean number of nocturia, mean duration of the first period and sleep quality." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not published. |
| Other bias | Unclear risk | Comment: abstract only |
| Methods | Design: RCT, parallel, double‐blind, placebo controlled. Randomisation ratio: 1:1:1:1:1 (placebo, desmopressin 10 µg, 25 µg, 50 µg, or 100 µg). Setting: multicentre/Canada and the USA. Dates when study was conducted: June 2007 to February 2008. | |
| Participants | Inclusion criteria: men and women aged > 18 years, mean ≥ 2 voids/night determined via a 3‐day frequency volume chart (serum sodium ≥ 135 mmol/L, serum creatinine within normal limits, and estimated glomerular filtration rate ≥ 60 mL/minutes), people on stable doses of medication for overactive bladder or BPH (or both) for 3 months could be included. Exclusion criteria: all individuals with urinary retention or PVR volume > 150 mL (or both); or history of urologic malignancies, neurogenic detrusor activity, or current genitourinary tract pathology that could interfere with voiding in addition to men with evidence of bladder outflow obstruction or urine flow < 5 mL/s (or both); or if surgery for bladder outflow obstruction/BPH had been performed within 6 months and women with potential for pregnancy, use of a pessary for pelvic prolapse, or presence of unexplained pelvic mass. Total number of participants randomly assigned: 799 (757 intention‐to‐treat population). Experimental group:
Control group
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 10 µg, 25 µg, 50 µg, and 100 µg ODT once daily, 1 h before bedtime. Control: placebo (ODT) once daily, 1 h before bedtime. Duration: 4 weeks. | |
| Outcomes | Coprimary efficacy endpoints:
Secondary efficacy endpoints:
Safety:
| |
| Funding sources | Ferring Pharmaceuticals. | |
| Declarations of interest | JP Weiss has been working with Ferring Pharmascience for more than 10 years acting in the capacity of paid consultant and scientific advisor. NR Zinner has been involved in clinical trials and has received speaker honoraria and consultancy fees from Ferring Pharmaceuticals and Astellas. BM Klein and JP Nørgaard are employees of Ferring Pharmaceuticals. | |
| Notes |
Protocol: NCT00477490. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: central randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatments were packaged according to the computer generated randomization code to ensure that patients, investigators, and the sponsor remained fully blinded." Comment: remote central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Desmopressin and placebo were supplied by Ferring Pharmaceutical A/S and were indistinguishable with respect to appearance, smell, taste, and packaging. Treatments were packaged according to the computer generated randomization code to ensure that patients, investigators, and the sponsor remained fully blinded." Comment: blinding of participants and personnel described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Treatments were packaged according to the computer generated randomization code to ensure that patients, investigators, and the sponsor remained fully blinded." Comment: outcome assessors blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "The ITT [intention‐to‐treat] population included 757 subjects and 710 (89%) completed the study. Across treatment groups, 6‐16% of subjects discontinued prematurely. The most common reasons for discontinuation were withdrawal of consent (4%), AEs [adverse events] (2%), and lost to follow‐up (2%)." Comment: 42 of 799 (5.2%) randomised participants not included in analysis, but data for men were not reported separately. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 42/799 (5.2%) randomised participants not included in analysis, but data for men not reported separately. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Selective reporting (reporting bias) | Low risk | Comment: protocol (NCT01262456) published, and all predefined review outcomes were well described. |
| Other bias | Unclear risk | Comment: no stratification based on gender |
| Methods | Design: RCT, parallel, double‐blind, placebo controlled. Randomisation ratio: 1:1:1 (placebo, desmopressin 50 µg or 75 µg). Setting: multicentre/Canada and the USA. Dates when study was conducted: February 2011 to January 2012. | |
| Participants | Inclusion criteria: men aged ≥ 18 years with nocturia (≥ 2 voids/night determined via a 3‐day frequency volume chart). Exclusion criteria: severe daytime voiding dysfunction (> 1 urge incontinence or urgency episode daily or > 8 daytime voids/day in the 3‐day diary); suspicion of bladder outlet obstruction or a urine flow < 5 mL/s; surgery for bladder outlet obstruction or BPH within 6 months of screening; urinary retention or PVR volume > 250 mL (confirmed by ultrasound if investigator suspected retention) (or both); history of urologic malignancies, neurogenic detrusor activity, or current genitourinary tract pathology that could interfere with voiding, polydipsia, and hyponatraemia (serum sodium < 135 mmol/L). Total number of participants randomly assigned: 395. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 50 µg and 75 µg (ODT) once daily. Control: placebo (ODT) once daily. Duration: 3 months. | |
| Outcomes | Coprimary efficacy endpoints:
Secondary efficacy endpoints:
Exploratory endpoints:
Safety and tolerability:
| |
| Funding sources | Supported by Ferring Pharmaceuticals. | |
| Declarations of interest | Jeffrey P Weiss: financial interest or other relationship (or both) with Ferring, Pfizer, Allergan, Astellas, and Lilly; Sender Herschorn: financial interest or other relationship (or both) with Ferring, Astellas, Pfizer, Allergan, Watson, American Medical Systems, Eli Lilly, Cook, and Gynecare; Egbert A van der Meulen and Cerasela D Albei: financial interest or other relationship (or both) with Ferring. | |
| Notes | Protocol: NCT01262456. Language of article: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer generated list prepared before study enrollment. Randomization was stratified by age." Comment: method of sequence generation described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Desmopressin and placebo ODT were supplied by Ferring Pharmaceuticals, and were indistinguishable with respect to appearance, smell, taste and packaging." Comment: participant and investigator blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: placebo‐controlled study, participants and investigator blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/243 (0.9%) participants in desmopressin group and 0/142 (0.0%) participants in placebo group not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 33/243 (13.5%) participants in desmopressin group and 15/142 (10.5%) participants in placebo group not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/243 (0.9%) participants in desmopressin group and 0/142 (0.0%) participants in placebo group not included in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All primary, secondary and exploratory end points were analyzed based on the full analysis set which included all randomized and exposed patients with at least 1 efficacy assessment after dosing initiation." Comment: all participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol (NCT01262456) published, and all predefined review outcomes were well described. |
| Other bias | Low risk | Comment: not detected |
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel trial. Randomisation ratio: 1:1:1:1:1 (placebo, desmopressin 10 µg, 25 µg, 50 µg, or 100 µg). Setting: multicentre (36)/Japan. Dates when study was conducted: July 2010 to April 2011. | |
| Participants | Inclusion criteria: adults aged 55 to 75 years, with mean ≥ 2 voids/night in a 3‐day frequency volume chart. Exclusion criteria: bladder obstruction or a urine flow < 5 mL/s, surgical treatment of bladder obstruction or prostatic hyperplasia ≤ 6 months before study, symptoms of BPH, overactive bladder or interstitial cystitis, mean number of nocturnal voids > 4/night in a consecutive 3‐day period during screening, or hyponatraemia (serum sodium < 135 mEq/L) after desmopressin administration. Total number of participants randomly assigned: 139. Experimental group:
Control group:
| |
| Interventions | Run‐in period: no. Experimental: desmopressin 10 µg, 25 µg, 50 µg, and 100 µg ODT once daily, 1 h before bedtime. Control: placebo once daily, 1 h before bedtime. Duration: 4 weeks. | |
| Outcomes | Primary endpoint:
Secondary endpoints:
Safety and tolerability:
| |
| Funding sources | Ferring Pharmaceuticals A/S. | |
| Declarations of interest | Osamu Yamaguchi and Osamu Nishizawa are consultants to Ferring Pharmaceuticals. Osamu Nishizawa, Kristian Vinter Juul, and Jens Peter Nørgaard are employees of Ferring Pharmaceuticals. | |
| Notes | Protocol: NCT01184859. Language: English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer‐generated randomization list and stratified by age and gender to ensure balanced groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Desmopressin ODT and placebo were supplied by Ferring Pharmaceuticals A/S and were indistinguishable with respect to appearance, smell, taste and packaging." "Treatments were packaged according to the computer‐generated randomization code to ensure that patients, investigators and the sponsor remained fully blinded." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Desmopressin ODT and placebo were supplied by Ferring Pharmaceuticals A/S and were indistinguishable with respect to appearance, smell, taste and packaging." "Treatments were packaged according to the computer‐generated randomization code to ensure that patients, investigators and the sponsor remained fully blinded." |
| Incomplete outcome data (attrition bias) | High risk | Comment: 30/112 (26.7%) participants in desmopressin group and 5/27 (18.5%) participants in placebo group were not included in analysis. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 24/112 (21.4%) participants in desmopressin group and 5/27 (18.5%) participants in placebo group were not included in analysis. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 30/112 (26.7%) participants in desmopressin group and 5/27 (18.5%) participants in placebo group were not included in analysis. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 19/112 (16.9%) participants in desmopressin group and 4/27 (14.8%) participants in placebo group were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol (NCT01184859) published, and predefined study outcomes were well described. |
| Other bias | Unclear risk | Comment: no stratification based on gender |
BPH: benign prostatic hyperplasia; FDA: US Food and Drug Administration; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; ODT: orally disintegrating tablet; PSA: prostate‐specific antigen; PVR: postvoid residual; Qmax: maximum flow rate; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Duplicate | |
| Wrong participant population (men and women) | |
| Wrong comparator | |
| Duplicate | |
| Wrong intervention | |
| Wrong study design | |
| Wrong study design | |
| Duplicate | |
| Wrong participant population (not defined) | |
| Wrong outcomes | |
| Wrong study design | |
| Wrong study design | |
| Duplicate | |
| Wrong participant population (men and women) | |
| Duplicate | |
| Duplicate | |
| Wrong participant population |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, controlled, double‐blind study. |
| Participants | People with ≥ 2 voids/night. |
| Interventions | Not reported. |
| Outcomes | Improvement in initial period of undisturbed sleep and sleep quality. |
| Notes | Abstract only. |
| Methods | Parallel, randomised, double‐blind (participants and investigator) trial. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: desmopressin 25 µg/50 µg. Control: placebo. Duration: 12 weeks. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | June 2013 (final data collection date for primary outcome measure). |
| Methods | NA. |
| Participants | NA. |
| Interventions | NA. |
| Outcomes | NA. |
| Notes | Title only (only indexed in Cochrane Central Register of Controlled Trials (CENTRAL). |
| Methods | NA. |
| Participants | NA. |
| Interventions | NA. |
| Outcomes | NA. |
| Notes | Title only (no Supplement 5 issue in Journal of Urology). |
NA: not available.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Trial of desmopressin orally disintegrating tablets for nocturia due to nocturnal polyuria in Japanese male subjects. |
| Methods | Parallel, randomised, double‐blind (participant, investigator) trial. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: desmopressin 25 µg/50 µg. Control: placebo. Duration: 12 weeks. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 14 September 2016. |
| Contact information | DK0‐[email protected]. |
| Notes | Study is currently recruiting participants. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| Analysis 1.1 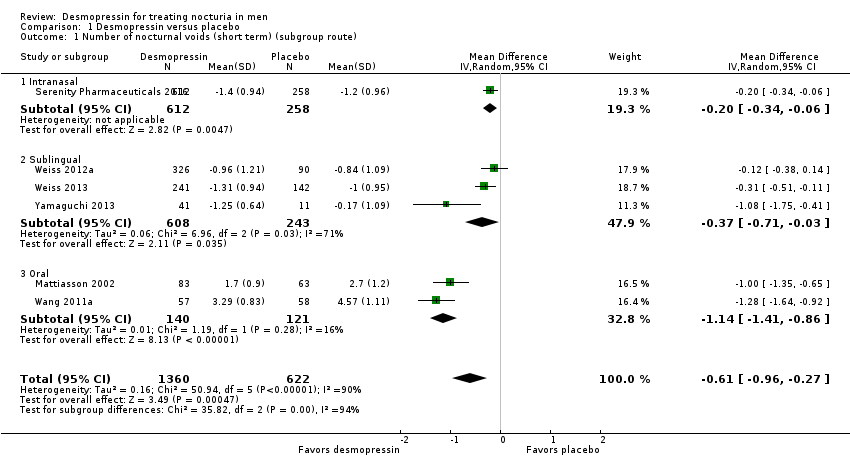 Comparison 1 Desmopressin versus placebo, Outcome 1 Number of nocturnal voids (short term) (subgroup route). | ||||
| 1.1 Intranasal | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 1.2 Sublingual | 3 | 851 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.71, ‐0.03] |
| 1.3 Oral | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.41, ‐0.86] |
| 2 Number of nocturnal voids (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.17, ‐0.53] |
| Analysis 1.2  Comparison 1 Desmopressin versus placebo, Outcome 2 Number of nocturnal voids (intermediate term). | ||||
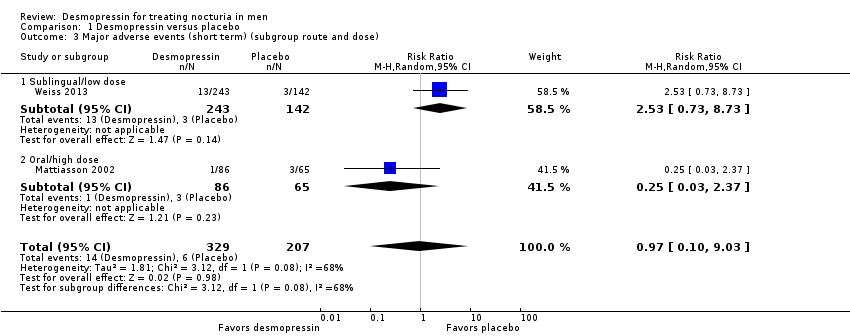
| 3 Major adverse events (short term) (subgroup route and dose) Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.10, 9.03] |
| Analysis 1.3  Comparison 1 Desmopressin versus placebo, Outcome 3 Major adverse events (short term) (subgroup route and dose). | ||||
| 3.1 Sublingual/low dose | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| 3.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| 4 Major adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| Analysis 1.4  Comparison 1 Desmopressin versus placebo, Outcome 4 Major adverse events (intermediate term). | ||||
| 5 Duration of first sleep episode (short term) Show forest plot | 4 | 652 | Mean Difference (IV, Random, 95% CI) | 54.61 [13.97, 95.25] |
| Analysis 1.5 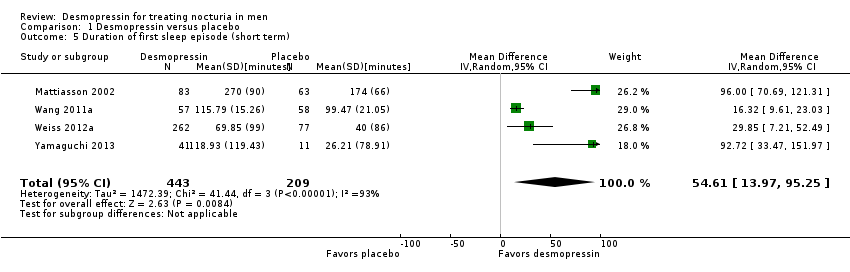 Comparison 1 Desmopressin versus placebo, Outcome 5 Duration of first sleep episode (short term). | ||||
| 6 Duration of first sleep episode (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 18.40 [11.60, 25.20] |
| Analysis 1.6 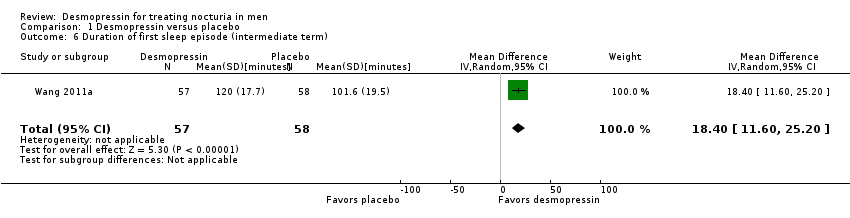 Comparison 1 Desmopressin versus placebo, Outcome 6 Duration of first sleep episode (intermediate term). | ||||
| 7 Time to first void (short term) [minutes] Show forest plot | 1 | 383 | Mean Difference (IV, Random, 95% CI) | 40.8 [17.07, 64.53] |
| Analysis 1.7 ![Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes].](/cdsr/doi/10.1002/14651858.CD012059.pub2/media/CDSR/CD012059/image_n/nCD012059-CMP-001-07.png) Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes]. | ||||
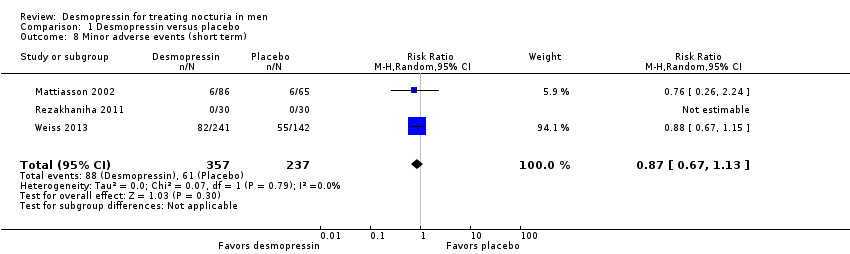
| 8 Minor adverse events (short term) Show forest plot | 3 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| Analysis 1.8 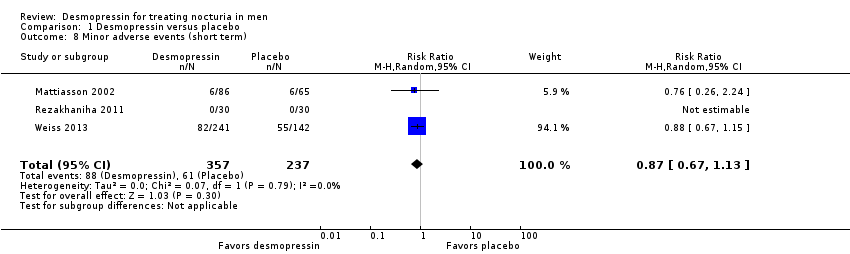 Comparison 1 Desmopressin versus placebo, Outcome 8 Minor adverse events (short term). | ||||
| 9 Minor adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.49] |
| Analysis 1.9  Comparison 1 Desmopressin versus placebo, Outcome 9 Minor adverse events (intermediate term). | ||||
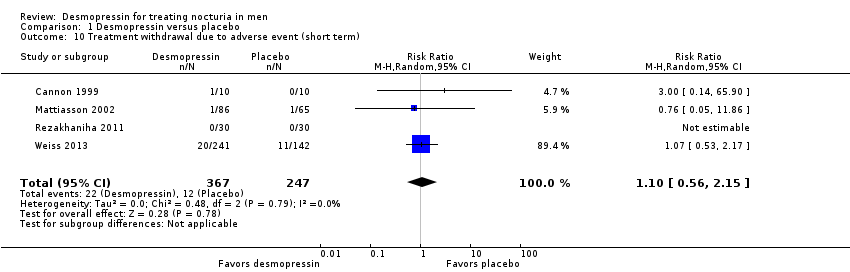
| 10 Treatment withdrawal due to adverse event (short term) Show forest plot | 4 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.15] |
| Analysis 1.10  Comparison 1 Desmopressin versus placebo, Outcome 10 Treatment withdrawal due to adverse event (short term). | ||||
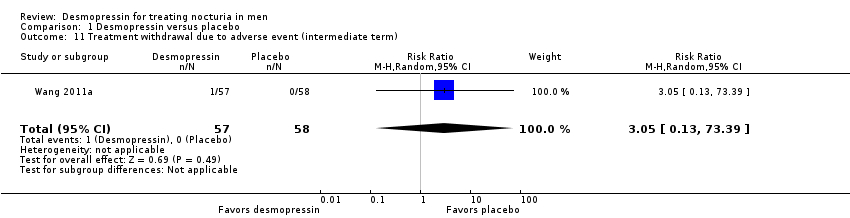
| 11 Treatment withdrawal due to adverse event (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| Analysis 1.11 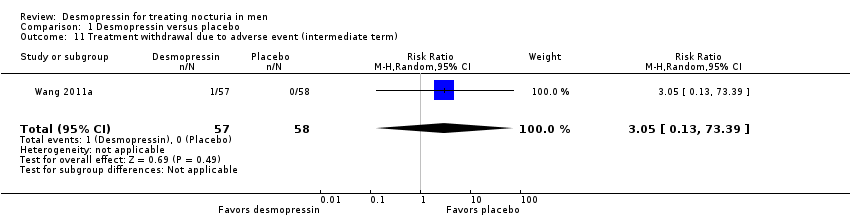 Comparison 1 Desmopressin versus placebo, Outcome 11 Treatment withdrawal due to adverse event (intermediate term). | ||||
| 12 Number of nocturnal voids (short term) (subgroup dose) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.98, ‐0.33] |
| Analysis 1.12 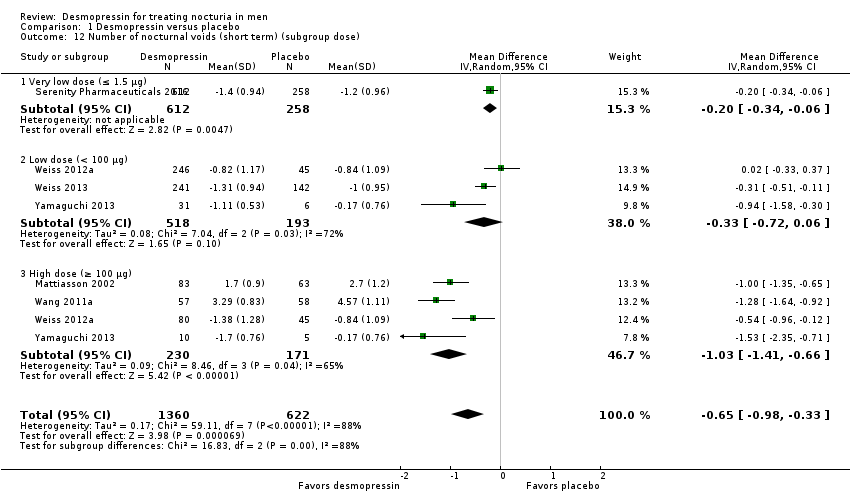 Comparison 1 Desmopressin versus placebo, Outcome 12 Number of nocturnal voids (short term) (subgroup dose). | ||||
| 12.1 Very low dose (≤ 1.5 μg) | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 12.2 Low dose (< 100 μg) | 3 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.72, 0.06] |
| 12.3 High dose (≥ 100 μg) | 4 | 401 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.41, ‐0.66] |
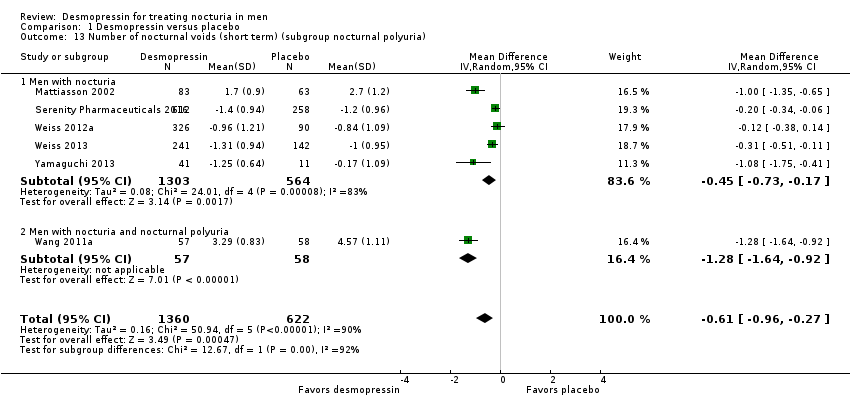
| 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| Analysis 1.13 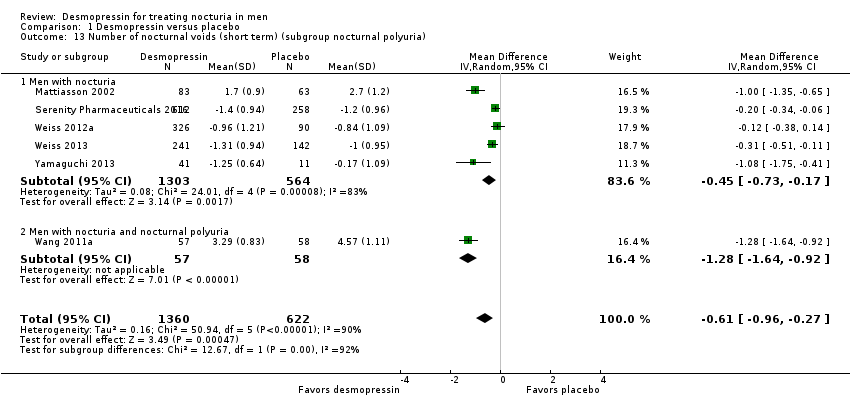 Comparison 1 Desmopressin versus placebo, Outcome 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria). | ||||
| 13.1 Men with nocturia | 5 | 1867 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.73, ‐0.17] |
| 13.2 Men with nocturia and nocturnal polyuria | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.64, ‐0.92] |
| 14 Number of nocturnal voids (short term) (sensitivity run‐in period) Show forest plot | 4 | 966 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.17, ‐0.14] |
| Analysis 1.14  Comparison 1 Desmopressin versus placebo, Outcome 14 Number of nocturnal voids (short term) (sensitivity run‐in period). | ||||
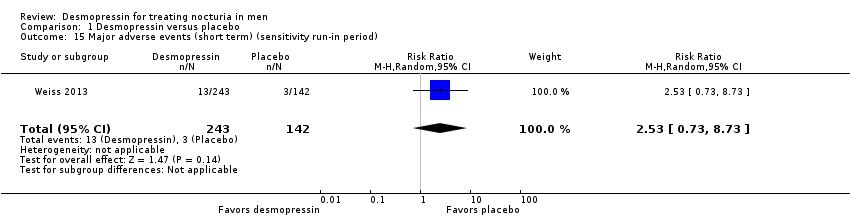
| 15 Major adverse events (short term) (sensitivity run‐in period) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| Analysis 1.15 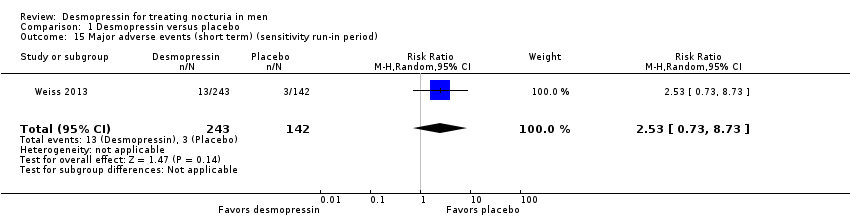 Comparison 1 Desmopressin versus placebo, Outcome 15 Major adverse events (short term) (sensitivity run‐in period). | ||||
| 16 Number of nocturnal voids (short term) (sensitivity clinical dosage) Show forest plot | 6 | 1586 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.15, ‐0.38] |
| Analysis 1.16 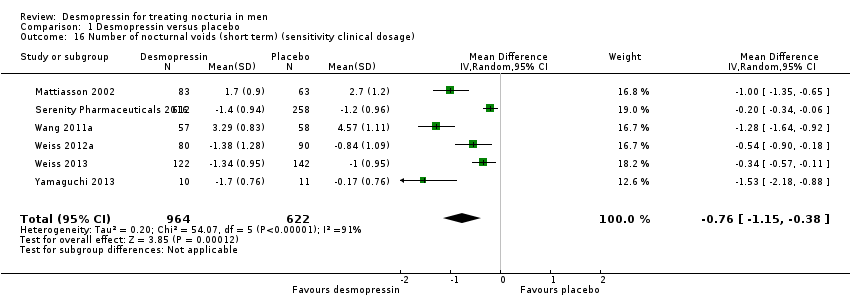 Comparison 1 Desmopressin versus placebo, Outcome 16 Number of nocturnal voids (short term) (sensitivity clinical dosage). | ||||
| 17 Major adverse events (short term) (sensitivity clinical dosage) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.71] |
| Analysis 1.17  Comparison 1 Desmopressin versus placebo, Outcome 17 Major adverse events (short term) (sensitivity clinical dosage). | ||||
| 17.1 Sublingual/low dose | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.71, 10.11] |
| 17.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of first sleep episode (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 90.0 [1.95, 178.05] |
| Analysis 2.1  Comparison 2 Desmopressin versus behaviour modification, Outcome 1 Duration of first sleep episode (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
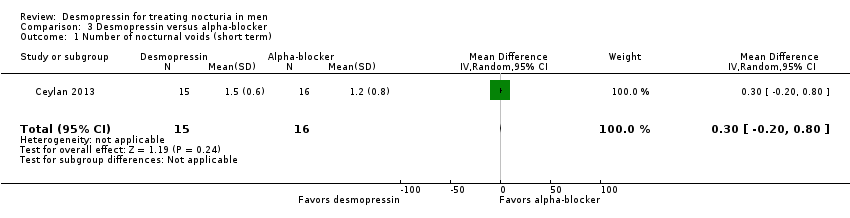
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.20, 0.80] |
| Analysis 3.1 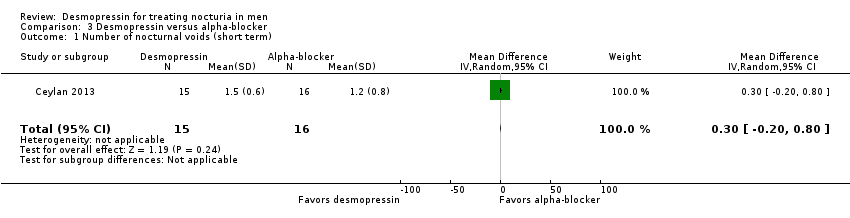 Comparison 3 Desmopressin versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term). | ||||
| 2 Quality of life (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.35, 0.35] |
| Analysis 3.2 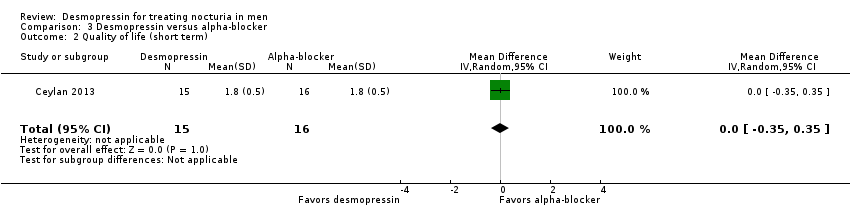 Comparison 3 Desmopressin versus alpha‐blocker, Outcome 2 Quality of life (short term). | ||||
| 3 Major adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.3 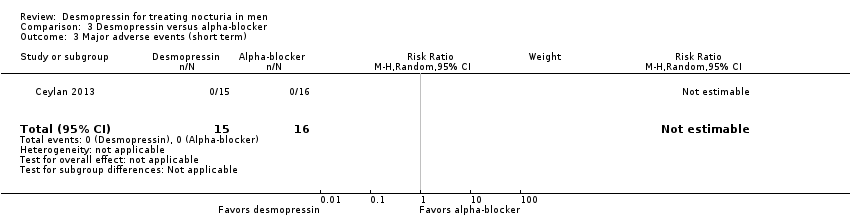 Comparison 3 Desmopressin versus alpha‐blocker, Outcome 3 Major adverse events (short term). | ||||
| 4 Minor adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.07, 15.57] |
| Analysis 3.4  Comparison 3 Desmopressin versus alpha‐blocker, Outcome 4 Minor adverse events (short term). | ||||
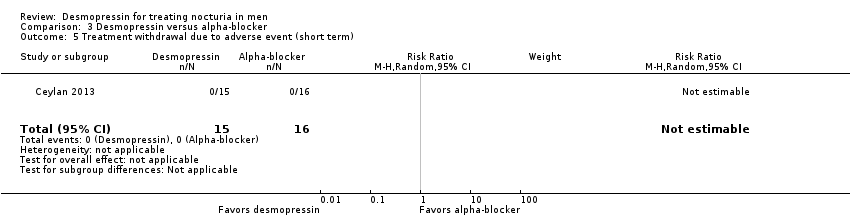
| 5 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.5  Comparison 3 Desmopressin versus alpha‐blocker, Outcome 5 Treatment withdrawal due to adverse event (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| Analysis 4.1 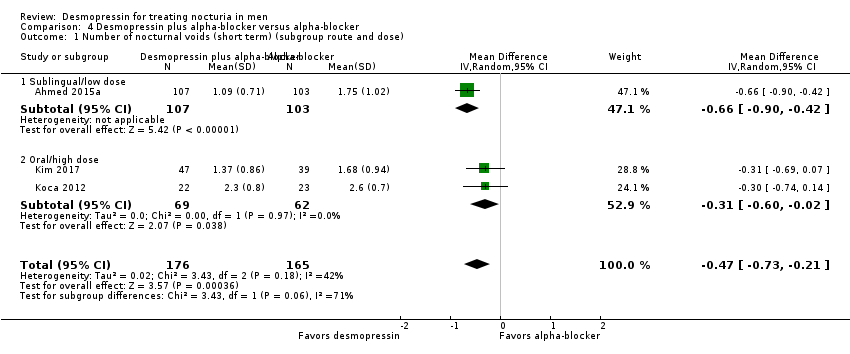 Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term) (subgroup route and dose). | ||||
| 1.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.90, ‐0.42] |
| 1.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.60, ‐0.02] |
| 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| Analysis 4.2  Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria). | ||||
| 2.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.74, 0.14] |
| 2.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.86, ‐0.18] |
| 3 Quality of life (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| Analysis 4.3  Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 3 Quality of life (short term) (subgroup route and dose). | ||||
| 3.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.49, 0.03] |
| 3.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.85, ‐0.03] |
| 4 Quality of life (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| Analysis 4.4 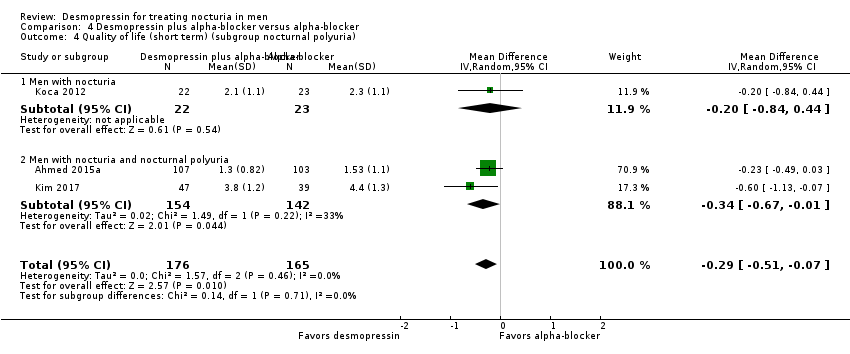 Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 4 Quality of life (short term) (subgroup nocturnal polyuria). | ||||
| 4.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.84, 0.44] |
| 4.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.67, ‐0.01] |
| 5 Major adverse events (short term) (subgroup route and dose) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| Analysis 4.5  Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 5 Major adverse events (short term) (subgroup route and dose). | ||||
| 5.1 Sublingual/low dose | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Oral/high dose | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 6 Major adverse events (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| Analysis 4.6  Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 6 Major adverse events (short term) (subgroup nocturnal polyuria). | ||||
| 6.1 Men with nocturia | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Men with nocturia and nocturnal polyuria | 2 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 7 Minor adverse events (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.15, 16.82] |
| Analysis 4.7 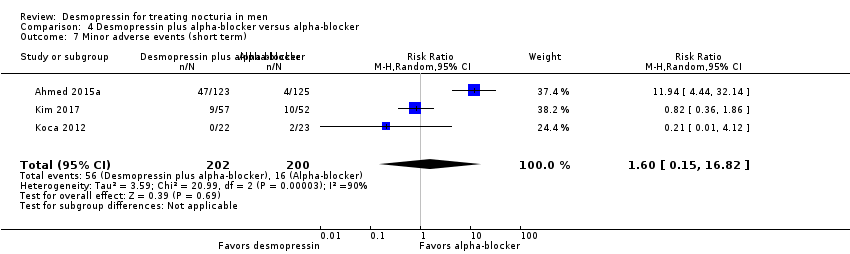 Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 7 Minor adverse events (short term). | ||||
| 8 Treatment withdrawal due to adverse event (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.46, 17.66] |
| Analysis 4.8  Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 8 Treatment withdrawal due to adverse event (short term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
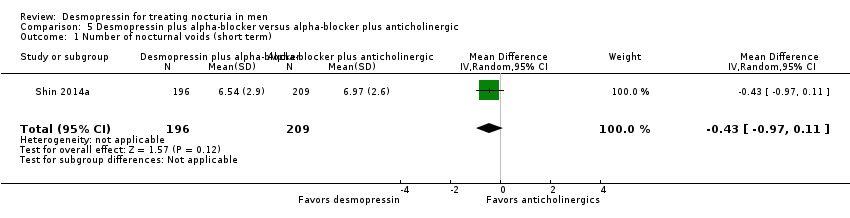
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.97, 0.11] |
| Analysis 5.1  Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 1 Number of nocturnal voids (short term). | ||||
| 2 Major adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 5.2  Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 2 Major adverse events (short term). | ||||
| 3 Minor adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |
| Analysis 5.3  Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 3 Minor adverse events (short term). | ||||
| 4 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |
| Analysis 5.4  Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 4 Treatment withdrawal due to adverse event (short term). | ||||
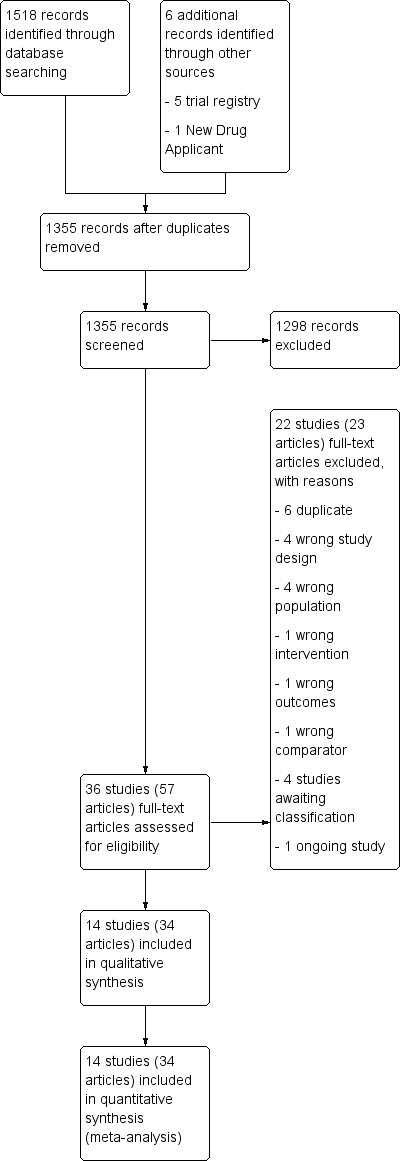
PRISMA flow diagram.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Comparison 1 Desmopressin versus placebo, Outcome 1 Number of nocturnal voids (short term) (subgroup route).

Comparison 1 Desmopressin versus placebo, Outcome 2 Number of nocturnal voids (intermediate term).
Comparison 1 Desmopressin versus placebo, Outcome 3 Major adverse events (short term) (subgroup route and dose).

Comparison 1 Desmopressin versus placebo, Outcome 4 Major adverse events (intermediate term).

Comparison 1 Desmopressin versus placebo, Outcome 5 Duration of first sleep episode (short term).

Comparison 1 Desmopressin versus placebo, Outcome 6 Duration of first sleep episode (intermediate term).
![Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes].](/es/cdsr/doi/10.1002/14651858.CD012059.pub2/media/CDSR/CD012059/image_n/nCD012059-CMP-001-07.png)
Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes].
Comparison 1 Desmopressin versus placebo, Outcome 8 Minor adverse events (short term).

Comparison 1 Desmopressin versus placebo, Outcome 9 Minor adverse events (intermediate term).
Comparison 1 Desmopressin versus placebo, Outcome 10 Treatment withdrawal due to adverse event (short term).
Comparison 1 Desmopressin versus placebo, Outcome 11 Treatment withdrawal due to adverse event (intermediate term).

Comparison 1 Desmopressin versus placebo, Outcome 12 Number of nocturnal voids (short term) (subgroup dose).
Comparison 1 Desmopressin versus placebo, Outcome 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria).

Comparison 1 Desmopressin versus placebo, Outcome 14 Number of nocturnal voids (short term) (sensitivity run‐in period).
Comparison 1 Desmopressin versus placebo, Outcome 15 Major adverse events (short term) (sensitivity run‐in period).

Comparison 1 Desmopressin versus placebo, Outcome 16 Number of nocturnal voids (short term) (sensitivity clinical dosage).

Comparison 1 Desmopressin versus placebo, Outcome 17 Major adverse events (short term) (sensitivity clinical dosage).

Comparison 2 Desmopressin versus behaviour modification, Outcome 1 Duration of first sleep episode (short term).
Comparison 3 Desmopressin versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 2 Quality of life (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 3 Major adverse events (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 4 Minor adverse events (short term).
Comparison 3 Desmopressin versus alpha‐blocker, Outcome 5 Treatment withdrawal due to adverse event (short term).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term) (subgroup route and dose).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 3 Quality of life (short term) (subgroup route and dose).
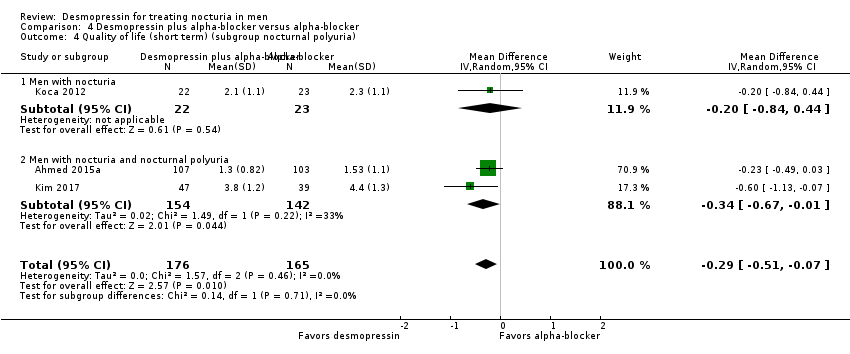
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 4 Quality of life (short term) (subgroup nocturnal polyuria).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 5 Major adverse events (short term) (subgroup route and dose).
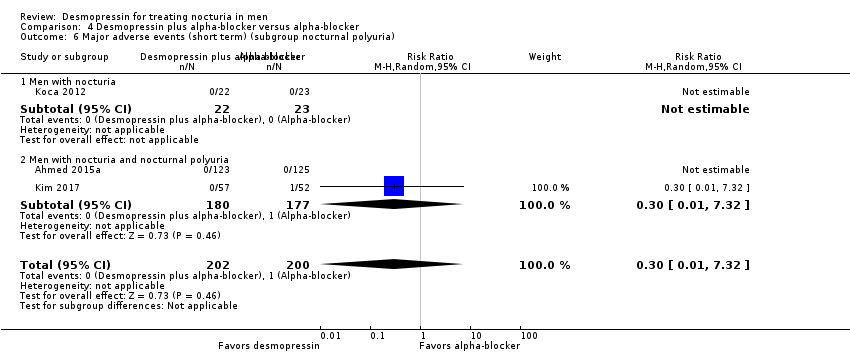
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 6 Major adverse events (short term) (subgroup nocturnal polyuria).
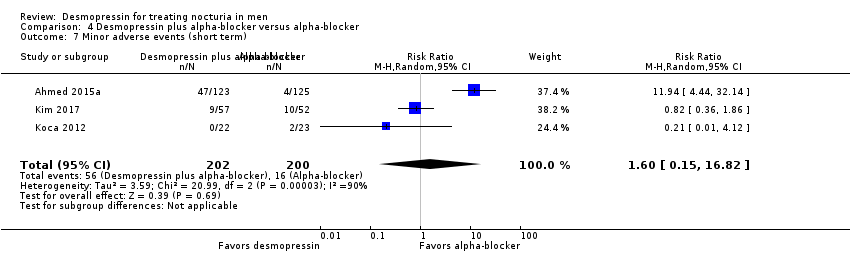
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 7 Minor adverse events (short term).
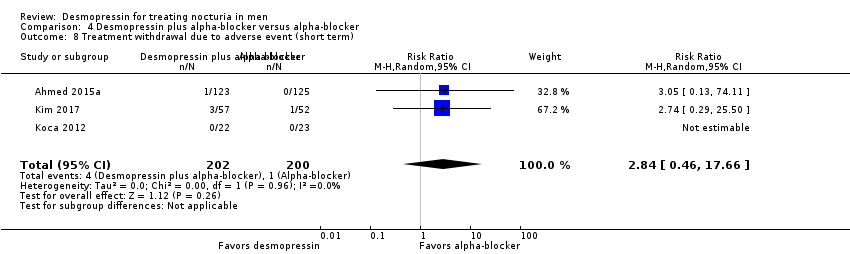
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 8 Treatment withdrawal due to adverse event (short term).
Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 1 Number of nocturnal voids (short term).
Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 2 Major adverse events (short term).

Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 3 Minor adverse events (short term).

Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 4 Treatment withdrawal due to adverse event (short term).
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 1982 | ⊕⊕⊝⊝ | ‐ | The mean number of nocturnal voids ranged from 1.9 to 4.57. | MD 0.61 lower |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 536 | ⊕⊝⊝⊝ | RR 0.97 | Study population | |
| 29 per 1000 | 1 fewer per 1000 | ||||
| Duration of first sleep episode | 652 | ⊕⊝⊝⊝ | ‐ | The mean duration of first sleep episode ranged from 26.21 to 174 minutes. | MD 54.61 minutes higher |
| Time to first void | 383 | ⊕⊕⊝⊝ | ‐ | The mean time to first void was 72.9 minutes. | MD 40.8 minutes higher |
| Minor adverse event | 594 | ⊕⊕⊝⊝ | RR 0.87 | Study population | |
| 257 per 1000 | 33 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 614 | ⊕⊕⊝⊝ | RR 1.10 | Study population | |
| 49 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in at least 50% of the studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 115 | ⊕⊕⊝⊝ | ‐ | Mean number of nocturnal voids was 4.14 voids. | MD 0.85 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode | 115 | ⊕⊕⊕⊝ | ‐ | Mean duration of first sleep episode was 101.6 minutes. | MD 18.4 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 115 | ⊕⊕⊝⊝ | RR 0.86 | Study population | |
| 328 per 1000 | 46 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: fluid restriction during nighttime | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with behaviour modification | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Duration of first sleep episode | 60 | ⊕⊕⊝⊝ | ‐ | Mean duration of first sleep episode was 150 minutes. | MD 90 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment withdrawal due to adverse event ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias in almost all domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 31 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 1.2 voids. | MD 0.3 voids more |
| Quality of life | 31 | ⊕⊕⊕⊝ | ‐ | Mean quality of life was 1.8 bothersome. | MD 0 |
| Major adverse events | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 31 | ⊕⊝⊝⊝ | RR 1.07 | Study population | |
| 63 per 1000 | 4 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: alpha‐blocker alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids assessed with: voiding diary | 341 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids ranged from 1.68 to 2.6. | MD 0.47 voids fewer |
| Quality of life assessed with: IPSS and N‐QoL | 341 | ⊕⊕⊕⊝ | ‐ | Mean quality of life ranged from 1.53 to 4.4. | MD 0.29 lower |
| Major adverse events | 402 | ⊕⊕⊝⊝ | RR 0.30 | Study population | |
| 5 per 1000 | 3 fewer per 1000 | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 402 | ⊕⊝⊝⊝ | RR 1.60 | Study population | |
| 80 per 1000 | 48 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 402 | ⊕⊕⊝⊝ | RR 2.84 | Study population | |
| 5 per 1000 | 9 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: anticholinergic + alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker + anticholinergic | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids | 405 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 6.97 voids. | MD 0.43 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 427 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
| Study | Setting | Trial period | Description of participants | Intervention(s) and comparator(s) | Duration of intervention |
| Outpatient/Egypt | 2011 to 2014 | People with LUTS/BPH aged ≥ 50 years with nocturia (≥ 2 voids/night), nocturnal polyuria (nocturnal urine volume > 30% of 24‐hour urine volume) | I: desmopressin 60 μg ODT + tamsulosin | 3 months | |
| C: tamsulosin | |||||
| UK | NR | Men aged > 50 years with nocturnal polyuria (using 48‐hour inpatient monitoring or 1‐week frequency volume chart) | I: desmopressin nasal spray 20 μg | 4 weeks | |
| C: placebo | |||||
| Outpatient/Turkey | 2011 | Men with advanced age, complaints of LUTS and nocturia (≥ 3 times/night) | I: desmopressin nasal spray 20 μg | 2 months | |
| C: doxazosin | |||||
| Multicentre/South Korea | NR | Men aged 40 to 65 years with LUTS (IPSS > 13), nocturia (≥ 2 episodes/night), and nocturnal polyuria (NPI > 33%) | I: desmopressin 0.2 mg oral + alpha‐blocker | 8 weeks | |
| C: placebo + alpha‐blocker | |||||
| Outpatient/Turkey | NR | Men aged 50 to 70 years with LUTS and nocturia (≥ 2/night) | I: desmopressin 0.2 mg oral with alfuzosin | 3 months | |
| C: alfuzosin | |||||
| Multicentre/Denmark, | NR | Men aged ≥ 18 years with nocturia (2 voids/night, nocturia index scores > 1) | I: desmopressin 0.1 mg/0.2 mg/0.4 mg oral; dose titration | 3 weeks | |
| C: placebo | |||||
| Outpatient/single centre/Iran | 2008 to 2009 | Older men (mean age about 63 to 64 years) with voiding ≥ 2/night | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/USA and Canada | NR | Men or women aged ≥ 50 years with nocturia (≥ 2 nocturic episodes/night) | I: desmopressin nasal spray 0.75 μg, 1.0 μg, or 1.5 μg | 12 weeks | |
| C: placebo | |||||
| South Korea | 2010 to 2013 | Men aged ≥ 50 years with LUTS due to bladder outlet obstruction (Qmax ≤ 15 mL/second, IPSS ≥ 14) and nocturia (≥ 1 void/night) | I: desmopressin 0.2 mg oral + tamsulosin | 4 weeks | |
| C: solifenacin + tamsulosin | |||||
| Single centre/Taiwan | 2007 to 2009 | Men aged ≥ 65 years with BPH (IPSS > 13), nocturia (≥ 2 voids/night), and nocturnal polyuria (nocturnal urine volume > 30%) | I: desmopressin 0.1 mg oral | 12 months | |
| C: placebo | |||||
| Outpatient/single centre/China | 2009 to 2010 | Older men (age not reported) | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2007 to 2008 | Men and women aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2010 to 2013 | Men aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 50 μg, 75 µg ODT | 3 months | |
| C: placebo | |||||
| Multicentre/Japan | 2010 to 2011 | Men and women aged 55 to 75 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo | |||||
| BPH: benign prostatic hyperplasia; C: comparator; I: intervention; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; NPI: nocturnal polyuria index; NR: not reported; ODT: orally disintegrating tablet; Qmax: maximum flow rate. | |||||
| Intervention(s) and comparator(s) | Sample size | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| I: desmopressin + tamsulosin | 100 | 397/273 | 139 | 123 | 123 | 107 | 77.0 | 3 months | |
| C: tamsulosin | 100 | 134 | 125 | 125 | 103 | 76.9 | |||
| Total: | 273 | 248 | 248 | 210 | 76.9 | ||||
| I: desmopressin | ‐ | ‐/‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 8 weeks (cross‐over study design) | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |||
| Total: | 20 | ‐ | 18 | 18 | 90.0 | ||||
| I: desmopressin | ‐ | 84/31 | 15 | 15 | 15 | 15 | 100.0 | 2 months | |
| C: doxazosin | ‐ | 16 | 16 | 16 | 16 | 100.0 | |||
| Total: | 31 | 31 | 31 | 31 | 100.0 | ||||
| I: desmopressin + alpha‐blocker | ‐ | 121/109 | 57 | 57 | 47 | 47 | 82.4 | 8 weeks | |
| C: placebo + alpha‐blocker | ‐ | 52 | 52 | 39 | 39 | 75.0 | |||
| Total: | 109 | 109 | 86 | 86 | 78.9 | ||||
| I: desmopressin + alfuzosin | ‐ | ‐/49 | ‐ | ‐ | 22 | 22 | ‐ | 3 months | |
| C: alfuzosin | ‐ | ‐ | ‐ | 23 | 23 | ‐ | |||
| Total: | 49 | ‐ | 45 | 45 | 91.8 | ||||
| I: desmopressin | 55 | 341/224 | 86 | 86 | 86 | 81 | 94.2 | 3 weeks | |
| C: placebo | 55 | 65 | 65 | 65 | 62 | 95.4 | |||
| Total: | 151 | 151 | 151 | 143 | 94.7 | ||||
| I: desmopressin | ‐ | 93/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 0.75 μg | ‐ | 3565/1707 | 458 | 448 | 448/252 | 401 | 87.5 | 12 weeks | |
| I2: desmopressin 1.0 μg | ‐ | 188 | 183 | 183/109 | 163 | 86.7 | |||
| I3: desmopressin 1.5 μg | ‐ | 452 | 439 | 439/251 | 387 | 85.6 | |||
| C: placebo | ‐ | 458 | 446 | 446/258 | 408 | 89.0 | |||
| Total: | 1556 | 1516 | 1516/870 | 1359 | 87.3 | ||||
| I: desmopressin + tamsulosin | ‐ | 435/427 | 205 | 205 | 205 | 196 | 95.6 | 8 weeks (cross‐over study design) | |
| C: tamsulosin + solifenacin | ‐ | 222 | 222 | 222 | 209 | 94.1 | |||
| Total: | 427 | 427 | 427 | 405 | 94.8 | ||||
| I: desmopressin | 45 | ‐/136 | NR | ‐ | 57 | 57 | ‐ | 12 months | |
| C: placebo | 45 | NR | ‐ | 58 | 58 | ‐ | |||
| Total: | 126 | ‐ | 115 | 115 | 91.3 | ||||
| I: desmopressin | ‐ | ‐/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 10 µg | ‐ | 1412/799 | 163 | 155 | 155/82 | 144 | 88.3 | 4 weeks | |
| I2: desmopressin 25 µg | ‐ | 158 | 152 | 152/87 | 148 | 93.7 | |||
| I3: desmopressin 50 µg | ‐ | 158 | 148 | 148/77 | 138 | 87.3 | |||
| I4: desmopressin 100 µg | ‐ | 160 | 146 | 146/80 | 135 | 84.4 | |||
| C: placebo | ‐ | 160 | 156 | 156/90 | 145 | 90.6 | |||
| Total: | 799 | 757 | 757/416 | 710 | 88.9 | ||||
| I1: desmopressin 50 µg | 130 | 1013/395 | ‐ | 119 | 119 | 100 | ‐ | 3 months | |
| I2: desmopressin 75 µg | 130 | ‐ | 124 | 124 | 103 | ‐ | |||
| C: placebo | 130 | ‐ | 142 | 142 | 120 | ‐ | |||
| Total: | 395 | 385 | 385 | 323 | 81.8 | ||||
| I1: desmopressin 10 µg | ‐ | 177/139 | 28 | 28 | 23/11 | 23 | 82.1 | 3 months | |
| I2: desmopressin 25 µg | ‐ | 25 | 25 | 22/11 | 22 | 88.0 | |||
| I3: desmopressin 50 µg | ‐ | 29 | 29 | 21/10 | 21 | 72.4 | |||
| I4: desmopressin 100 µg | ‐ | 30 | 30 | 23/11 | 23 | 76.6 | |||
| C: placebo | ‐ | 27 | 27 | 23/11 | 23 | 85.1 | |||
| Total: | 139 | 139 | 112/54 | 112 | 80.5 | ||||
| Overall total | Men | ‐ | ‐ | ‐ | 2966 | ‐ | |||
| Women | ‐ | 1045 | |||||||
| Total: | 4195 | 4011 | |||||||
| ‐ denotes not reported; C: comparator; I: intervention; ITT: intention‐to‐treat; n: number of participants. 1Follow‐up under randomised conditions until end of trial or if not available, duration of intervention; extended follow‐up refers to follow‐up of participants once the original study was terminated as specified in the power calculation. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| 1.1 Intranasal | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 1.2 Sublingual | 3 | 851 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.71, ‐0.03] |
| 1.3 Oral | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.41, ‐0.86] |
| 2 Number of nocturnal voids (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.17, ‐0.53] |
| 3 Major adverse events (short term) (subgroup route and dose) Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.10, 9.03] |
| 3.1 Sublingual/low dose | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| 3.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| 4 Major adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| 5 Duration of first sleep episode (short term) Show forest plot | 4 | 652 | Mean Difference (IV, Random, 95% CI) | 54.61 [13.97, 95.25] |
| 6 Duration of first sleep episode (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 18.40 [11.60, 25.20] |
| 7 Time to first void (short term) [minutes] Show forest plot | 1 | 383 | Mean Difference (IV, Random, 95% CI) | 40.8 [17.07, 64.53] |
| 8 Minor adverse events (short term) Show forest plot | 3 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 9 Minor adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.49] |
| 10 Treatment withdrawal due to adverse event (short term) Show forest plot | 4 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.15] |
| 11 Treatment withdrawal due to adverse event (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| 12 Number of nocturnal voids (short term) (subgroup dose) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.98, ‐0.33] |
| 12.1 Very low dose (≤ 1.5 μg) | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 12.2 Low dose (< 100 μg) | 3 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.72, 0.06] |
| 12.3 High dose (≥ 100 μg) | 4 | 401 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.41, ‐0.66] |
| 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| 13.1 Men with nocturia | 5 | 1867 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.73, ‐0.17] |
| 13.2 Men with nocturia and nocturnal polyuria | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.64, ‐0.92] |
| 14 Number of nocturnal voids (short term) (sensitivity run‐in period) Show forest plot | 4 | 966 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.17, ‐0.14] |
| 15 Major adverse events (short term) (sensitivity run‐in period) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| 16 Number of nocturnal voids (short term) (sensitivity clinical dosage) Show forest plot | 6 | 1586 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.15, ‐0.38] |
| 17 Major adverse events (short term) (sensitivity clinical dosage) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.71] |
| 17.1 Sublingual/low dose | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.71, 10.11] |
| 17.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of first sleep episode (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 90.0 [1.95, 178.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.20, 0.80] |
| 2 Quality of life (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.35, 0.35] |
| 3 Major adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Minor adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.07, 15.57] |
| 5 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 1.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.90, ‐0.42] |
| 1.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.60, ‐0.02] |
| 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 2.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.74, 0.14] |
| 2.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.86, ‐0.18] |
| 3 Quality of life (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 3.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.49, 0.03] |
| 3.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.85, ‐0.03] |
| 4 Quality of life (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 4.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.84, 0.44] |
| 4.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.67, ‐0.01] |
| 5 Major adverse events (short term) (subgroup route and dose) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 5.1 Sublingual/low dose | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Oral/high dose | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 6 Major adverse events (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 6.1 Men with nocturia | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Men with nocturia and nocturnal polyuria | 2 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 7 Minor adverse events (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.15, 16.82] |
| 8 Treatment withdrawal due to adverse event (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.46, 17.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.97, 0.11] |
| 2 Major adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Minor adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |
| 4 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |

