Desmopressin untuk merawat kerap kencing malam pada lelaki
Abstract
Background
Nocturia is the bothersome symptom of awakening one or more times per night to void. Desmopressin is a commonly used medication for treating nocturia.
Objectives
To assess the effects of desmopressin as compared to other interventions in the treatment of nocturia in men.
Search methods
We performed a comprehensive search of the medical literature with no restrictions on the language of publication or publication status. The date of the latest search of all databases was August 2017.
Selection criteria
We included randomised or quasi‐randomised trials. Inclusion criteria were men with nocturia defined as one or more voids per night. We excluded trials of children or adults with primary or secondary enuresis or underlying distinct disorders.
Data collection and analysis
Two review authors independently classified studies and abstracted data from the included studies. We performed statistical analyses using a random‐effects model and interpreted data according to the Cochrane Handbook for Systematic Reviews of Interventions. We compared desmopressin with placebo, behavioural modification, alpha‐blockers, in combination with alpha‐blockers against alpha‐blockers alone, and in combination with alpha‐blocker against alpha‐blocker plus anticholinergic. Our outcomes were mean number of voids, quality of life, adverse events, and sleep disturbance.
Main results
We included 14 studies with 2966 randomised men across five comparisons. The studies recruited from urology outpatients clinics and defined nocturia as two or more voids per night. The average age of study participants ranged between 57 and 74 years.
Desmopressin versus placebo: based on short‐term follow‐up (up to three months), desmopressin may result in a small, possibly unimportant effect on the number of nocturnal voids (mean difference (MD) ‐0.61, 95% confidence interval (CI) ‐0.96 to ‐0.27; low‐quality evidence). We are uncertain about the effect of desmopressin on major adverse events at short‐term follow‐up (risk ratio (RR) 0.97, 95% CI 0.10 to 9.03; very low‐quality evidence). For intermediate‐term follow‐up (three to 12 months), desmopressin may reduce the number of nocturnal voids in an appreciable number of men (MD ‐0.85, 95% CI ‐1.17 to ‐0.53; low‐quality evidence). There was one major adverse event in the desmopressin group at intermediate‐term follow‐up in one trial of 115 men (RR 3.05, 95% CI 0.13 to 73.39 for both outcomes; low‐quality evidence). We found no evidence on quality of life. Subgroup analyses suggest a larger effect on nocturnal voiding with oral, higher‐dose formulations of desmopressin and in men with documented nocturnal polyuria.
Desmopressin versus behaviour modification: there were no data regarding the effect on the number of nocturnal voids, quality of life, or major adverse events.
Desmopressin versus alpha‐blocker: based on short‐term follow‐up in one small trial, desmopressin likely has a similar effect on the number of nocturnal voids (MD 0.30, 95% CI ‐0.20 to 0.80; moderate‐quality evidence) and quality of life measured on the International Prostate Symptom Score (IPSS) scale (MD 0.00, 95% CI ‐0.35 to 0.35; moderate‐quality evidence). There were no major adverse events in either group.
Desmopressin plus alpha‐blocker versus alpha‐blocker alone: based on short‐term follow‐up, combination therapy likely results in a small, unimportant effect on the number of nocturnal voids (MD ‐0.47, 95% CI ‐0.73 to ‐0.21; moderate‐quality evidence) and quality of life (MD ‐0.29, 95% CI ‐0.51 to ‐0.07; moderate‐quality evidence). The risk of major adverse events may be similar (0.5% versus 0.3%; RR 0.30, 95% CI 0.01 to 7.32; low‐quality evidence).
Desmopressin plus alpha‐blocker versus alpha‐blocker plus an anticholinergic: based on short‐term follow‐up, combination therapy likely results in little or no difference in the number of nocturnal voids (MD ‐0.43, 95% CI ‐0.97 to 0.11; moderate‐quality evidence). We found no evidence on quality of life or sleep duration. There were no major adverse events in either study group.
Authors' conclusions
Desmopressin may reduce the number of nocturnal voids by a small amount compared to placebo in intermediate‐term (three to 12 months) follow‐up without increasing major adverse events. We found insufficient evidence to determine the effects of desmopressin when compared with behaviour modification. The effect on the number of nocturnal voids in the short term is likely to be similar to that of alpha‐blockers, with very infrequent major adverse events. There appears to be little added benefit in the combined use of desmopressin with an alpha‐blocker. The findings of this review were limited by short‐term follow‐up, study limitations, and imprecision.
PICO
Ringkasan bahasa mudah
Desmopressin untuk merawat kerap kencing malam pada lelaki dengan gejala saluran kencing yang lebih rendah
Soalan ulasan
Adakah desmopressin berkesan untuk merawat kerap kencing malam pada lelaki?
Latar belakang
Kerap kencing malam adalah gejala yang mengganggu untuk bangun satu atau lebih kali setiap malam untuk kencing. Ia biasa berlaku pada lelaki yang lebih berusia. Lebih separuh daripada lelaki berusia lebih dari 70 tahun menderitai kencing malam dan terdapat banyak punca untuk ini. Desmopressin adalah ubat yang mengawal jumlah dan kepekatan air kencing dalam badan. Ia juga digunakan untuk merawat kerap kencing malam.
Ciri‐ciri kajian
Carian adalah terkini sehingga 1 Ogos 2017. Penyelidik telah mengenal pasti 14 kajian klinikal, melibatkan 2966 lelaki. Penyelidik membandingkan desmopressin sahaja atau digabungkan dengan ubat lain yang digunakan untuk masalah kencing (seperti penghalang alpha atau antikolinergik) terhadap plasebo, perubahan tingkah laku atau ubat‐ubatan yang digunakan untuk masalah kencing sahaja atau gabungan. Kebanyakan kajian hanya mendaftarkan lelaki yang lebih berusia.
Keputusan utama
Rawatan dengan desmopressin untuk tiga hingga 12 bulan boleh mengurangkan kekerapan lelaki kencing pada waktu malam dengan jumlah yang agak kecil berbanding dengan plasebo. Kesan sampingan yang serius tidak meningkat. Penyelidik tidak tahu cara pengunaan desmopressin berbanding perubahan dalam tingkah laku. Kesan desmopressin mengenai kekerapan lelaki kencing pada waktu malam adalah mungkin serupa dengan penghalang alpha apabila diberi sehingga tiga bulan (iaitu rawatan jangka pendek) tanpa kesan sampingan yang besar. Menambah desmopressin kepada alpha‐penyekat nampaknya lebih berkesan berbanding daripada alpha‐penyekat sahaja atau alpha‐blocker digabungkan dengan anticholinergic.
Kualiti bukti
Penyelidik menilai kualiti bukti untuk menjadi rendah pada kebanyakan kes, bermaksud bahawa penyelidik tidak dapat sepenuhnya mempercayai hasilnya. Kajian yang disertakan dirancang dengan kurang baik, kecil, dan hanya mengikuti orang untuk waktu yang singkat (biasanya tiga bulan atau kurang).
Authors' conclusions
Summary of findings
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 1982 | ⊕⊕⊝⊝ | ‐ | The mean number of nocturnal voids ranged from 1.9 to 4.57. | MD 0.61 lower |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 536 | ⊕⊝⊝⊝ | RR 0.97 | Study population | |
| 29 per 1000 | 1 fewer per 1000 | ||||
| Duration of first sleep episode | 652 | ⊕⊝⊝⊝ | ‐ | The mean duration of first sleep episode ranged from 26.21 to 174 minutes. | MD 54.61 minutes higher |
| Time to first void | 383 | ⊕⊕⊝⊝ | ‐ | The mean time to first void was 72.9 minutes. | MD 40.8 minutes higher |
| Minor adverse event | 594 | ⊕⊕⊝⊝ | RR 0.87 | Study population | |
| 257 per 1000 | 33 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 614 | ⊕⊕⊝⊝ | RR 1.10 | Study population | |
| 49 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in at least 50% of the studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 115 | ⊕⊕⊝⊝ | ‐ | Mean number of nocturnal voids was 4.14 voids. | MD 0.85 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode | 115 | ⊕⊕⊕⊝ | ‐ | Mean duration of first sleep episode was 101.6 minutes. | MD 18.4 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 115 | ⊕⊕⊝⊝ | RR 0.86 | Study population | |
| 328 per 1000 | 46 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: fluid restriction during nighttime | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with behaviour modification | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Duration of first sleep episode | 60 | ⊕⊕⊝⊝ | ‐ | Mean duration of first sleep episode was 150 minutes. | MD 90 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment withdrawal due to adverse event ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias in almost all domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 31 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 1.2 voids. | MD 0.3 voids more |
| Quality of life | 31 | ⊕⊕⊕⊝ | ‐ | Mean quality of life was 1.8 bothersome. | MD 0 |
| Major adverse events | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 31 | ⊕⊝⊝⊝ | RR 1.07 | Study population | |
| 63 per 1000 | 4 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: alpha‐blocker alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids assessed with: voiding diary | 341 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids ranged from 1.68 to 2.6. | MD 0.47 voids fewer |
| Quality of life assessed with: IPSS and N‐QoL | 341 | ⊕⊕⊕⊝ | ‐ | Mean quality of life ranged from 1.53 to 4.4. | MD 0.29 lower |
| Major adverse events | 402 | ⊕⊕⊝⊝ | RR 0.30 | Study population | |
| 5 per 1000 | 3 fewer per 1000 | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 402 | ⊕⊝⊝⊝ | RR 1.60 | Study population | |
| 80 per 1000 | 48 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 402 | ⊕⊕⊝⊝ | RR 2.84 | Study population | |
| 5 per 1000 | 9 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: anticholinergic + alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker + anticholinergic | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids | 405 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 6.97 voids. | MD 0.43 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 427 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
Background
Description of the condition
Nocturia is defined as waking at night one or more times to pass urine with each void being preceded and followed by sleep. This is distinct from nocturnal enuresis, which is defined as voiding during sleep (Van Kerrebroeck 2002). Although somewhat controversial, clinically significant nocturia is defined as one or more voids per night (Abrams 2002). Nocturia is one of the most bothersome lower urinary tract symptoms (LUTS), with a correlation between degree of severity and increased bother (Bliwise 2009).
Pathophysiology
Nocturia occurs when nocturnal urine volume exceeds the maximal voiding volume, which is reflective of the functional bladder capacity (Bosch 2013; Cornu 2012). The maximal voiding volume may be different at night than it is during the day. Nocturia has both urologic and non‐urologic causes that are behavioural, physiological, or pathologic in nature. Overall, the causes fall into four main categories: reduced bladder capacity secondary to anatomical or functional factors, which may or may not only occur at night; overall increase in urine production (24‐hour polyuria); nocturnal polyuria; or any primary or secondary sleep disorder (Bosch 2013; Cornu 2012). Benign prostatic hyperplasia (BPH) contributes to, but is not the sole cause of, LUTS, including nocturia. Benign prostatic hyperplasia can cause bladder outlet obstruction, which may induce secondary bladder overactivity and reduction in functional bladder capacity, resulting in nocturia (Yoshimura 2003). Men may also experience nocturia with or without detrusor overactivity or postvoid residual urine (Berges 2014).
Epidemiology
Nocturia can affect any population at any age, however its incidence generally increases with age. Nocturia defined as one or more micturitions at night affects 20.4% to 43.9% of men aged 20 to 40 years and 68.9% to 93% in men older than 70 years (Bosch 2013).
Risk factors
Risk factors for nocturia are dependent upon the underlying causes, which can be overlapping. General risk factors include advancing age, higher body mass index, alcohol consumption, smoking, hypertension, cardiovascular disease, cerebrovascular disease, diabetes mellitus, and metabolic syndrome (Yoshimura 2003). Men with LUTS suggestive of BPH are more susceptible to nocturia than are men without LUTS. Advancing age, smaller functional bladder capacity, increased nocturnal urine volume, and severity of urgency are predictors of frequent nocturnal voiding (Yoshimura 2003). Overall, age is considered the most important risk factor (Yoshimura 2012).
Diagnosis
Initial assessment of nocturia includes patient history, review of current medications, physical exam including a digital rectal examination in men, urinalysis, measurement of postvoid residual volumes, and validated symptom questionnaires such as the Nocturia‐Quality of Life questionnaire (N‐QoL) and the International Prostate Symptom Score (IPSS) (Abraham 2004). Other tests that may be necessary include uroflowmetry, urodynamic evaluation of bladder function, cystoscopy, bladder biopsies, sleep laboratory evaluation, and advanced cardiology tests (Oelke 2014a). A potential dilemma in diagnosing nocturia in men is that it is often not the only symptom mentioned during medical consultations, and it is rarely the symptom that prompted seeking medical evaluation. Its evaluation is therefore often limited to patient‐reported numbers of voids per night as an item on the IPSS and subjective history. The International Continence Society encourages the use of the frequency volume chart, an important diagnostic tool that involves the person recording the time and volume of all voids as well as incontinence episodes and the number of incontinence pads used over a specific time, which is usually seven days (Van Kerrebroeck 2002).
Treatment
Treatment is dependent upon the cause of nocturia as well as the person's individual treatment goals. Goals that are cited as being important include decreasing the number of nocturnal voiding episodes, prolongation of undisturbed sleep to greater than four hours, and improvement of sleep quality (Oelke 2014a). Treatment for nocturia can be divided into behavioural modification, medical management, and surgical modalities.
Behavioural treatment
Although treatment should be centred on the cause of nocturia, behavioural modifications appear to be universally beneficial regardless of underlying aetiology. Lifestyle modification is therefore often used as first‐line therapy (Van Kerrebroeck 2010). Behavioural treatments include restriction of fluids before going to sleep, avoiding caffeinated or alcoholic beverages in the evening, pre‐emptive voiding immediately before going to bed, strategic medication timing, leg elevation in the case of lower extremity oedema, moderate physical exercise, and keeping warm in bed. Lifestyle modifications have been associated with up to 50% of people reporting improvement in nocturia (Soda 2010).
Medical management
Medical management includes therapy with a single drug or a combination of drugs from different classes. Nocturia has historically been considered a symptom secondary to underlying bladder or prostate dysfunction, therefore therapies have been targeted towards these conditions. Current therapies include antimuscarinics, alpha‐1 adrenergic receptor blockers, 5‐alpha‐reductase inhibitors, phosphodiesterase type 5 inhibitors, anti‐inflammatory drugs, and desmopressin.
The use of alpha‐1 adrenergic receptor blockers and 5‐alpha‐reductase inhibitors are in the context of BPH management, with only a few studies focusing on nocturia as a primary outcome. Overall, the evidence supporting the efficacy of alpha‐1 adrenergic receptor blockers and 5‐alpha‐reductase inhibitors in treating nocturia is low with inconsistent evidence that at best accounts for minor improvements (Schneider 2009). In one large study analysing 3047 men randomised to one of four groups (doxazosin, finasteride, doxazosin plus finasteride, and placebo), the doxazosin and doxazosin plus finasteride groups showed statistically significant mean reductions in the number of nocturia episodes by 0.77 for doxazosin and 0.80 for doxazosin plus finasteride at one year (Johnson 2007). The placebo group had a decrease in the mean number of nocturia episodes by 0.61 and the finasteride group had a decrease of 0.60 at one year. These results were modest, and whether they are clinically significant is highly questionable.
Tadalafil is a phosphodiesterase type 5 inhibitor that also has approval for treatment of LUTS/BPH. Integrated data from four randomised, placebo‐controlled studies investigating the use of daily tadalafil for nocturia found a mean decrease in the number of episodes of 0.4 in the placebo group and 0.5 in the tadalafil group (Oelke 2014b). While the difference between groups was statistically significant, the difference was very small and unlikely to be indicative of a clinically meaningful improvement.
Most studies on antimuscarinics such as solifenacin and tolterodine are in the context of overactive bladder management, and evidence that they are effective for the specific management of nocturia is limited. The clinical impact of these agents is also questionable, as they result in a reduction of half a void or less per night (Smith 2011). The population that may benefit most from these agents are people with severe overactive bladder who have frequent nighttime awakenings associated with urgency.
Few studies have been done on anti‐inflammatory drugs for nocturia. One study evaluating celecoxib in men with BPH with refractory nocturia showed promising results (Falahatkar 2008). Men in the active treatment group had a statistically significant decrease in nocturnal frequency from (mean ± standard deviation (SD)) 5.17 ± 2.1 episodes to 2.5 ± 1.9 episodes, a dramatic response that is better than any other treatment modality. However, this study included only 80 men and was for a period of only four weeks. Without more reports with longer follow‐up and larger study populations, the evidence to support the use of anti‐inflammatory drugs for nocturia in men remains unclear.
Surgical management
Surgical treatment may be appropriate for men with nocturia due to LUTS related to benign prostatic obstruction, although no surgical therapy is specifically indicated for nocturia (Cornu 2012). Surgical options to reduce prostatic obstruction include transurethral resection of the prostate, transurethral incision of the prostate, transurethral microwave therapy of the prostate, and prostatectomy. Transurethral resection of the prostate has been associated with a one‐point reduction in nocturia, which correlates to one less nightly episode (Wada 2014; Yoshimura 2003). Prostatectomy has been associated with a decrease in nighttime voiding frequency of 0.8 (mean ± SD): from 3.4 ± 1.2 episodes to 2.6 ± 0.99 episodes (Margel 2007). Other interventions such as botulinum toxin detrusor injection, sacral neuromodulation, or tibial electric nerve stimulation are used in the context of overactive bladder where nocturia is regarded a secondary outcome (Cornu 2012).
Description of the intervention
Desmopressin is the synthetic analogue of the human hormone vasopressin, which has been used clinically in a variety of formulations since 1974. Vasopressin, also known as antidiuretic hormone, is produced by the posterior pituitary gland, and its role is to maintain serum osmolality and volume via modulation of free water excretion. Vasopressin is released in states of hyperosmolality and hypovolaemia, which is detected by chemoreceptors and baroreceptors located in the hypothalamus and carotid sinus, respectively. It acts on the V2 receptors in the distal collecting tubules, which subsequently results in translocation of aquaporin channels associated with cytosolic vesicles to the apical membrane of collecting duct cells. Free water is then passively reabsorbed from the nephron back into the systemic circulation via basolateral membrane channels. Vasopressin also plays a minor role in increasing systemic vascular resistance and increasing urea reabsorption in the medullary collecting tubule (Shoskes 2011). It is the most frequently tested medication for the specific treatment of nocturia, but has traditionally been used to treat central diabetes insipidus, bleeding disorders such as Von Willebrand disease, and primary nocturnal enuresis. The US Food and Drug Administration (FDA) approved desmopressin acetate nasal spray with the trademark name of NOCTIVA in March 2017 for nocturia due to nocturnal polyuria in adults who awaken at least twice per night to void (Serenity Pharmaceuticals 2017).
Adverse events of the intervention
The most frequently encountered adverse events include headache, hyponatraemia, insomnia, dry mouth, hypertension, abdominal pain, peripheral oedema, and nausea (Friedman 2013).
How the intervention might work
Desmopressin is effective in the treatment of polyuric states, and nocturnal polyuria is very common in people with nocturia (as high as 82.9%) (Chang 2006). Desmopressin may help alleviate nocturnal voiding by inducing antidiuresis resulting in increased urine osmolality and decreased urine output.
Why it is important to do this review
Nocturia may decrease quality of life and is associated with a high degree of bother. Reduced sleep due to nocturia has been associated with diurnal fatigue, decreased concentration, lower performance at work, and accidents from cognitive and motor impairment (Asplund 2005; Chartier‐Kastler 2006). In the elderly population, nocturia is associated with an increased risk of bone fractures due to nighttime falls (Nakagawa 2010). Although various treatment modalities have been used to treat LUTS successfully, nocturia remains one of the most elusive and problematic symptoms.
There are also inherent risks in recommending a medication to treat a symptom if the underlying disease process is not thoughtfully considered. Desmopressin can also cause hyponatraemia, which is often asymptomatic but in limited cases can have deleterious effects. With regard to assessing efficacy, a common outcome measure is the decrease in the number of nocturnal voids, however there is no existing consensus on its clinical significance. To better assess clinically meaningful efficacy it is therefore crucial to critically analyse and synthesise the symptoms, quality of life measures, and sleep parameters in existing clinical trials (Cornu 2012).
One systematic review on this topic exists (Ebell 2014). However, no review has used GRADE to assess the quality of evidence supporting the use of desmopressin for the treatment of nocturia in men. Given the modest efficacy and questionable clinical significance of the multiple medical and surgical treatments for nocturia in men, such an investigation into the effects of desmopressin based on the totality of available evidence as summarised in a rigorous systematic review is critically important. In an era of rising health costs and an increased emphasis on evidence‐based medicine, the findings of this review will be especially relevant to policymakers and healthcare providers. Furthermore, as a drug can gain regulatory approval based on statistical significance, it is important to know that this does not automatically mean that the drug has a clinically significant impact. This is of particular concern when the effect size is small or when the potential adverse events are considerable, or both (Fralick 2017).
Objectives
To assess the effects of desmopressin as compared to other interventions in the treatment of nocturia in men.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised trials regardless of their publication status or language of publication.
Types of participants
Men with nocturia defined as one or more voids per night (Abrams 2002). We excluded trials of children or adults with primary or secondary enuresis or underlying distinct disorders such as Cushing disease, multiple sclerosis, or autonomic dysfunction.
We mostly included studies focused on this specific population of participants. In addition, we included studies in which a subset of participants met our inclusion criteria, if relevant data were reported separately to permit data analysis.
Types of interventions
We planned to investigate the following comparisons of experimental intervention versus comparator intervention.
Experimental interventions
-
Desmopressin.
-
Desmopressin plus other drug treatment for LUTS.
Comparator interventions
-
Placebo.
-
Behavioural modifications.
-
Drug treatments for LUTS.
-
Surgery for LUTS.
Comparisons
-
Desmopressin versus placebo.
-
Desmopressin versus behavioural modification.
-
Desmopressin versus drug treatment for LUTS.
-
Desmopressin plus other drug treatment for LUTS versus drug treatment for LUTS alone.
-
Desmopressin versus surgery for LUTS.
We required that concomitant interventions be the same in the experimental and comparator groups to establish fair comparisons.
Types of outcome measures
We did not use the measurement of the outcomes specified in this review as an eligibility criterion.
Primary outcomes
-
Number of nocturnal voids.
-
Quality of life.
-
Major adverse events.
Secondary outcomes
-
Duration of first sleep episode.
-
Time to first void.
-
Minor adverse events.
-
Treatment withdrawal due to adverse event.
Method and timing of outcome measurement
We used clinically important differences (CID) for each outcome to rate the quality of the evidence in the 'Summary of findings' tables (Johnston 2010).
Number of nocturnal voids
-
Measured by voiding diary (final value or change from baseline).
-
Minimal clinically important differences (MCIDs) for outcomes when treating nocturia have not been defined. We considered improvement of nocturia of at least one episode per night as CID for nocturia (Krader 2012).
Quality of life
-
Assessed using condition‐specific, validated health measures (such as IPSS and N‐QoL; final value or change from baseline).
-
We used an MCID of one point for IPSS‐QoL to assess efficacy and comparative effectiveness (Brasure 2016). While no thresholds were established for N‐QoL and Impact of Nighttime Urination, we used a CID of eight points for both questionnaires to assess efficacy and comparative effectiveness (Serenity Pharmaceuticals 2016).
Major adverse events
-
Such as symptomatic hyponatraemia, arrhythmia, need for hospital admission, respiratory insufficiency.
Duration of first sleep episode
-
Measured by voiding diary (final value or change from baseline).
Time to first void
-
Measured by voiding diary (final value or change from baseline).
Minor adverse events
-
Such as asymptomatic hyponatraemia requiring treatment, headache, nausea, insomnia, dry mouth, hypertension, abdominal pain, and peripheral oedema.
Treatment withdrawal due to adverse event
-
Defined as treatment discontinuation from any cause at any time after men were randomised to intervention/comparator groups.
The CID for the duration of first sleep episode, time to first void, major and minor adverse events, and treatment withdrawal due to adverse event have not been defined. We considered improvement of at least one hour per night as the CID for duration of first sleep episode and time to first void. We considered the CID for major and minor adverse events, and treatment withdrawal due to adverse event as a relative risk reduction of at least 25% (Guyatt 2011a).
We separately considered outcomes measured up to three months (short term), more than three months up to 12 months (intermediate term), and more than 12 months (long term).
Main outcomes for 'Summary of findings' tables
We presented 'Summary of findings' tables for the following outcomes.
-
Number of nocturnal voids.
-
Quality of life.
-
Major adverse events.
-
Duration of first sleep episode.
-
Time to first void.
-
Minor adverse events.
-
Treatment withdrawal due to adverse event.
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status.
Electronic searches
We searched the following sources from inception of each database. We initially searched the following sources from inception to 22 June 2015. The date of last search of all databases was 1 August 2017.
-
The Cochrane Library (from 1991 via Wiley for the search strategy, see Appendix 2);
-
Cochrane Database of Systematic Reviews (CDSR);
-
Cochrane Central Register of Controlled Trials (CENTRAL);
-
Database of Abstracts of Reviews of Effects (DARE);
-
Health Technology Assessment (HTA) Database.
-
-
MEDLINE (from 1946 via Ovid; Appendix 3).
-
EMBASE (from 1974 via Ovid; Appendix 4).
-
Scopus (from 1966; Appendix 5).
-
Google Scholar (Appendix 6).
-
Web of Science (from 1900; Appendix 7).
-
Western Pacific Region Index Medicus (from 1950; Appendix 8).
We also searched the following clinical trial registries. The date of last search of all clinical trial registries was 1 August 2017.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; Appendix 9).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/; Appendix 10).
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We also contacted study authors of included trials to identify any further studies that we may have missed.
We contacted Ferring Pharmaceuticals, the sponsor of several trials, for ongoing or unpublished trials. We also searched for unpublished studies by handsearching the abstract proceedings of the annual meetings of the American Urological Association, European Association of Urology, International Continence Society, and the British Association of Urological Surgeons from 2012 to 2017.
Data collection and analysis
Selection of studies
We used reference management software to identify and remove potential duplicate records (EndNote). Two review authors (JH, JHJ) independently scanned the abstract, title, or both, of the remaining records to determine which studies should be assessed further in Covidence. In the second stage, two review authors (JH, JHJ) investigated all potentially relevant records as full text; mapped records to studies; and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any discrepancies were resolved through consensus or recourse to a third review author (PD). We documented reasons for exclusion of studies that may have reasonably been expected to have been included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data abstraction form that we pilot tested ahead of time.
Two review authors (JH, JHJ) independently abstracted the following information for studies that fulfilled our inclusion criteria, which we summarised in the Characteristics of included studies table.
-
Study design.
-
Study dates (if dates were not available, we reported this as such).
-
Study settings and country.
-
Participant inclusion and exclusion criteria.
-
Participant details, baseline demographics.
-
Number of participants by study and study arm.
-
Details of relevant experimental and comparator interventions such as desmopressin dose, route, frequency, and duration.
-
Definitions of relevant outcomes, and method and timing of outcome measurement as well as any relevant subgroups.
-
Study funding sources.
-
Declarations of interest by primary investigators.
We extracted outcomes data relevant to this review as needed for the calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for completion of a two‐by‐two table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and SDs or data needed to calculate this information.
Any disagreements were resolved by discussion or by consulting a third review author (PD).
We attempted to contact authors of included studies to obtain key missing data as needed and documented these communications.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximised the yield of information by mapping all publications to unique studies and collating all available data using the most complete data set aggregated across all known publications.
Assessment of risk of bias in included studies
Two review authors (JHJ, PD) independently assessed the risk of bias of each included study on a per‐outcome basis. We resolved all disagreements by discussion and consensus.
We assessed risk of bias using the Cochrane 'Risk of bias' assessment tool employing the following domains (Higgins 2011b).
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other sources of bias.
We judged 'Risk of bias' domains as 'low risk', 'high risk', or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We presented a 'Risk of bias' summary figure to illustrate these findings.
For selection bias (random sequence generation and allocation concealment) and reporting bias (selective reporting), we evaluated risk of bias at the level of the trial.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome. We considered all outcomes similarly susceptible to bias and assessed them as one group.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis. Since judgements were identical across some outcomes, we collapsed these ratings into groups when reporting our findings in the 'Risk of bias' tables.
We further summarised the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. We identified no cluster‐randomised trials. For cross‐over trials, we only considered data up to the time point that cross‐over occurred in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We sought to obtain missing data from study authors and to perform intention‐to‐treat analyses if data were available; we otherwise performed available‐case analyses, which we identified as such. We investigated attrition rates, for example dropouts, losses to follow‐up, and withdrawals, and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we planned not to report outcome results as the pooled effect estimate in a meta‐analysis but to provide a narrative description of the results of each study only.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted the I2 statistic as follows.
-
0% to 40%: may not be important.
-
30% to 60%: may indicate moderate heterogeneity.
-
50% to 90%: may indicate substantial heterogeneity.
-
75% to 100%: considerable heterogeneity.
In the setting of heterogeneity, we planned to determine the possible reasons for the heterogeneity by examining individual study and subgroup characteristics. However, due to a paucity of studies for each comparison, this was not feasible.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting.
For 10 or more studies investigating a particular outcome, we planned to use funnel plots to assess small‐study effects. However, the number of included studies was consistently too low to permit this type of analysis.
Data synthesis
We summarised data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used the Mantel‐Haenszel method for dichotomous outcomes, and the inverse variance method for continuous outcomes. We performed all analyses in Review Manager 5 software (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses with investigation of interactions limited to the primary outcomes.
-
Routes of administration of desmopressin (intranasal versus sublingual versus oral) to evaluate whether the treatment effects depended on the routes of administration. The differences in the systemic absorption of the three administration routes are reflected in the bioavailability (Fransén 2009).
-
Desmopressin dose (less than 10 μg versus 10 μg or greater to less than 100 μg versus 100 μg or greater) to evaluate whether the efficacy of desmopressin depends on the dose. Statistically significant benefits have been seen with doses as low as 25 μg, however a greater magnitude of benefit has been shown with doses of 100 μg or higher with no associated statistically significant increase in adverse events (Ebell 2014).
-
In men with or without nocturnal polyuria to evaluate whether desmopressin has more benefit in men with nocturnal polyuria (Berges 2014).
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses if there were sufficient studies (RevMan 2014).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes limited to the primary outcomes.
-
Restricting the analysis by considering risk of bias, excluding studies at 'high risk' or 'unclear risk'.
-
Restricting the analysis by considering risk of bias, excluding studies with run‐in periods.
-
Restricting the analysis by considering the clinically recommended dosage of desmopressin, excluding lower dose levels.
'Summary of findings' tables
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which considers five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (JH, JHJ) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT (GRADEpro GDT), resolving any discrepancies by consensus, or, if needed, by recourse to a third review author (PD). For each comparison, we presented a summary of the evidence for the main outcomes in 'Summary of findings' tables, which provided key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences, for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011b; Schünemann 2011).
Results
Description of studies
Results of the search
Our comprehensive literature search identified 1524 records that included six records found through the trial registries and the New Drug Applications submitted to the FDA. After removal of duplicates, we screened the titles and abstracts of 1355 records and excluded 1298. We screened 57 full‐text articles and excluded 22 studies (23 articles). Seventeen studies did not meet the inclusion criteria or were not relevant to our review question (Ahmed 2015b; Asplund 1999; Cho 2015; Cho 2016; Fu 2011; Gilbert 2011; Holm‐Larsen 2013a; Kaminetsky 2016; Lam 2017; Malli 2014; Moon 2002; Moon 2003; Shin 2014b; van Kerrebroeck 2007; Wang 2011b; Weiss 2012b; Yassin 2010). We identified four studies awaiting classification (Holm‐Larsen 2013b; NCT01694498; Salvatore 1996; Weiss 2001), and one ongoing trial (NCT02904759). Fourteen studies (34 articles) ultimately met the inclusion criteria and were included in the qualitative synthesis of this review (Ahmed 2015a; Cannon 1999; Ceylan 2013; Kim 2017; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Shin 2014a; Wang 2011a; Wang 2012; Weiss 2012a; Weiss 2013; Yamaguchi 2013). The flow of literature through this assessment process is shown in the PRISMA flowchart (Figure 1).
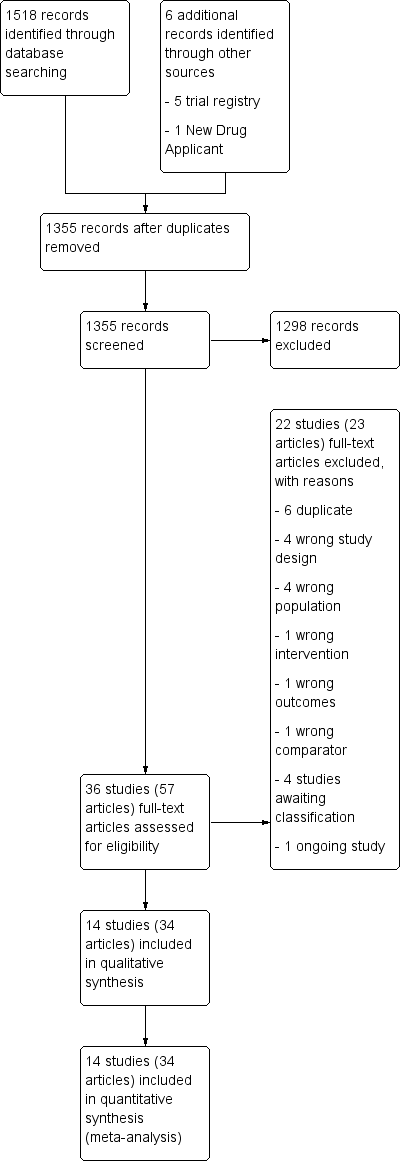
PRISMA flow diagram.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies table; Table 1; Table 2).
| Study | Setting | Trial period | Description of participants | Intervention(s) and comparator(s) | Duration of intervention |
| Outpatient/Egypt | 2011 to 2014 | People with LUTS/BPH aged ≥ 50 years with nocturia (≥ 2 voids/night), nocturnal polyuria (nocturnal urine volume > 30% of 24‐hour urine volume) | I: desmopressin 60 μg ODT + tamsulosin | 3 months | |
| C: tamsulosin | |||||
| UK | NR | Men aged > 50 years with nocturnal polyuria (using 48‐hour inpatient monitoring or 1‐week frequency volume chart) | I: desmopressin nasal spray 20 μg | 4 weeks | |
| C: placebo | |||||
| Outpatient/Turkey | 2011 | Men with advanced age, complaints of LUTS and nocturia (≥ 3 times/night) | I: desmopressin nasal spray 20 μg | 2 months | |
| C: doxazosin | |||||
| Multicentre/South Korea | NR | Men aged 40 to 65 years with LUTS (IPSS > 13), nocturia (≥ 2 episodes/night), and nocturnal polyuria (NPI > 33%) | I: desmopressin 0.2 mg oral + alpha‐blocker | 8 weeks | |
| C: placebo + alpha‐blocker | |||||
| Outpatient/Turkey | NR | Men aged 50 to 70 years with LUTS and nocturia (≥ 2/night) | I: desmopressin 0.2 mg oral with alfuzosin | 3 months | |
| C: alfuzosin | |||||
| Multicentre/Denmark, | NR | Men aged ≥ 18 years with nocturia (2 voids/night, nocturia index scores > 1) | I: desmopressin 0.1 mg/0.2 mg/0.4 mg oral; dose titration | 3 weeks | |
| C: placebo | |||||
| Outpatient/single centre/Iran | 2008 to 2009 | Older men (mean age about 63 to 64 years) with voiding ≥ 2/night | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/USA and Canada | NR | Men or women aged ≥ 50 years with nocturia (≥ 2 nocturic episodes/night) | I: desmopressin nasal spray 0.75 μg, 1.0 μg, or 1.5 μg | 12 weeks | |
| C: placebo | |||||
| South Korea | 2010 to 2013 | Men aged ≥ 50 years with LUTS due to bladder outlet obstruction (Qmax ≤ 15 mL/second, IPSS ≥ 14) and nocturia (≥ 1 void/night) | I: desmopressin 0.2 mg oral + tamsulosin | 4 weeks | |
| C: solifenacin + tamsulosin | |||||
| Single centre/Taiwan | 2007 to 2009 | Men aged ≥ 65 years with BPH (IPSS > 13), nocturia (≥ 2 voids/night), and nocturnal polyuria (nocturnal urine volume > 30%) | I: desmopressin 0.1 mg oral | 12 months | |
| C: placebo | |||||
| Outpatient/single centre/China | 2009 to 2010 | Older men (age not reported) | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2007 to 2008 | Men and women aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2010 to 2013 | Men aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 50 μg, 75 µg ODT | 3 months | |
| C: placebo | |||||
| Multicentre/Japan | 2010 to 2011 | Men and women aged 55 to 75 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo |
BPH: benign prostatic hyperplasia; C: comparator; I: intervention; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; NPI: nocturnal polyuria index; NR: not reported; ODT: orally disintegrating tablet; Qmax: maximum flow rate.
| Intervention(s) and comparator(s) | Sample size | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| I: desmopressin + tamsulosin | 100 | 397/273 | 139 | 123 | 123 | 107 | 77.0 | 3 months | |
| C: tamsulosin | 100 | 134 | 125 | 125 | 103 | 76.9 | |||
| Total: | 273 | 248 | 248 | 210 | 76.9 | ||||
| I: desmopressin | ‐ | ‐/‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 8 weeks (cross‐over study design) | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |||
| Total: | 20 | ‐ | 18 | 18 | 90.0 | ||||
| I: desmopressin | ‐ | 84/31 | 15 | 15 | 15 | 15 | 100.0 | 2 months | |
| C: doxazosin | ‐ | 16 | 16 | 16 | 16 | 100.0 | |||
| Total: | 31 | 31 | 31 | 31 | 100.0 | ||||
| I: desmopressin + alpha‐blocker | ‐ | 121/109 | 57 | 57 | 47 | 47 | 82.4 | 8 weeks | |
| C: placebo + alpha‐blocker | ‐ | 52 | 52 | 39 | 39 | 75.0 | |||
| Total: | 109 | 109 | 86 | 86 | 78.9 | ||||
| I: desmopressin + alfuzosin | ‐ | ‐/49 | ‐ | ‐ | 22 | 22 | ‐ | 3 months | |
| C: alfuzosin | ‐ | ‐ | ‐ | 23 | 23 | ‐ | |||
| Total: | 49 | ‐ | 45 | 45 | 91.8 | ||||
| I: desmopressin | 55 | 341/224 | 86 | 86 | 86 | 81 | 94.2 | 3 weeks | |
| C: placebo | 55 | 65 | 65 | 65 | 62 | 95.4 | |||
| Total: | 151 | 151 | 151 | 143 | 94.7 | ||||
| I: desmopressin | ‐ | 93/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 0.75 μg | ‐ | 3565/1707 | 458 | 448 | 448/252 | 401 | 87.5 | 12 weeks | |
| I2: desmopressin 1.0 μg | ‐ | 188 | 183 | 183/109 | 163 | 86.7 | |||
| I3: desmopressin 1.5 μg | ‐ | 452 | 439 | 439/251 | 387 | 85.6 | |||
| C: placebo | ‐ | 458 | 446 | 446/258 | 408 | 89.0 | |||
| Total: | 1556 | 1516 | 1516/870 | 1359 | 87.3 | ||||
| I: desmopressin + tamsulosin | ‐ | 435/427 | 205 | 205 | 205 | 196 | 95.6 | 8 weeks (cross‐over study design) | |
| C: tamsulosin + solifenacin | ‐ | 222 | 222 | 222 | 209 | 94.1 | |||
| Total: | 427 | 427 | 427 | 405 | 94.8 | ||||
| I: desmopressin | 45 | ‐/136 | NR | ‐ | 57 | 57 | ‐ | 12 months | |
| C: placebo | 45 | NR | ‐ | 58 | 58 | ‐ | |||
| Total: | 126 | ‐ | 115 | 115 | 91.3 | ||||
| I: desmopressin | ‐ | ‐/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 10 µg | ‐ | 1412/799 | 163 | 155 | 155/82 | 144 | 88.3 | 4 weeks | |
| I2: desmopressin 25 µg | ‐ | 158 | 152 | 152/87 | 148 | 93.7 | |||
| I3: desmopressin 50 µg | ‐ | 158 | 148 | 148/77 | 138 | 87.3 | |||
| I4: desmopressin 100 µg | ‐ | 160 | 146 | 146/80 | 135 | 84.4 | |||
| C: placebo | ‐ | 160 | 156 | 156/90 | 145 | 90.6 | |||
| Total: | 799 | 757 | 757/416 | 710 | 88.9 | ||||
| I1: desmopressin 50 µg | 130 | 1013/395 | ‐ | 119 | 119 | 100 | ‐ | 3 months | |
| I2: desmopressin 75 µg | 130 | ‐ | 124 | 124 | 103 | ‐ | |||
| C: placebo | 130 | ‐ | 142 | 142 | 120 | ‐ | |||
| Total: | 395 | 385 | 385 | 323 | 81.8 | ||||
| I1: desmopressin 10 µg | ‐ | 177/139 | 28 | 28 | 23/11 | 23 | 82.1 | 3 months | |
| I2: desmopressin 25 µg | ‐ | 25 | 25 | 22/11 | 22 | 88.0 | |||
| I3: desmopressin 50 µg | ‐ | 29 | 29 | 21/10 | 21 | 72.4 | |||
| I4: desmopressin 100 µg | ‐ | 30 | 30 | 23/11 | 23 | 76.6 | |||
| C: placebo | ‐ | 27 | 27 | 23/11 | 23 | 85.1 | |||
| Total: | 139 | 139 | 112/54 | 112 | 80.5 | ||||
| Overall total | Men | ‐ | ‐ | ‐ | 2966 | ‐ | |||
| Women | ‐ | 1045 | |||||||
| Total: | 4195 | 4011 | |||||||
‐ denotes not reported; C: comparator; I: intervention; ITT: intention‐to‐treat; n: number of participants.
1Follow‐up under randomised conditions until end of trial or if not available, duration of intervention; extended follow‐up refers to follow‐up of participants once the original study was terminated as specified in the power calculation.
2Study included men and women.
Source of data
The literature search identified all 14 trials included in the review. Of the 14 included studies, we found one study by the New Drug Application submitted to the FDA (Serenity Pharmaceuticals 2016), and the remaining studies were published trials. We contacted all trial authors of the included trials except two studies supported by pharmaceutical companies (Serenity Pharmaceuticals 2016; Yamaguchi 2013). We received a reply from six trial authors (Kim 2017; Mattiasson 2002; Rezakhaniha 2011; Shin 2014a; Weiss 2012a; Weiss 2013).
Study design and settings
Two trials were cross‐over randomised controlled trials (Cannon 1999; Shin 2014a). The remaining studies were parallel‐group randomised controlled trials (Ahmed 2015a; Ceylan 2013; Kim 2017; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Wang 2012; Weiss 2012a; Weiss 2013; Yamaguchi 2013). One trial was single‐blinded (Ahmed 2015a). Four trials were reported as double‐blinded, and stated that participants and investigators were blinded (Serenity Pharmaceuticals 2016; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Three trials were reported to be double‐blinded, but it was unclear which groups were actually blinded (Cannon 1999; Mattiasson 2002; Wang 2011a). The lead author of Kim 2017 confirmed that participants and personnel were blinded. Five trials provided no information regarding blinding (Ceylan 2013; Koca 2012; Rezakhaniha 2011; Shin 2014a; Wang 2012). Four trials had run‐in periods (Cannon 1999; Mattiasson 2002; Serenity Pharmaceuticals 2016; Shin 2014a). None of the trials were terminated early (for benefit).
All studies were likely conducted in an outpatient urology clinic setting. Five of 14 trials explicitly stated that the trial was conducted in an outpatient urology clinic setting (Ahmed 2015a; Ceylan 2013; Koca 2012; Rezakhaniha 2011; Wang 2012). Six trials were multicentre studies (Kim 2017; Mattiasson 2002; Serenity Pharmaceuticals 2016; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Seven trials were performed in Asia (Ahmed 2015a; Kim 2017; Rezakhaniha 2011; Shin 2014a; Wang 2011a; Wang 2012; Yamaguchi 2013), and the remaining studies were conducted in Europe and the USA. Trials were performed during the time frame of 1999 to 2016. Thirteen trials were published in English, and one was published in Turkish (Koca 2012), which was translated into English using Google Translate.
Participants
This review included a total of 4195 randomised participants (range 20 to 1556), of which a total of 4011 were subsequently accounted for in the analysis. Of the 4011 participants, 1045 were women (26.0%), as three trials included women (Serenity Pharmaceuticals 2016; Weiss 2012a; Yamaguchi 2013). The ethnic group representation was clearly reported in only one trial (Serenity Pharmaceuticals 2016: 78.4% white; 12.1% African‐American; 2% Asian; 6.6% Hispanic and others). The mean age of the men ranged from 57 to 74 years; four studies did not report age (Cannon 1999; Wang 2012; Weiss 2012a; Yamaguchi 2013). One trial reported the mean prostate volume, which was 45.7 mL for the treatment group and 47.0 mL for the comparator group (Ahmed 2015a). Two trials reported mean prostate specific antigen, which ranged from 1.8 ng/mL to 2.6 ng/mL (Ahmed 2015a; Ceylan 2013). Five studies reported mean baseline IPSS score, peak urinary flow rate, and postvoid residual (Ahmed 2015a; Ceylan 2013; Kim 2017; Koca 2012; Shin 2014a). Mean baseline IPSS score ranged from 12.1 to 24.9. Mean baseline maximum flow rate (Qmax) ranged from 10.3 mL/second to 17.8 mL/second. The mean baseline postvoid residual ranged from 32.9 mL to 61.8 mL. The mean IPSS score after intervention ranged from 6.5 to 23.2. The mean Qmax after intervention ranged from 13.3 mL/second to 22.7 mL/second. The mean postvoid residual after intervention ranged from 14.0 mL to 41.4 mL.
Eleven of 14 studies included participants aged over 40 years, Ahmed 2015a; Cannon 1999; Kim 2017; Koca 2012; Serenity Pharmaceuticals 2016; Shin 2014a; Wang 2011a; Yamaguchi 2013, or with old age (not defined; Ceylan 2013; Rezakhaniha 2011; Wang 2012). Three trials included participants aged over 18 years (Mattiasson 2002; Weiss 2012a; Weiss 2013). Three trials included men and women and reported the men's data separately (Serenity Pharmaceuticals 2016; Weiss 2012a; Yamaguchi 2013).
Major exclusion criteria from trials included prior treatment with desmopressin or other BPH medical therapy within three months, evidence of severe daytime voiding dysfunction, suspicion of bladder outlet obstruction or a urine flow less than 5 mL/second, prior genitourinary surgeries, urinary retention or elevated postvoid residual greater than 250 mL (or both), history of genitourinary malignancies, positive urine cultures, hyponatraemia, uncontrolled diabetes, uncontrolled hypertension, diuretic use, voiding dysfunctions arising from well‐defined causes other than BPH, or polydipsia. For the two of three trials that included women (Serenity Pharmaceuticals 2016; Weiss 2012a), potential for pregnancy, use of a pessary or pelvic prolapse, and presence of an unexplained pelvic mass were exclusion criteria.
Diagnosis
Most studies defined nocturia as two or more voids per night (Ahmed 2015a; Ceylan 2013; Kim 2017; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013), and only one study used one or more voids per night as an inclusion criterion (Shin 2014a). Two trials did not report a definition of nocturia (Cannon 1999; Wang 2012). Four trials only included participants with nocturnal polyuria defined as the overproduction of urine at night (i.e. excess of a third or 30% of total daily urine volume; Ahmed 2015a; Cannon 1999; Kim 2017; Wang 2011a).
Interventions and comparators
Three included studies administered desmopressin as a nasal spray (Cannon 1999; Ceylan 2013; Serenity Pharmaceuticals 2016), while the remaining 11 studies administered an oral or sublingual formulation. Two studies used desmopressin 20 μg nasal spray (Cannon 1999; Ceylan 2013), and one trial used a new formulation of nasal spray submitted for FDA approval with a desmopressin dose ranging from 0.75 μg to 1.5 μg (Serenity Pharmaceuticals 2016). Four trials administered desmopressin as a sublingual agent (orally disintegrating tablet), with a dose range from 10 μg to 100 μg (Ahmed 2015a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). The remaining studies administered desmopressin as an oral agent (dose range from 0.1 mg to 0.4 mg). Three studies included participants given sublingual desmopressin less than the clinical dosage (60 μg) as an experimental group (Weiss 2012a: 10/25/50 μg, Weiss 2013: 50 μg, Yamaguchi 2013: 10/25/50 μg). Ten of 14 studies used desmopressin as monotherapy (Cannon 1999; Ceylan 2013; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Wang 2012; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Four trials investigated the combination of desmopressin with alpha‐blocker therapy (Ahmed 2015a; Kim 2017; Koca 2012; Shin 2014a). One study had dose titration of desmopressin prior to randomisation, and only participants who obtained a 20% reduction in nocturnal diuresis were enrolled in the study (active run‐in; Mattiasson 2002).
Comparators were behaviour modification and four agents, namely placebo, alpha‐blocker, desmopressin plus alpha‐blocker, and desmopressin plus anticholinergic. Placebo was administered in accordance with the frequencies of desmopressin administration (Cannon 1999; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Wang 2012 encouraged liquid restriction during nighttime for participants in the control group. A variety of alpha‐blockers (alfuzosin, doxazosin, and tamsulosin) were administered orally as monotherapy, Ahmed 2015a; Ceylan 2013; Koca 2012, and combination therapy with placebo, Kim 2017, or anticholinergic, Shin 2014a. Alfuzosin (10 mg; Koca 2012), doxazosin (2 mg or 4 mg; Ceylan 2013), and tamsulosin (0.2 mg or 0.4 mg; Ahmed 2015a; Shin 2014a) were used as comparator drug agents. Solifenacin (5 mg; Shin 2014a) was used as a comparator in combination therapy with an alpha‐blocker.
The duration of interventions ranged from three weeks to 12 months. All included studies reported short‐term outcomes across five comparisons. Only one study reported intermediate‐term outcomes for the comparison of desmopressin versus placebo (Wang 2011a). No studies reported long‐term follow‐up.
Comparisons
We included five comparisons in this review, of which eight studies compared desmopressin to placebo (Cannon 1999; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). One study compared desmopressin to behavioural modifications (Wang 2012). One study compared desmopressin to an alpha‐blocker (Ceylan 2013). Three studies compared the addition of desmopressin to alpha‐blocker therapy versus an alpha‐blocker alone (Ahmed 2015a; Kim 2017; Koca 2012). One study compared desmopressin with an alpha‐blocker to an alpha‐blocker with an anticholinergic (Shin 2014a). No trials compared desmopressin to surgery.
Outcomes
We identified all primary outcomes in each of the included studies for four comparisons. The number of nocturnal voids and major adverse events were reported in all comparisons except desmopressin versus behaviour modification. Quality of life outcomes were reported in the comparisons of desmopressin versus alpha‐blocker and desmopressin plus alpha‐blocker versus alpha‐blocker. Eight trials reported health‐related quality of life using the IPSS (Ahmed 2015a; Ceylan 2013; Kim 2017; Koca 2012; Shin 2014a; Wang 2011a; Weiss 2012a; Weiss 2013). We were able to use the information regarding quality of life outcomes for the comparisons of desmopressin versus alpha‐blocker and desmopressin plus alpha‐blocker versus alpha‐blocker. The authors of included studies did not use validated score systems such as Common Terminology Criteria for Adverse Events to assess adverse events (National Cancer Institute), therefore we judged the severity of adverse events using the available information described in the studies.
For secondary outcomes, the duration of the first sleep episode was reported in the comparisons of desmopressin versus placebo and desmopressin versus behaviour modification. None of the studies reported time to first void. The outcomes of minor adverse events and treatment withdrawal due to adverse event were reported in all comparisons except desmopressin versus behaviour modification.
Funding source and conflicts of interest
Seven studies specified funding sources (Kim 2017; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Shin 2014a; Weiss 2012a; Weiss 2013; Yamaguchi 2013): five were supported by pharmaceutical companies; one was funded by government; and one reported no funding source. Six studies reported no conflicts of interest (Ahmed 2015a; Ceylan 2013; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Shin 2014a), and five reported having relationships with pharmaceutical companies (Cannon 1999; Serenity Pharmaceuticals 2016; Weiss 2012a; Weiss 2013; Yamaguchi 2013).
Excluded studies
We excluded 17 studies (18 articles) after evaluation of the full publication (see Characteristics of excluded studies table). Six studies were duplicates (Ahmed 2015b; Cho 2016; Kaminetsky 2016; Shin 2014b; Wang 2011b; Weiss 2012b). Four studies had the wrong study design (Gilbert 2011; Holm‐Larsen 2013a; Moon 2002; Moon 2003). Four studies had the wrong study population (Asplund 1999; Lam 2017; van Kerrebroeck 2007; Yassin 2010). Of the remaining studies, one had the wrong intervention (Fu 2011); one had the wrong comparator (Cho 2015); and one had the wrong outcome (Malli 2014).
Studies awaiting classification
There are four studies awaiting classification (see Characteristics of studies awaiting classification table) (Holm‐Larsen 2013b; NCT01694498; Salvatore 1996; Weiss 2001).
Ongoing studies
We found one ongoing study (see Characteristics of ongoing studies table) (NCT02904759).
Risk of bias in included studies
Detailed results of the 'Risk of bias' assessment are provided in Figure 2 and Figure 3, and judgements for individual domains are provided in the Characteristics of included studies table.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
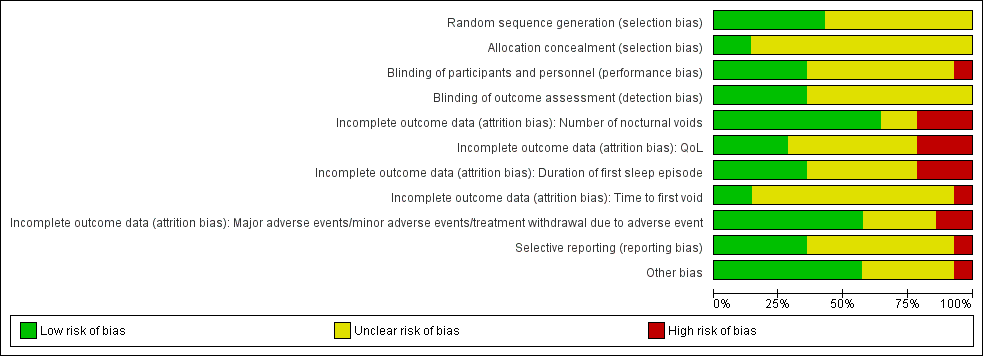
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
The sequence generation for participation allocation was adequate in six trials and rated at low risk of bias (Ahmed 2015a; Kim 2017; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). In the remaining trials, the method of sequence generation was unclear or not specified.
Allocation concealment
Two trials had adequate allocation concealment and were at low risk of bias (Kim 2017; Weiss 2012a). In the remaining trials, treatment allocation was unclear.
Blinding
Blinding of participants and personnel
Five trials were at low risk of bias (Kim 2017; Serenity Pharmaceuticals 2016; Weiss 2012a; Weiss 2013; Yamaguchi 2013), and one study was at high risk of bias (Ahmed 2015a). The remaining studies were at unclear risk of bias.
Blinding of outcome assessment
Five studies were at low risk of bias (Kim 2017; Serenity Pharmaceuticals 2016; Weiss 2012a; Weiss 2013; Yamaguchi 2013). The remaining studies were at unclear risk of bias.
Incomplete outcome data
Number of nocturnal voids
Nine trials were at low risk of bias for number of nocturnal voids (Ahmed 2015a; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Shin 2014a; Wang 2011a; Wang 2012; Weiss 2013). Three studies were at high risk of bias (Ceylan 2013; Kim 2017; Yamaguchi 2013). The remaining studies were at unclear risk of bias.
Quality of life
Four trials were at low risk of bias (Ahmed 2015a; Koca 2012; Wang 2011a; Wang 2012). Three studies were at high risk of bias (Ceylan 2013; Kim 2017; Yamaguchi 2013). The remaining studies were at unclear risk of bias.
Duration of first sleep episode
Five trials were at low risk of bias (Ahmed 2015a; Mattiasson 2002; Rezakhaniha 2011; Wang 2011a; Wang 2012). Three studies were at high risk of bias (Ceylan 2013; Kim 2017; Yamaguchi 2013). The remaining studies were at unclear risk of bias.
Time to first void
Two studies were at low risk of bias (Serenity Pharmaceuticals 2016; Weiss 2013). One study was at high risk of bias (Ceylan 2013). The remaining (11 of 14) studies were at unclear risk of bias.
Major adverse events, minor adverse events, and treatment withdrawal due to adverse event
Most studies (eight of 14) were at low risk of bias (Ahmed 2015a; Koca 2012; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Shin 2014a; Wang 2011a; Weiss 2013). Two studies were at high risk of bias (Ceylan 2013; Kim 2017). The remaining studies were at unclear risk of bias.
Selective reporting
We identified published protocols for four included studies and rated the risk of reporting bias for these studies as low (Kim 2017; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Although there was a published protocol for a fifth study, we rated this study as at high risk of reporting bias due to important deficits in outcome reporting (Serenity Pharmaceuticals 2016). One study did not have a published protocol, but we rated it at low risk of bias due to its comprehensive outcome reporting (Shin 2014a). We judged the risk of selective reporting bias as unclear for the remaining studies.
Other potential sources of bias
We rated eight trials as at low risk of bias (Ahmed 2015a; Ceylan 2013; Kim 2017; Koca 2012; Rezakhaniha 2011; Shin 2014a; Wang 2011a; Weiss 2013). We rated one study as at high risk of bias due to the exclusion of participants who did not respond in the active run‐in period (Mattiasson 2002). The remaining five trials were at unclear risk of bias: two trials had placebo or active run‐in periods (Cannon 1999; Serenity Pharmaceuticals 2016); three trials had a participant population that consisted of almost 50% women (which we were able to selectively exclude from the analysis); we noted that the investigators did not stratify randomisation based on gender, which may have resulted in prognostic imbalances (Serenity Pharmaceuticals 2016; Weiss 2012a; Yamaguchi 2013); and one trial, reported as abstract only, had insufficient information to assess for other biases and was rated as at unclear risk of bias (Wang 2012).
Effects of interventions
See: Summary of findings for the main comparison Desmopressin versus placebo for men with nocturia (short term); Summary of findings 2 Desmopressin versus placebo for men with nocturia (intermediate term); Summary of findings 3 Desmopressin versus behaviour modifications for men with nocturia (short term); Summary of findings 4 Desmopressin versus alpha‐blocker for men with nocturia (short term); Summary of findings 5 Desmopressin plus alpha‐blocker versus alpha‐blocker for men with nocturia (short term); Summary of findings 6 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic for men with nocturia (short term)
Desmopressin versus placebo
We included eight studies comparing desmopressin versus placebo with short‐term follow‐up (Cannon 1999; Mattiasson 2002; Rezakhaniha 2011; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013; summary of findings Table for the main comparison), and one study with intermediate‐term follow‐up in this comparison (Wang 2011a; summary of findings Table 2). There were no studies with long‐term follow‐up.
Primary outcomes
1. Number of nocturnal voids
We included six studies with 1982 men (intervention 1360, control 622) for short‐term follow‐up (Mattiasson 2002; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). Desmopressin may result in a small, possibly unimportant effect on the number of nocturnal voids (mean difference (MD) ‐0.61, 95% confidence interval (CI) ‐0.96 to ‐0.27) (Analysis 1.1). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and inconsistency.
We included one study with 115 men (intervention 57, control 58) for intermediate‐term follow‐up (Wang 2011a). Desmopressin may reduce the number of nocturnal voids in an appreciable number of men (MD ‐0.85, 95% CI ‐1.17 to ‐0.53) (Analysis 1.2). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
2. Quality of life
None of the included studies reported quality of life.
3. Major adverse events
We included two studies with 536 men (intervention 329, control 207) for short‐term follow‐up (Mattiasson 2002; Weiss 2013). We are uncertain about the effect of desmopressin on major adverse events (risk ratio (RR) 0.97, 95% CI 0.10 to 9.03) (Analysis 1.3). We rated the quality of evidence as very low according to GRADE, downgrading for study limitations, inconsistency, and imprecision.
We included one study with 115 men (intervention 57, control 58) for intermediate‐term follow‐up (Wang 2011a). Desmopressin may result in little or no difference in major adverse events (RR 3.05, 95% CI 0.13 to 73.39) (Analysis 1.4). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
Secondary outcomes
1. Duration of first sleep episode
We included four studies with 652 men (intervention 443, control 209) for short‐term follow‐up (Mattiasson 2002; Wang 2011a; Weiss 2012a; Yamaguchi 2013). Desmopressin may improve the duration of first sleep episode, but we are very uncertain of this finding (MD 54.61 minutes, 95% CI 13.97 to 95.25) (Analysis 1.5). We rated the quality of evidence as very low according to GRADE, downgrading for study limitations, inconsistency, and imprecision.
We included one study with 115 men (intervention 57, control 58) for intermediate‐term follow‐up (Wang 2011a). Desmopressin likely results in a small effect, but this may not represent a clinically important improvement in the duration of first sleep episode (MD 18.4 minutes, 95% CI 11.6 to 25.2) (Analysis 1.6). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
2. Time to first void
We included one study with 383 men (intervention 241, control 142) for short‐term follow‐up (Weiss 2013). Desmopressin may result in a small, possibly unimportant effect on time to first void (MD 40.80, 95% CI 17.07 to 64.53) (Analysis 1.7). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
3. Minor adverse events
We included three studies with 594 men (intervention 357, control 237) for short‐term follow‐up (Mattiasson 2002; Rezakhaniha 2011; Weiss 2013). Desmopressin may result in little or no difference in minor adverse events (RR 0.87, 95% CI 0.67 to 1.13) (Analysis 1.8). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
We included one study with 115 men (intervention 57, control 58) for intermediate‐term follow‐up (Wang 2011a). Desmopressin may result in little or no difference in minor adverse events (RR 0.86, 95% CI 0.49 to 1.49) (Analysis 1.9). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
4. Treatment withdrawal due to adverse event
We included four studies with 614 men (intervention 367, control 247) for short‐term follow‐up (Cannon 1999; Mattiasson 2002; Rezakhaniha 2011; Weiss 2013). Desmopressin may result in little or no difference in treatment withdrawal due to adverse events (RR 1.10, 95% CI 0.56 to 2.15) (Analysis 1.10). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
We included one study with 115 men (intervention 57, control 58) for intermediate‐term follow‐up (Wang 2011a). Desmopressin may result in little or no difference in treatment withdrawal due to adverse events (RR 3.05, 95% CI 0.13 to 73.39) (Analysis 1.11). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
Subgroup analysis
We were able to perform preplanned subgroups for short‐term outcomes only.
1. Routes of administration (intranasal, sublingual, or oral)
Number of nocturnal voids
The mean change in the number of nocturnal voids for intranasal was MD ‐0.20 (95% CI ‐0.34 to ‐0.06), for sublingual was MD ‐0.37 (95% CI ‐0.71 to ‐0.03), and for oral was MD ‐1.14 (95% CI ‐1.41 to ‐0.86). The test for interaction was significant (P < 0.001, I2 = 94.4%) (Analysis 1.1).
Major adverse events
The test for interaction was non‐significant for major adverse events (sublingual: RR 2.53, 95% CI 0.73 to 8.73; oral: RR 0.25, 95% CI 0.03 to 2.37) (P = 0.08, I2 = 68.0%) (Analysis 1.3).
2. Desmopressin dose (very low, low, or high dose)
Number of nocturnal voids
The mean change in the number of nocturnal voids for the very low dose was MD ‐0.20 (95% CI ‐0.34 to ‐0.06), for low dose was MD ‐0.33 (95% CI ‐0.72 to 0.06), and for high dose was MD ‐1.03 (95% CI ‐1.41 to ‐0.66). The test for interaction was significant (P < 0.001, I2 = 88.1%) (Analysis 1.12).
Major adverse events
The test for interaction was non‐significant for major adverse events (low dose: RR 2.53, 95% CI 0.73 to 8.73; high dose: RR 0.25, 95% CI 0.03 to 2.37) (P = 0.08, I2 = 68.0%) (Analysis 1.3).
3. Presence of nocturnal polyuria (with or without)
Two studies defined men with nocturia and nocturnal polyuria as inclusion criteria (Cannon 1999; Wang 2011a). The remaining studies included men with nocturia without any description of nocturnal polyuria.
Number of nocturnal voids
When comparing men with nocturnal polyuria versus without nocturnal polyuria, we found an MD of ‐1.28 (95% CI ‐1.64 to ‐0.92) with nocturnal polyuria versus an MD of ‐0.45 (95% CI ‐0.73 to ‐0.17) without nocturnal polyuria. The test for interaction was significant (P < 0.001, I2 = 92.1%) (Analysis 1.13).
Major adverse events
As eligible studies in this analysis did not include men with nocturia and nocturnal polyuria, we were unable to perform a subgroup analysis.
Sensitivity analysis
1. Restricting the analysis by considering risk of bias, excluding studies at 'high risk' or 'unclear risk'
We rated all included studies as at high or unclear risk of bias and were therefore unable to perform a sensitivity analysis.
2. Restricting the analysis by considering risk of bias, excluding studies with run‐in periods
Number of nocturnal voids
We included four studies with 966 men (intervention 665, control 301) for short‐term follow‐up (Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). The MD for this sensitivity analysis was ‐0.66 (95% CI ‐1.17 to ‐0.14) compared to an MD of ‐0.61 (95% CI ‐0.96 to ‐0.27) for the main analysis (Analysis 1.14).
The results of the main and sensitivity analyses were the same for intermediate‐term follow‐up.
Major adverse events
We included one study with 385 men (intervention 243, control 142) for short‐term follow‐up (Weiss 2013). Sensitivity analyses (RR 2.53, 95% CI 0.73 to 8.73) did not alter the treatment effect compared to the main analysis (RR 0.97, 95% CI 0.10 to 9.03) (Analysis 1.15).
3. Restricting the analysis by considering clinically recommended dosage of desmopressin, excluding lower dose levels
Number of nocturnal voids
We included six studies with 1586 men (intervention 964, control 622) for short‐term follow‐up (Mattiasson 2002; Serenity Pharmaceuticals 2016; Wang 2011a; Weiss 2012a; Weiss 2013; Yamaguchi 2013). The MD for this sensitivity analysis was ‐0.76 (95% CI ‐1.15 to ‐0.38) compared to an MD of ‐0.61 (95% CI ‐0.96 to ‐0.27) for the main analysis (Analysis 1.16).
The results of the main and sensitivity analyses were the same for intermediate‐term follow‐up.
Major adverse events
We included two studies with 417 men (intervention 210, control 207) for short‐term follow‐up (Mattiasson 2002; Weiss 2013). Sensitivity analyses (RR 0.98, 95% CI 0.10 to 9.71) did not alter the treatment effect substantially compared to the main analysis (RR 0.97, 95% CI 0.10 to 9.03) (Analysis 1.17).
Desmopressin versus behaviour modification
We found one study with 60 men (intervention 30, control 30) comparing desmopressin versus behaviour modification for short‐term follow‐up (Wang 2012; summary of findings Table 3).
Primary outcomes
1. Number of nocturnal voids
The study did not report number of nocturnal voids.
2. Quality of life
The study did not report quality of life.
3. Major adverse events
The study did not report major adverse events.
Secondary outcomes
1. Duration of first sleep episode
Desmopressin may result in a small, possibly unimportant effect on duration of first sleep episode (MD 90.00 minutes, 95% CI 1.95 to 178.05) (Analysis 2.1). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
2. Time to first void
The study did not report time to first void.
3. Minor adverse events
The study did not report minor adverse events.
4. Treatment withdrawal due to adverse event
The study did not report treatment withdrawal due to adverse event.
Subgroup and sensitivity analysis
We were unable to perform any of the planned secondary analyses due to a paucity of included studies or lack of relevant data in the included studies.
Desmopressin versus alpha‐blocker
We included one study with 31 men (intervention 15, control 16) comparing desmopressin versus alpha‐blocker for all review outcomes in short‐term follow‐up (Ceylan 2013; summary of findings Table 4) There were no studies with intermediate‐ or long‐term follow‐up.
Primary outcomes
1. Number of nocturnal voids
Desmopressin likely results in little or no difference in the number of nocturnal voids (MD 0.30, 95% CI ‐0.20 to 0.80) (Analysis 3.1). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
2. Quality of life
Desmopressin likely results in little or no difference in quality of life (MD 0.00, 95% CI ‐0.35 to 0.35) (Analysis 3.2). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
3. Major adverse events
No major adverse events were reported in either study group.
Secondary outcomes
1. Duration of first sleep episode
The study did not report duration of first sleep episode.
2. Time to first void
The study did not report time to first void.
3. Minor adverse events
We are uncertain about the effect on minor adverse events (RR 1.07, 95% CI 0.07 to 15.57) (Analysis 3.4). We rated the quality of evidence as very low according to GRADE, downgrading for study limitations and twice for imprecision.
4. Treatment withdrawal due to adverse event
No treatment withdrawals due to adverse events were reported in either study group.
Subgroup and sensitivity analysis
We were unable to perform any of the planned secondary analyses due to a paucity of included studies or lack of relevant data in the included studies.
Desmopressin plus alpha‐blocker versus alpha‐blocker
We included three studies comparing desmopressin plus alpha‐blocker versus alpha‐blocker with short‐term follow‐up (Ahmed 2015a; Kim 2017; Koca 2012; summary of findings Table 5). There were no studies with intermediate‐ or long‐term follow‐up.
Primary outcomes
1. Number of nocturnal voids
We included three studies with 341 men (intervention 176, control 165) (Ahmed 2015a; Kim 2017; Koca 2012). Desmopressin combined with an alpha‐blocker likely results in a small, possibly unimportant effect on the number of nocturnal voids (MD ‐0.47, 95% CI ‐0.73 to ‐0.21) (Analysis 4.1). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
2. Quality of life
We included three studies with 341 men (intervention 176, control 165) (Ahmed 2015a; Kim 2017; Koca 2012). Desmopressin combined with an alpha‐blocker likely results in a small, possibly unimportant effect on quality of life (MD ‐0.29, 95% CI ‐0.51 to ‐0.07) (Analysis 4.3). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
3. Major adverse events
We included three studies with 402 men (intervention 202, control 200) (Ahmed 2015a; Kim 2017; Koca 2012). Desmopressin combined with an alpha‐blocker may result in little to no difference in major adverse events (RR 0.30, 95% CI 0.01 to 7.32) (Analysis 4.5). There was only one adverse event in the control group in one study (Kim 2017). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
Secondary outcomes
1. Duration of first sleep episode
No study reported duration of first sleep episode.
2. Time to first void
No study reported time to first void.
3. Minor adverse events
We included three studies with 402 men (intervention 202, control 200) (Ahmed 2015a; Kim 2017; Koca 2012). We are uncertain about the effect on minor adverse events (RR 1.60, 95% CI 0.15 to 16.82) (Analysis 4.7). We rated the quality of evidence as very low according to GRADE, downgrading for study limitations and twice for imprecision.
4. Treatment withdrawal due to adverse event
We included three studies with 402 men (intervention 202, control 200) (Ahmed 2015a; Kim 2017; Koca 2012). Desmopressin combined with an alpha‐blocker may result in little to no difference in treatment withdrawal due to adverse events (RR 2.84, 95% CI 0.46 to 17.66) (Analysis 4.8). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
Subgroup analysis
1. Desmopressin route of administration and dose (intranasal/very low, sublingual/low, or oral/high)
Number of nocturnal voids
The mean change in the number of nocturnal voids for sublingual/low dose was MD ‐0.66 (95% CI ‐0.90 to ‐0.42) and for oral/high dose was MD ‐0.31 (95% CI ‐0.60 to ‐0.02). The test for interaction was non‐significant (P = 0.06, I2 = 70.8%) (Analysis 4.1).
Quality of life
The mean change in quality of life score for sublingual/low dose was MD ‐0.23 (95% CI ‐0.49 to 0.03) and for oral/high dose was MD ‐0.44 (95% CI ‐0.85 to ‐0.03). The test for interaction was non‐significant (P = 0.41, I2 = 0.0%) (Analysis 4.3).
Major adverse events
We were unable to estimate the subgroup difference due to no events in the sublingual/low‐dose group (Analysis 4.5).
2. Presence of nocturnal polyuria (with or without)
Number of nocturnal voids
When comparing men with nocturnal polyuria versus without nocturnal polyuria, we found an MD of ‐0.30 (95% CI ‐0.74 to 0.14) with nocturnal polyuria versus an MD of ‐0.52 (95% CI ‐0.86 to ‐0.18) without nocturnal polyuria. The test for interaction was non‐significant (P = 0.44, I2 = 0.0%) (Analysis 4.2).
Quality of life
We found an MD of ‐0.20 (95% CI ‐0.84 to 0.44) for men with nocturnal polyuria versus an MD of ‐0.34 (95% CI ‐0.67 to ‐0.01) for men without nocturnal polyuria. The test for interaction was non‐significant (P = 0.71, I2 = 0.0%) (Analysis 4.4).
Major adverse events
We were unable to estimate the subgroup difference due to no events in men with nocturia (Analysis 4.6).
Sensitivity analysis
The results of the main and sensitivity analyses were the same.
Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic
We included one study with short‐term follow‐up in this comparison (Shin 2014a; summary of findings Table 6). There were no studies with intermediate‐ or long‐term follow‐up.
Primary outcomes
1. Number of nocturnal voids
We included 405 men (intervention 196, control 209). Desmopressin combined with alpha‐blocker likely results in little or no difference in the number of nocturnal voids (MD ‐0.43, 95% CI ‐0.97 to 0.11) (Analysis 5.1). We rated the quality of evidence as moderate according to GRADE, downgrading for study limitations.
2. Quality of life
The study did not report quality of life.
3. Major adverse events
We included 427 men (intervention 205, control 222). No major adverse events were reported in either study group.
Secondary outcomes
1. Duration of first sleep episode
The study did not report duration of first sleep episode.
2. Time to first void
The study did not report time to first void.
3. Minor adverse events
We included 427 men (intervention 205, control 222). Desmopressin combined with alpha‐blocker may result in a small, possibly unimportant effect on minor adverse events (RR 0.22, 95% CI 0.05 to 0.98) (Analysis 5.3). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
4. Treatment withdrawal due to adverse event
We included 427 men (intervention 205, control 222). Desmopressin combined with alpha‐blocker may result in a small, possibly unimportant effect on treatment withdrawal due to adverse events (RR 0.22, 95% CI 0.05 to 0.98) (Analysis 5.4). We rated the quality of evidence as low according to GRADE, downgrading for study limitations and imprecision.
Subgroup and sensitivity analysis
We were unable to perform any of the planned secondary analyses due to a paucity of included studies or lack of relevant data in the included studies.
Desmopressin versus surgery
We found no trials comparing desmopressin versus surgery.
Discussion
Summary of main results
We included 14 unique studies with 2966 male participants with nocturia across five comparisons. We included all comparisons with short‐term follow‐up and one comparison (desmopressin versus placebo) with intermediate‐term follow‐up. The mean age of the male participants ranged from 57 to 74 years.
Desmopressin versus placebo
Based on short‐term follow‐up, desmopressin may reduce the number of nocturnal voids, but this may not represent a clinically important reduction to patients. We are uncertain about the effect of desmopressin on major adverse events.
For intermediate‐term follow‐up, desmopressin may reduce number of nocturnal voids in comparison to placebo in an appreciable number of men. Desmopressin may result in little or no difference in major adverse events.
None of the studies reported quality of life.
Subgroup analyses suggest a larger effect with oral, higher‐dose formulations of desmopressin and in men with documented nocturnal polyuria. Sensitivity analyses based on risk of bias, run‐in periods, and clinical dosage of desmopressin did not alter the treatment effect substantially.
Desmopressin versus behaviour modification
We found no evidence on primary outcomes for the comparison of desmopressin versus behaviour modification.
Desmopressin versus alpha‐blocker
Based on short‐term follow‐up, desmopressin likely has a similar effect to alpha‐blocker on the number of nocturnal voids and quality of life. No major adverse events were reported in either study group.
Desmopressin plus alpha‐blocker versus alpha‐blocker
Based on short‐term follow‐up, combination therapy likely results in a small, possibly unimportant effect compared with desmopressin alone on the number of nocturnal voids and quality of life. Combination therapy was not associated with a meaningful impact on major adverse events.
Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic
Based on short‐term follow‐up, desmopressin plus alpha‐blocker likely results in little or no difference compared with an alpha‐blocker plus anticholinergic in the number of nocturnal voids. We found no evidence on quality of life. No major adverse events were reported in either study group.
Desmopressin versus surgery
We found no studies comparing desmopressin to surgical interventions.
Overall completeness and applicability of evidence
-
The focus of this systematic review was male participants with nocturia irrespective of aetiology but excluding men with known metabolic disorders potentially associated with a secondary nocturnal diuresis, such as Cushing disease. We included men with and without nocturnal polyuria, since desmopressin has been used in both patient populations.
-
This review included three different formulations of desmopressin with different bioavailability. While we labelled the dosage as high, low, and very low in this review, actual bioavailability may be different.
-
The existing body of evidence was largely limited to short‐term outcomes, with 13 of 14 studies reporting outcomes of three months' duration or less. Given the usually chronic nature of men's nocturia complaints, such short‐term outcomes appear to be insufficient to provide assurance of long‐term effectiveness and safety.
-
Desmopressin is known to lower serum sodium levels. We made a conscious decision not to include serum sodium levels as an outcome of this review given that their levels per se are not patient important. Instead, we focused on both major and minor adverse events, expecting that clinically important serum sodium level reductions would have been reflected in the event rates for these outcomes. The findings of this review did not suggest increased rates for either outcomes, although the quality of evidence was only low or very low. However, participants in clinical trials are commonly monitored more closely than in routine clinical practice, thereby potentially reducing the risk of adverse events and limiting the applicability of our findings. In addition, as previously mentioned, the time‐horizon of most included studies was short, thereby negating the opportunity for us to document any long‐term effects of desmopressin on serum sodium levels. Large observational studies may therefore provide additional insight into the 'real‐life' safety profile of this drug when used longer term.
-
We identified trials that appeared to be eligible for inclusion but did not provide data in such a way as to allow analyses, for example results reporting by gender group. Our efforts to obtain additional information from the manufacturers and trial sponsors were largely unsuccessful. This raises concerns about selective reporting and potential publication bias, although we found insufficient indication to downgrade the quality of evidence for publication bias.
-
Given that one‐third of trials had active run‐in periods, study findings may have been biased in favour of the intervention, since people with adverse events or limited compliance may have been excluded up‐front.
-
We performed subgroup analyses based on the presence or absence of nocturnal polyuria. Four trials included men with documented nocturnal polyuria, defined as more than 30% or one‐third of a person's 24‐hour urine output excreted during their sleeping time. However, the other trials did not describe whether the men had nocturnal polyuria or not. Given that this analysis did not exactly match our intended analysis, we are less confident in its findings. These analyses may also have been underpowered to detect a difference.
-
There were small differences in the pooled effect estimates among subgroups, which may represent true differences or spurious findings due to prognostic imbalances in the absence of stratified randomisation.
Quality of the evidence
We rated the quality of evidence as low or moderate for most outcomes and comparisons. Our confidence in the estimates of effect were lowered for the following reasons.
-
Study limitation: issues surrounding allocation concealment and blinding.
-
Inconsistency: high I2 values, which we were unable to explain through secondary analyses.
-
Imprecision: few events resulting in wide CIs.
Potential biases in the review process
-
In order to reduce the potential for publication bias, we followed a published protocol (Han 2016), and performed a comprehensive and updated literature search without publication or language restriction. In addition, we searched trial registries for unpublished, planned, or ongoing studies. We found a few abstract proceedings of the annual meetings of the American Urological Association, European Association of Urology, International Continence Society, and the British Association of Urological Surgeons from 2012 to 2017 and one ongoing trial. Should any results of such studies become available, we will include them in updates of this review.
-
We contacted study authors on several occasions, who provided feedback to some of our queries. However, we received additional information from only two out of six study authors. This may represent a source of bias with potential under‐reporting of the true treatment burden.
-
We included only randomised controlled trials in this review, which were all relatively small and had a short time‐horizon. To understand infrequent and long‐term adverse events better, additional non‐randomised studies are likely necessary but were outside the scope of this review.
Agreements and disagreements with other studies or reviews
A systematic review assessing the efficacy and safety of desmopressin for nocturia in adults was published in 2014 (Ebell 2014). The review included 10 studies with 2191 participants, of which several studies included a significant portion of women. We excluded studies with only women from our review, as we focused on the use of desmopressin to treat nocturia in men. One study comparing desmopressin in addition to alpha‐blocker therapy versus alpha‐blocker therapy alone was published after the systematic review by Ebell and colleagues (Ahmed 2015a). Similar to our review, Ebell 2014 found a small improvement in nocturia mainly based on a small decrease in the number of nocturnal voids and increase in duration of first sleep episode (Ebell 2014). The authors viewed desmopressin as generally safe with an overall favourable adverse effect profile and very rare major adverse events. In congruence with our review, their results were limited to the short term, and they acknowledged the need for additional, well‐designed studies of longer duration. These reviews did not use GRADE or assess the quality of evidence on a per‐outcome basis.
Another review discussing the medical treatment of nocturia in men with LUTS found that desmopressin was more effective than placebo in decreasing nocturnal voiding frequency and increasing duration of undisturbed sleep (Sakalis 2017). However, of the included studies, three trials had mixed populations of men and women. Given the review's clearly defined focus on men and the unique pathophysiology of men, we believe these studies introduced meaningful heterogeneity and should have been excluded. Furthermore, another included study compared furosemide (a diuretic) with desmopressin to placebo, and the contribution of each drug could not be isolated. This review provided no quality of evidence ratings and did not use an explicit CID (Jung 2017). They found an MD of ‐0.87 (95% CI ‐1.15 to ‐0.60) comparing desmopressin to placebo; the proposed CID is a reduction of at least one episode per night (Krader 2012).
PRISMA flow diagram.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Comparison 1 Desmopressin versus placebo, Outcome 1 Number of nocturnal voids (short term) (subgroup route).

Comparison 1 Desmopressin versus placebo, Outcome 2 Number of nocturnal voids (intermediate term).
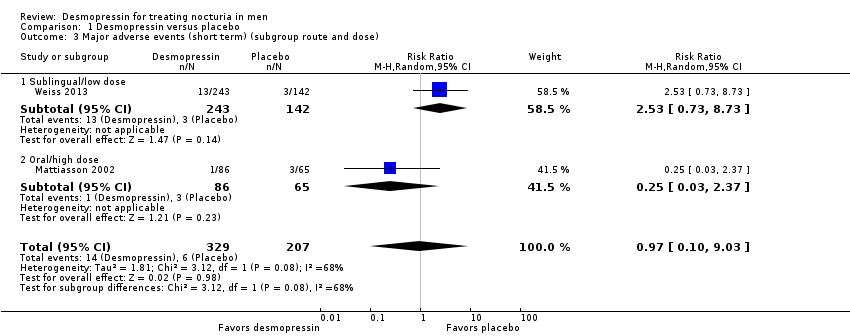
Comparison 1 Desmopressin versus placebo, Outcome 3 Major adverse events (short term) (subgroup route and dose).

Comparison 1 Desmopressin versus placebo, Outcome 4 Major adverse events (intermediate term).

Comparison 1 Desmopressin versus placebo, Outcome 5 Duration of first sleep episode (short term).

Comparison 1 Desmopressin versus placebo, Outcome 6 Duration of first sleep episode (intermediate term).
![Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes].](/es/cdsr/doi/10.1002/14651858.CD012059.pub2/media/CDSR/CD012059/image_n/nCD012059-CMP-001-07.png)
Comparison 1 Desmopressin versus placebo, Outcome 7 Time to first void (short term) [minutes].
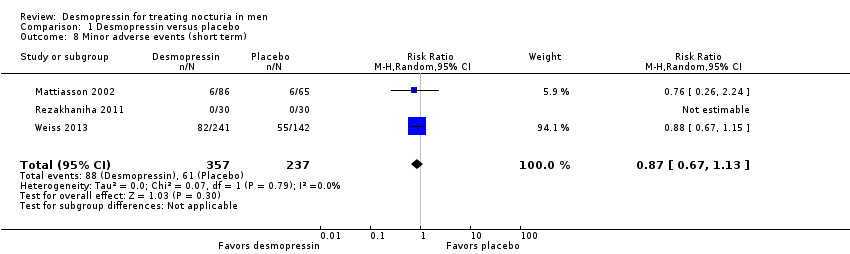
Comparison 1 Desmopressin versus placebo, Outcome 8 Minor adverse events (short term).

Comparison 1 Desmopressin versus placebo, Outcome 9 Minor adverse events (intermediate term).
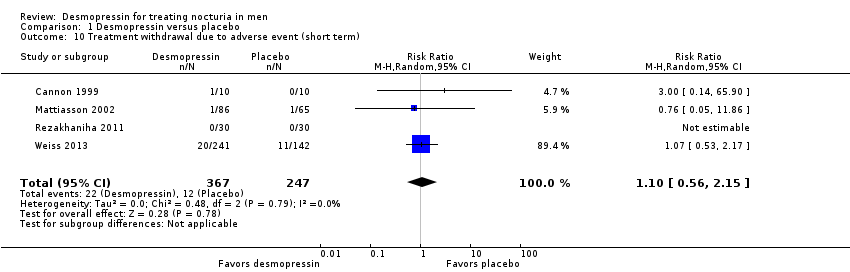
Comparison 1 Desmopressin versus placebo, Outcome 10 Treatment withdrawal due to adverse event (short term).
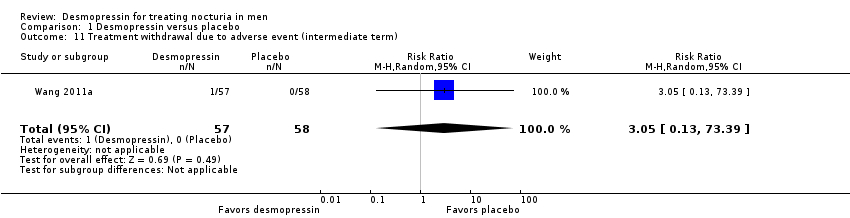
Comparison 1 Desmopressin versus placebo, Outcome 11 Treatment withdrawal due to adverse event (intermediate term).

Comparison 1 Desmopressin versus placebo, Outcome 12 Number of nocturnal voids (short term) (subgroup dose).
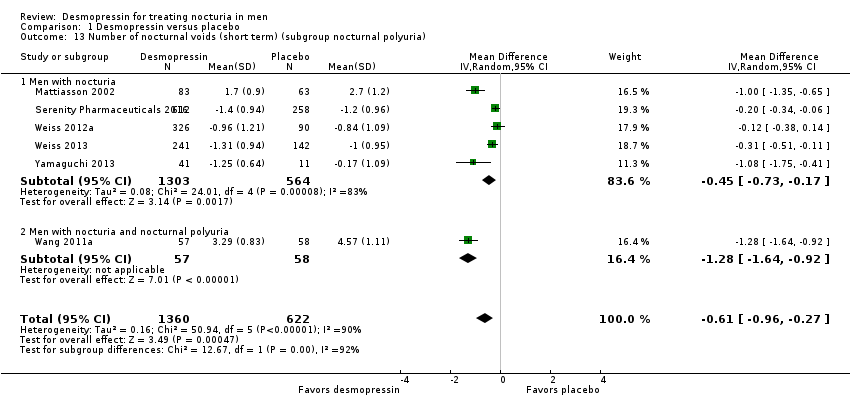
Comparison 1 Desmopressin versus placebo, Outcome 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria).

Comparison 1 Desmopressin versus placebo, Outcome 14 Number of nocturnal voids (short term) (sensitivity run‐in period).
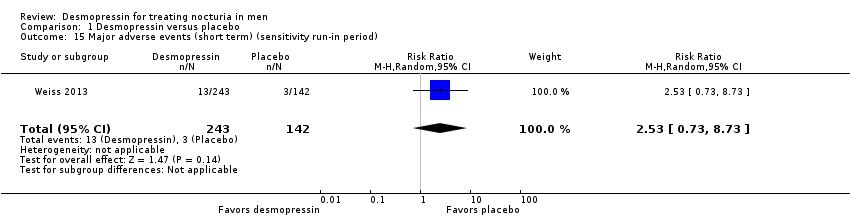
Comparison 1 Desmopressin versus placebo, Outcome 15 Major adverse events (short term) (sensitivity run‐in period).

Comparison 1 Desmopressin versus placebo, Outcome 16 Number of nocturnal voids (short term) (sensitivity clinical dosage).

Comparison 1 Desmopressin versus placebo, Outcome 17 Major adverse events (short term) (sensitivity clinical dosage).

Comparison 2 Desmopressin versus behaviour modification, Outcome 1 Duration of first sleep episode (short term).
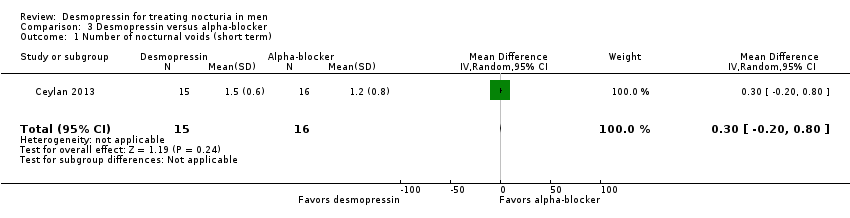
Comparison 3 Desmopressin versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 2 Quality of life (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 3 Major adverse events (short term).

Comparison 3 Desmopressin versus alpha‐blocker, Outcome 4 Minor adverse events (short term).
Comparison 3 Desmopressin versus alpha‐blocker, Outcome 5 Treatment withdrawal due to adverse event (short term).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 1 Number of nocturnal voids (short term) (subgroup route and dose).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 3 Quality of life (short term) (subgroup route and dose).
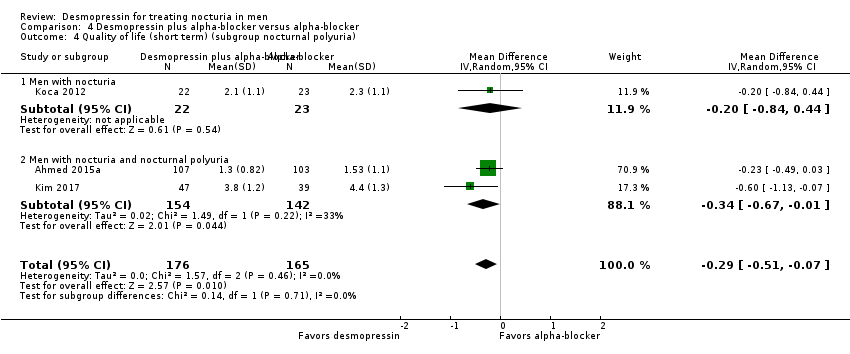
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 4 Quality of life (short term) (subgroup nocturnal polyuria).

Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 5 Major adverse events (short term) (subgroup route and dose).
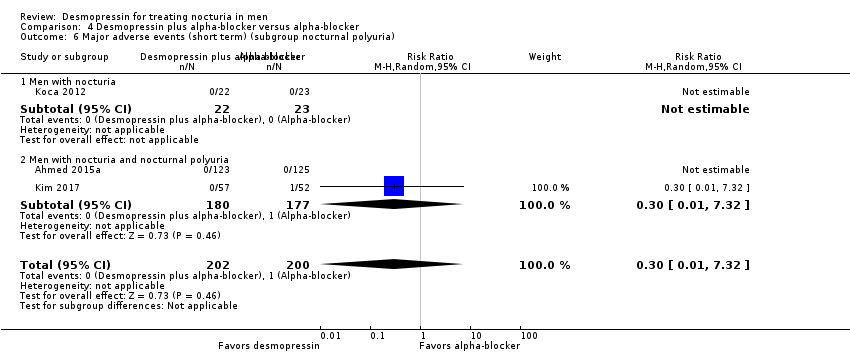
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 6 Major adverse events (short term) (subgroup nocturnal polyuria).
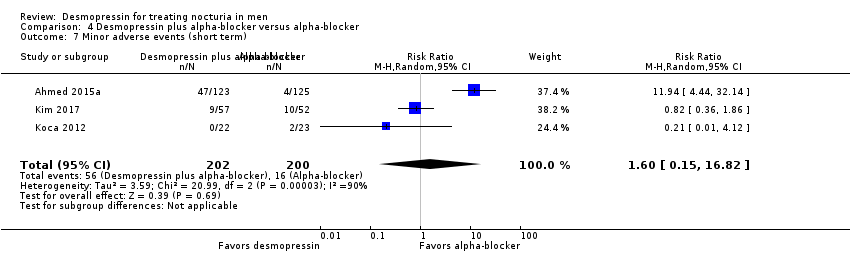
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 7 Minor adverse events (short term).
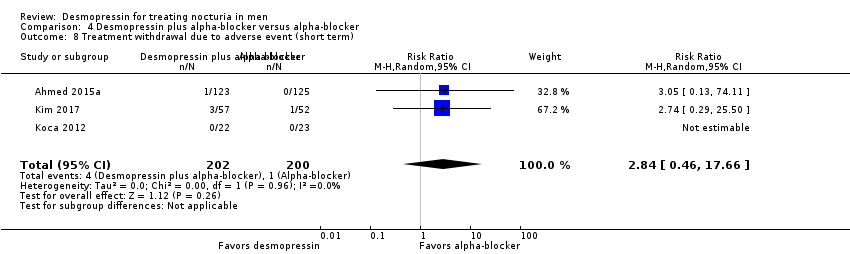
Comparison 4 Desmopressin plus alpha‐blocker versus alpha‐blocker, Outcome 8 Treatment withdrawal due to adverse event (short term).
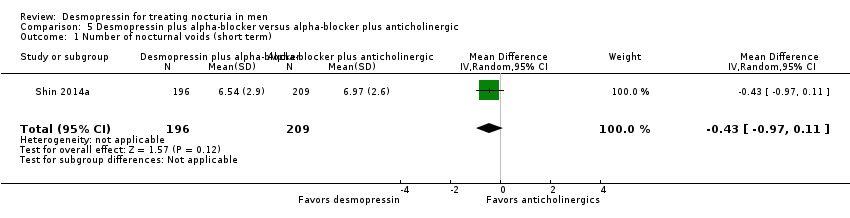
Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 1 Number of nocturnal voids (short term).
Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 2 Major adverse events (short term).

Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 3 Minor adverse events (short term).

Comparison 5 Desmopressin plus alpha‐blocker versus alpha‐blocker plus anticholinergic, Outcome 4 Treatment withdrawal due to adverse event (short term).
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 1982 | ⊕⊕⊝⊝ | ‐ | The mean number of nocturnal voids ranged from 1.9 to 4.57. | MD 0.61 lower |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 536 | ⊕⊝⊝⊝ | RR 0.97 | Study population | |
| 29 per 1000 | 1 fewer per 1000 | ||||
| Duration of first sleep episode | 652 | ⊕⊝⊝⊝ | ‐ | The mean duration of first sleep episode ranged from 26.21 to 174 minutes. | MD 54.61 minutes higher |
| Time to first void | 383 | ⊕⊕⊝⊝ | ‐ | The mean time to first void was 72.9 minutes. | MD 40.8 minutes higher |
| Minor adverse event | 594 | ⊕⊕⊝⊝ | RR 0.87 | Study population | |
| 257 per 1000 | 33 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 614 | ⊕⊕⊝⊝ | RR 1.10 | Study population | |
| 49 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in at least 50% of the studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: placebo | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with placebo | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 115 | ⊕⊕⊝⊝ | ‐ | Mean number of nocturnal voids was 4.14 voids. | MD 0.85 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode | 115 | ⊕⊕⊕⊝ | ‐ | Mean duration of first sleep episode was 101.6 minutes. | MD 18.4 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 115 | ⊕⊕⊝⊝ | RR 0.86 | Study population | |
| 328 per 1000 | 46 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 115 | ⊕⊕⊝⊝ | RR 3.05 | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: fluid restriction during nighttime | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with behaviour modification | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Duration of first sleep episode | 60 | ⊕⊕⊝⊝ | ‐ | Mean duration of first sleep episode was 150 minutes. | MD 90 minutes higher |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment withdrawal due to adverse event ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias in almost all domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin Control: alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin | ||||
| Number of nocturnal voids | 31 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 1.2 voids. | MD 0.3 voids more |
| Quality of life | 31 | ⊕⊕⊕⊝ | ‐ | Mean quality of life was 1.8 bothersome. | MD 0 |
| Major adverse events | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 31 | ⊕⊝⊝⊝ | RR 1.07 | Study population | |
| 63 per 1000 | 4 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 31 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains in the included study. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: alpha‐blocker alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids assessed with: voiding diary | 341 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids ranged from 1.68 to 2.6. | MD 0.47 voids fewer |
| Quality of life assessed with: IPSS and N‐QoL | 341 | ⊕⊕⊕⊝ | ‐ | Mean quality of life ranged from 1.53 to 4.4. | MD 0.29 lower |
| Major adverse events | 402 | ⊕⊕⊝⊝ | RR 0.30 | Study population | |
| 5 per 1000 | 3 fewer per 1000 | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 402 | ⊕⊝⊝⊝ | RR 1.60 | Study population | |
| 80 per 1000 | 48 more per 1000 | ||||
| Treatment withdrawal due to adverse event | 402 | ⊕⊕⊝⊝ | RR 2.84 | Study population | |
| 5 per 1000 | 9 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MD: mean difference; N‐QoL: Nocturia‐Quality of Life questionnaire; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear or high risk of bias for one or more domains among the included studies. | |||||
| Participants: men with nocturia Setting: likely outpatient Intervention: desmopressin + alpha‐blocker Control: anticholinergic + alpha‐blocker | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Assumed risk with alpha‐blocker + anticholinergic | Corresponding risk difference with desmopressin + alpha‐blocker | ||||
| Number of nocturnal voids | 405 | ⊕⊕⊕⊝ | ‐ | Mean number of nocturnal voids was 6.97 voids. | MD 0.43 voids fewer |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major adverse events | 427 | ⊕⊕⊝⊝ | Not estimable | Study population | |
| ‐ | ‐ | ||||
| Duration of first sleep episode ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Time to first void ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor adverse events | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| Treatment withdrawal due to adverse event | 427 | ⊕⊕⊝⊝ | RR 0.22 | Study population | |
| 45 per 1000 | 35 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for study limitations: unclear risk of bias for one or more domains in the included study. | |||||
| Study | Setting | Trial period | Description of participants | Intervention(s) and comparator(s) | Duration of intervention |
| Outpatient/Egypt | 2011 to 2014 | People with LUTS/BPH aged ≥ 50 years with nocturia (≥ 2 voids/night), nocturnal polyuria (nocturnal urine volume > 30% of 24‐hour urine volume) | I: desmopressin 60 μg ODT + tamsulosin | 3 months | |
| C: tamsulosin | |||||
| UK | NR | Men aged > 50 years with nocturnal polyuria (using 48‐hour inpatient monitoring or 1‐week frequency volume chart) | I: desmopressin nasal spray 20 μg | 4 weeks | |
| C: placebo | |||||
| Outpatient/Turkey | 2011 | Men with advanced age, complaints of LUTS and nocturia (≥ 3 times/night) | I: desmopressin nasal spray 20 μg | 2 months | |
| C: doxazosin | |||||
| Multicentre/South Korea | NR | Men aged 40 to 65 years with LUTS (IPSS > 13), nocturia (≥ 2 episodes/night), and nocturnal polyuria (NPI > 33%) | I: desmopressin 0.2 mg oral + alpha‐blocker | 8 weeks | |
| C: placebo + alpha‐blocker | |||||
| Outpatient/Turkey | NR | Men aged 50 to 70 years with LUTS and nocturia (≥ 2/night) | I: desmopressin 0.2 mg oral with alfuzosin | 3 months | |
| C: alfuzosin | |||||
| Multicentre/Denmark, | NR | Men aged ≥ 18 years with nocturia (2 voids/night, nocturia index scores > 1) | I: desmopressin 0.1 mg/0.2 mg/0.4 mg oral; dose titration | 3 weeks | |
| C: placebo | |||||
| Outpatient/single centre/Iran | 2008 to 2009 | Older men (mean age about 63 to 64 years) with voiding ≥ 2/night | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/USA and Canada | NR | Men or women aged ≥ 50 years with nocturia (≥ 2 nocturic episodes/night) | I: desmopressin nasal spray 0.75 μg, 1.0 μg, or 1.5 μg | 12 weeks | |
| C: placebo | |||||
| South Korea | 2010 to 2013 | Men aged ≥ 50 years with LUTS due to bladder outlet obstruction (Qmax ≤ 15 mL/second, IPSS ≥ 14) and nocturia (≥ 1 void/night) | I: desmopressin 0.2 mg oral + tamsulosin | 4 weeks | |
| C: solifenacin + tamsulosin | |||||
| Single centre/Taiwan | 2007 to 2009 | Men aged ≥ 65 years with BPH (IPSS > 13), nocturia (≥ 2 voids/night), and nocturnal polyuria (nocturnal urine volume > 30%) | I: desmopressin 0.1 mg oral | 12 months | |
| C: placebo | |||||
| Outpatient/single centre/China | 2009 to 2010 | Older men (age not reported) | I: desmopressin 0.1 mg oral | 8 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2007 to 2008 | Men and women aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo | |||||
| Multicentre/Canada and the USA | 2010 to 2013 | Men aged ≥ 18 years with nocturia (≥ 2 voids/night) | I: desmopressin 50 μg, 75 µg ODT | 3 months | |
| C: placebo | |||||
| Multicentre/Japan | 2010 to 2011 | Men and women aged 55 to 75 years with nocturia (≥ 2 voids/night) | I: desmopressin 10 µg, 25 µg, 50 µg, or 100 µg ODT | 4 weeks | |
| C: placebo | |||||
| BPH: benign prostatic hyperplasia; C: comparator; I: intervention; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; NPI: nocturnal polyuria index; NR: not reported; ODT: orally disintegrating tablet; Qmax: maximum flow rate. | |||||
| Intervention(s) and comparator(s) | Sample size | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| I: desmopressin + tamsulosin | 100 | 397/273 | 139 | 123 | 123 | 107 | 77.0 | 3 months | |
| C: tamsulosin | 100 | 134 | 125 | 125 | 103 | 76.9 | |||
| Total: | 273 | 248 | 248 | 210 | 76.9 | ||||
| I: desmopressin | ‐ | ‐/‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 8 weeks (cross‐over study design) | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |||
| Total: | 20 | ‐ | 18 | 18 | 90.0 | ||||
| I: desmopressin | ‐ | 84/31 | 15 | 15 | 15 | 15 | 100.0 | 2 months | |
| C: doxazosin | ‐ | 16 | 16 | 16 | 16 | 100.0 | |||
| Total: | 31 | 31 | 31 | 31 | 100.0 | ||||
| I: desmopressin + alpha‐blocker | ‐ | 121/109 | 57 | 57 | 47 | 47 | 82.4 | 8 weeks | |
| C: placebo + alpha‐blocker | ‐ | 52 | 52 | 39 | 39 | 75.0 | |||
| Total: | 109 | 109 | 86 | 86 | 78.9 | ||||
| I: desmopressin + alfuzosin | ‐ | ‐/49 | ‐ | ‐ | 22 | 22 | ‐ | 3 months | |
| C: alfuzosin | ‐ | ‐ | ‐ | 23 | 23 | ‐ | |||
| Total: | 49 | ‐ | 45 | 45 | 91.8 | ||||
| I: desmopressin | 55 | 341/224 | 86 | 86 | 86 | 81 | 94.2 | 3 weeks | |
| C: placebo | 55 | 65 | 65 | 65 | 62 | 95.4 | |||
| Total: | 151 | 151 | 151 | 143 | 94.7 | ||||
| I: desmopressin | ‐ | 93/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 0.75 μg | ‐ | 3565/1707 | 458 | 448 | 448/252 | 401 | 87.5 | 12 weeks | |
| I2: desmopressin 1.0 μg | ‐ | 188 | 183 | 183/109 | 163 | 86.7 | |||
| I3: desmopressin 1.5 μg | ‐ | 452 | 439 | 439/251 | 387 | 85.6 | |||
| C: placebo | ‐ | 458 | 446 | 446/258 | 408 | 89.0 | |||
| Total: | 1556 | 1516 | 1516/870 | 1359 | 87.3 | ||||
| I: desmopressin + tamsulosin | ‐ | 435/427 | 205 | 205 | 205 | 196 | 95.6 | 8 weeks (cross‐over study design) | |
| C: tamsulosin + solifenacin | ‐ | 222 | 222 | 222 | 209 | 94.1 | |||
| Total: | 427 | 427 | 427 | 405 | 94.8 | ||||
| I: desmopressin | 45 | ‐/136 | NR | ‐ | 57 | 57 | ‐ | 12 months | |
| C: placebo | 45 | NR | ‐ | 58 | 58 | ‐ | |||
| Total: | 126 | ‐ | 115 | 115 | 91.3 | ||||
| I: desmopressin | ‐ | ‐/60 | 30 | 30 | 30 | 30 | 100.0 | 8 weeks | |
| C: placebo | ‐ | 30 | 30 | 30 | 30 | 100.0 | |||
| Total: | 60 | 60 | 60 | 60 | 100.0 | ||||
| I1: desmopressin 10 µg | ‐ | 1412/799 | 163 | 155 | 155/82 | 144 | 88.3 | 4 weeks | |
| I2: desmopressin 25 µg | ‐ | 158 | 152 | 152/87 | 148 | 93.7 | |||
| I3: desmopressin 50 µg | ‐ | 158 | 148 | 148/77 | 138 | 87.3 | |||
| I4: desmopressin 100 µg | ‐ | 160 | 146 | 146/80 | 135 | 84.4 | |||
| C: placebo | ‐ | 160 | 156 | 156/90 | 145 | 90.6 | |||
| Total: | 799 | 757 | 757/416 | 710 | 88.9 | ||||
| I1: desmopressin 50 µg | 130 | 1013/395 | ‐ | 119 | 119 | 100 | ‐ | 3 months | |
| I2: desmopressin 75 µg | 130 | ‐ | 124 | 124 | 103 | ‐ | |||
| C: placebo | 130 | ‐ | 142 | 142 | 120 | ‐ | |||
| Total: | 395 | 385 | 385 | 323 | 81.8 | ||||
| I1: desmopressin 10 µg | ‐ | 177/139 | 28 | 28 | 23/11 | 23 | 82.1 | 3 months | |
| I2: desmopressin 25 µg | ‐ | 25 | 25 | 22/11 | 22 | 88.0 | |||
| I3: desmopressin 50 µg | ‐ | 29 | 29 | 21/10 | 21 | 72.4 | |||
| I4: desmopressin 100 µg | ‐ | 30 | 30 | 23/11 | 23 | 76.6 | |||
| C: placebo | ‐ | 27 | 27 | 23/11 | 23 | 85.1 | |||
| Total: | 139 | 139 | 112/54 | 112 | 80.5 | ||||
| Overall total | Men | ‐ | ‐ | ‐ | 2966 | ‐ | |||
| Women | ‐ | 1045 | |||||||
| Total: | 4195 | 4011 | |||||||
| ‐ denotes not reported; C: comparator; I: intervention; ITT: intention‐to‐treat; n: number of participants. 1Follow‐up under randomised conditions until end of trial or if not available, duration of intervention; extended follow‐up refers to follow‐up of participants once the original study was terminated as specified in the power calculation. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| 1.1 Intranasal | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 1.2 Sublingual | 3 | 851 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.71, ‐0.03] |
| 1.3 Oral | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.41, ‐0.86] |
| 2 Number of nocturnal voids (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.17, ‐0.53] |
| 3 Major adverse events (short term) (subgroup route and dose) Show forest plot | 2 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.10, 9.03] |
| 3.1 Sublingual/low dose | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| 3.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| 4 Major adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| 5 Duration of first sleep episode (short term) Show forest plot | 4 | 652 | Mean Difference (IV, Random, 95% CI) | 54.61 [13.97, 95.25] |
| 6 Duration of first sleep episode (intermediate term) Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 18.40 [11.60, 25.20] |
| 7 Time to first void (short term) [minutes] Show forest plot | 1 | 383 | Mean Difference (IV, Random, 95% CI) | 40.8 [17.07, 64.53] |
| 8 Minor adverse events (short term) Show forest plot | 3 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 9 Minor adverse events (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.49] |
| 10 Treatment withdrawal due to adverse event (short term) Show forest plot | 4 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.15] |
| 11 Treatment withdrawal due to adverse event (intermediate term) Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.13, 73.39] |
| 12 Number of nocturnal voids (short term) (subgroup dose) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.98, ‐0.33] |
| 12.1 Very low dose (≤ 1.5 μg) | 1 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 12.2 Low dose (< 100 μg) | 3 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.72, 0.06] |
| 12.3 High dose (≥ 100 μg) | 4 | 401 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.41, ‐0.66] |
| 13 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 6 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| 13.1 Men with nocturia | 5 | 1867 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.73, ‐0.17] |
| 13.2 Men with nocturia and nocturnal polyuria | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.64, ‐0.92] |
| 14 Number of nocturnal voids (short term) (sensitivity run‐in period) Show forest plot | 4 | 966 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.17, ‐0.14] |
| 15 Major adverse events (short term) (sensitivity run‐in period) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.73, 8.73] |
| 16 Number of nocturnal voids (short term) (sensitivity clinical dosage) Show forest plot | 6 | 1586 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.15, ‐0.38] |
| 17 Major adverse events (short term) (sensitivity clinical dosage) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.71] |
| 17.1 Sublingual/low dose | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.71, 10.11] |
| 17.2 Oral/high dose | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of first sleep episode (short term) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 90.0 [1.95, 178.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.20, 0.80] |
| 2 Quality of life (short term) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.35, 0.35] |
| 3 Major adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Minor adverse events (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.07, 15.57] |
| 5 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 1.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.90, ‐0.42] |
| 1.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.60, ‐0.02] |
| 2 Number of nocturnal voids (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 2.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.74, 0.14] |
| 2.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.86, ‐0.18] |
| 3 Quality of life (short term) (subgroup route and dose) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 3.1 Sublingual/low dose | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.49, 0.03] |
| 3.2 Oral/high dose | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.85, ‐0.03] |
| 4 Quality of life (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 4.1 Men with nocturia | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.84, 0.44] |
| 4.2 Men with nocturia and nocturnal polyuria | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.67, ‐0.01] |
| 5 Major adverse events (short term) (subgroup route and dose) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 5.1 Sublingual/low dose | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Oral/high dose | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 6 Major adverse events (short term) (subgroup nocturnal polyuria) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 6.1 Men with nocturia | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Men with nocturia and nocturnal polyuria | 2 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.32] |
| 7 Minor adverse events (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.15, 16.82] |
| 8 Treatment withdrawal due to adverse event (short term) Show forest plot | 3 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.46, 17.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of nocturnal voids (short term) Show forest plot | 1 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.97, 0.11] |
| 2 Major adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Minor adverse events (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |
| 4 Treatment withdrawal due to adverse event (short term) Show forest plot | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.98] |

