تاثیر طب سوزنی در تسکین درد نوروپاتیک در بزرگسالان
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
-
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011b; Moore 2011c), back pain (Moore 2010b), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
-
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
-
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014a; Straube 2008; Sultan 2008). A Cochrane Review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
-
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010d; Moore 2014b).
-
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. GRADE: assessing the evidence
Quality of the evidence
Two review authors (ZYJ, YY) independently rated the quality of the outcomes. We used the GRADE system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (GRADEpro GDT 2015), and the guidelines provided in Chapter 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect;
-
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
-
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
-
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Schünemann 2011b).
-
High: randomised trials; or double‐upgraded observational studies.
-
Moderate: downgraded randomised trials; or upgraded observational studies.
-
Low: double‐downgraded randomised trials; or observational studies.
-
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
-
limitations in the design and implementation of available studies suggesting high likelihood of bias;
-
indirectness of evidence (indirect population, intervention, control, outcomes);
-
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
-
imprecision of results (wide confidence intervals);
-
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
-
large magnitude of effect;
-
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
-
dose‐response gradient.
We decreased the grade rating by one (‐ 1) or two (‐ 2) (up to a maximum of ‐ 3 to 'very low') if we identified:
-
serious (‐ 1) or very serious (‐ 2) limitation to study quality;
-
important inconsistency (‐ 1);
-
some (‐ 1) or major (‐ 2) uncertainty about directness;
-
imprecise or sparse data (‐ 1);
-
high probability of reporting bias (‐ 1).
Appendix 3. Search strategy for CENTRAL (CRSO)
#1 MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES
#2 MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES
#3 MESH DESCRIPTOR Somatosensory Disorders EXPLODE ALL TREES
#4 (((pain* or discomfort*) adj5 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*))):TI,AB,KY
#5 (((neur* or nerv*) adj5 (compress* or damag*))):TI,AB,KY
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MESH DESCRIPTOR Acupuncture
#8 MESH DESCRIPTOR Acupuncture Therapy EXPLODE ALL TREES
#9 ((acupuncture or acupoint* or meridian*)):TI,AB,KY
#10 ((electroacupuncture or electro‐acupuncture)):TI,AB,KY
#11 ((acupressure* or mox* or needling or acup* point*)):TI,AB,KY
#12 #7 OR #8 OR #9 OR #10 OR #11
#13 #6 AND #12
Appendix 4. Search strategy for MEDLINE via Ovid
1 exp Neuralgia/
2 exp Peripheral Nervous System Diseases/
3 exp Somatosensory Disorders/
4 ((pain* or discomfort*) adj5 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).tw.
5 ((neur* or nerv*) adj5 (compress* or damag*)).tw.
6 or/1‐5 (199037)
7 Acupuncture/
8 exp Acupuncture Therapy/
9 (acupuncture or acupoint* or meridian*).tw.
10 (electroacupuncture or electro‐acupuncture).tw.
11 (acupressure* or mox* or needling or acup* point*).tw.
12 or/7‐11
13 6 and 12
14 randomized controlled trial.pt.
15 controlled clinical trial.pt.
16 randomized.ab.
17 placebo.ab.
18 drug therapy.fs.
19 randomly.ab.
20 trial.ab.
21 groups.ab.
22 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
23 exp animals/ not humans.sh.
24 22 not 23
25 13 and 24
Appendix 5. Search strategy for Embase via Ovid
1 exp Neuralgia/
2 exp Peripheral Nervous System Diseases/
3 exp Somatosensory Disorders/
4 ((pain* or discomfort*) adj5 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).tw.
5 ((neur* or nerv*) adj5 (compress* or damag*)).tw.
6 or/1‐5
7 Acupuncture/
8 exp Acupuncture Therapy/
9 (acupuncture or acupoint* or meridian*).tw.
10 (electroacupuncture or electro‐acupuncture).tw.
11 (acupressure* or mox* or needling or acup* point*).tw.
12 or/7‐11
13 6 and 12
14 random$.tw.
15 factorial$.tw.
16 crossover$.tw.
17 cross over$.tw.
18 cross‐over$.tw.
19 placebo$.tw.
20 (doubl$ adj blind$).tw.
21 (singl$ adj blind$).tw.
22 assign$.tw.
23 allocat$.tw.
24 volunteer$.tw.
25 Crossover Procedure/
26 double‐blind procedure.tw.
27 Randomized Controlled Trial/
28 Single Blind Procedure/
29 or/14‐28
30 (animal/ or nonhuman/) not human/
31 29 not 30
32 13 and 31
33 limit 32 to embase
Appendix 6. China National Knowledge Infrastructure (CNKI)
#1 Neuralgia*:ti,ab,kw
#2 Neurodynia*:ti,ab,kw
#3 Paroxysmal neuralgia*:ti,ab,kw
#4 Nerve pain*:ti,ab,kw
#5 Spontaneous pain*:ti,ab,kw
#6 Sciatic*:ti,ab,kw
#7 Sciatic neuritis*:ti,ab,kw
#8 Sciatica*:ti,ab,kw
#9 Causalgia*:ti,ab,kw
#10 Peripheral Nerve Disease*:ti,ab,kw
#11 Peripheral Nervous System Disease*:ti,ab,kw
#12 Peripheral Nervous System Disorder*:ti,ab,kw
#13 Peripheral Neuropathy:ti,ab,kw
#14 PNS Disease*:ti,ab,kw
#15 or/1‐14
#16 Acupuncture:ti,ab,kw
#17 Needl*:ti,ab,kw
#18 Moxibustion*:ti,ab,kw
#19 or/16‐18
#20 Randomized Controlled Trial:full text
#21 Controlled Clinical Trial:full text
#22 Random*:full text
#23 or/20‐22
#24 and/15,19,23
Appendix 7. Chinese BioMedical Literature Database (CBM)
#1 MeSH:Neuralgia/explode all trees
#2 MeSH:Sciatica/explode all trees
#3 MeSH:Causalgia/explode all trees
#4 MeSH:Peripheral Nervous System Diseases/explode all trees
#5 Neuralgia*:ti,ab,kw
#6 Neurodynia*:ti,ab,kw
#7 Paroxysmal neuralgia*:ti,ab,kw
#8 Nerve pain*:ti,ab,kw
#9 Spontaneous pain*:ti,ab,kw
#10 Sciatic*:ti,ab,kw
#11 Sciatic neuritis*:ti,ab,kw
#12 Sciatica*:ti,ab,kw
#13 Causalgia*:ti,ab,kw
#14 Peripheral Nerve Disease*:ti,ab,kw
#15 Peripheral Nervous System Disease*:ti,ab,kw
#16 Peripheral Nervous System Disorder*:ti,ab,kw
#17 Peripheral Neuropathy:ti,ab,kw
#18 PNS Disease*:ti,ab,kw
#19 or/1‐18
#20 MeSH:Acupuncture/explode all trees
#21 MeSH:Acupuncture Therapy/explode all trees
#22 MeSH:Needling Methods/explode all trees
#23 MeSH:Electroacupuncture/explode all trees
#24 MeSH:Needle Warming Therapy/explode all trees
#25 MeSH:Microwave Acupuncture/explode all trees
#26 MeSH:Specific Tissue Acupuncture/explode all trees
#27 MeSH:Specific Region Acupuncture/explode all trees
#28 MeSH:Manual Acupuncture/explode all trees
#29 MeSH:Air Acupuncture Therapy/explode all trees
#30 MeSH:Cutaneous Acupuncture/explode all trees
#31 MeSH:Laser Acupuncture/explode all trees
#32 MeSH:Fire‐Needle Therapy/explode all trees
#33 MeSH:Electric Stimulation Therapy/explode all trees
#34 MeSH:Di‐Needle Therapy/explode all trees
#35 MeSH:Pricking Blood Therapy/explode all trees
#36 MeSH:Long Needle Therapy/explode all trees
#37 MeSH:Flint Acupuncture/explode all trees
#38 Acupuncture:ti,ab,kw
#39 Needl*:ti,ab,kw
#40 Moxibustion*:ti,ab,kw
#40 or/20‐39
#41 MeSH:Randomized Controlled Trial/explode all trees
#42 MeSH:Randomized Controlled Trial/publication type
#43 MeSH:Controlled Clinical Trial/explode all trees
#44 MeSH:Controlled Clinical Trial/publication type
#45 Random*:ti,ab,kw
#46 or/41‐45
#47 and/19,40,46
Appendix 8. Wanfang Database
#1 Neuralgia*:ti,ab,kw
#2 Neurodynia*:ti,ab,kw
#3 Paroxysmal neuralgia*:ti,ab,kw
#4 Nerve pain*:ti,ab,kw
#5 Spontaneous pain*:ti,ab,kw
#6 Sciatic*:ti,ab,kw
#7 Sciatic neuritis*:ti,ab,kw
#8 Sciatica*:ti,ab,kw
#9 Causalgia*:ti,ab,kw
#10 Peripheral Nerve Disease*:ti,ab,kw
#11 Peripheral Nervous System Disease*:ti,ab,kw
#12 Peripheral Nervous System Disorder*:ti,ab,kw
#13 Peripheral Neuropathy:ti,ab,kw
#14 PNS Disease*:ti,ab,kw
#15 or/1‐14
#16 Acupuncture:ti,ab,kw
#17 Needl*:ti,ab,kw
#18 Moxibustion*:ti,ab,kw
#19 or/16‐18
#20 Randomized Controlled Trial:all fields
#21 Controlled Clinical Trial:all fields
#22 Random*:all fields
#23 or/20‐22
#24 and/15,19,23
Appendix 9. Chongqing Weipu (VIP)
#1 Neuralgia*:ti,ab
#2 Neurodynia*:ti,ab
#3 Paroxysmal neuralgia*:ti,ab
#4 Nerve pain*:ti,ab
#5 Spontaneous pain*:ti,ab
#6 Sciatic*:ti,ab
#7 Sciatic neuritis*:ti,ab
#8 Sciatica*:ti,ab
#9 Causalgia*:ti,ab
#10 Peripheral Nerve Disease*:ti,ab
#11 Peripheral Nervous System Disease*:ti,ab
#12 Peripheral Nervous System Disorder*:ti,ab
#13 Peripheral Neuropathy:ti,ab
#14 PNS Disease*:ti,ab
#15 or/1‐14
#16 Acupuncture:ti,ab
#17 Needl*:ti,ab
#18 Moxibustion*:ti,ab
#19 or/16‐18
#20 Randomized Controlled Trial:any fields
#21 Controlled Clinical Trial:any fields
#22 Random*:any fields
#23 or/20‐22
#24 and/15,19,23

Study flow diagram
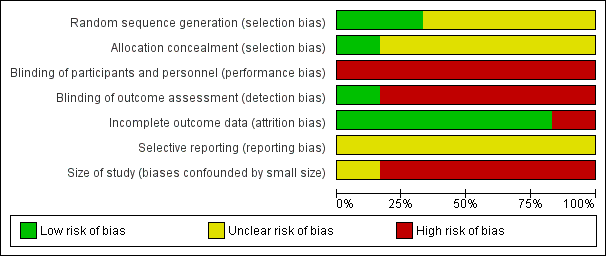
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Acupuncture alone versus other active therapy, Outcome 1 Any pain‐related outcomes: no clinical response ‐ defined by original study.
| Acupuncture versus sham acupuncture for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: sham acupuncture | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | Mean 6.2 | Mean 5.8 | The mean participant‐reported pain intensity in the intervention group was | 45 | ⊕⊝⊝⊝ | Acupuncture has no clinical significant beneficial effects on pain intensity compared to sham acupuncture. |
| Participant‐reported pain relief substantial (at least 50% pain relief over baseline) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | Mean 27.7 | Mean 37.7 | The mean bodily pain component of quality of life in the intervention groups was 10 higher | 45 | ⊕⊝⊝⊝ | Acupuncture has no beneficial effects on bodily pain compared to sham acupuncture. |
| CI: confidence interval; MD: mean difference; SF‐36: Short Form (36) Health Survey (SF‐36); VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGarrow 2014 recruited 59 participants initially; there were 14 withdrawals and only the 45 participants that completed treatment were included in the study's final results. | ||||||
| Acupuncture versus treatment as usual for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: treatment as usual | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect (Not applicable) | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participant‐reported pain relief | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| GRADE Working Group grades of evidence | ||||||
| Acupuncture versus other active therapy for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: other active therapy | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect (Not applicable) | No of Participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participant‐reported pain relief | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| GRADE Working Group grades of evidence | ||||||
| Acupuncture combined with other active therapy versus other active therapy for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: other active therapy alone | ||||||
| Outcomes | Other active therapy | Acupuncture combined with other active therapy | Relative effect | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | Mean 4.25 | Mean 3.23 | The mean participant‐reported pain intensity in the intervention groups was | 104 | ⊕⊝⊝⊝ | Acupuncture combined other active therapy has no clinical significant beneficial effects on pain intensity compared to other active therapy alone. |
| Participant‐reported pain relief substantial (at least 50% pain relief over baseline) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | Mean 35.17 | Mean 32.98 | The mean bodily pain component of quality of life in the intervention groups was 2.19lower | 104 | ⊕⊕⊝⊝ | Acupuncture combined other active therapy improved the quality of life compared to other active therapy alone. |
| CI: confidence interval; FACT/the GOG‐Ntx: Functional Assessment of Cancer Therapy/Gynaecologic Oncology Group/Neurotoxicity; MD: mean difference; VAS: Visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded twice for study limitations (risk of bias) due to high risk of performance and detection bias. | ||||||
| Acupuncture points used | Study ID |
| Taixi (KI3); Hegu (LI4); Taichong (LR3); Sanyinjiao (SP6); Zusanli (ST36) | |
| Shenmai (B62); Zulinqi (GB41); Zhaohai (K6); Lieque (L7); Neiguan (P6); Houxi (SI3); Waiguan (SJ5); Gongsun (SP4) | |
| Feishu (BL13); Geshu (BL17); Feiyang (BL58); Zulinqi (GB41); Zhiyang (GV9); Shendao (GV11); Shenzhu (GV12); Dazhui (GV14); Taichong (LR3); Sanyinjiao (SP6); Xuehai (SP10); Tianshu (ST25); Zusanli (ST36); Xiangu (ST43) | |
| The main points: Huantiao (GB30); Yanglingquan (GB34); Sanyinjiao (SP6); Zusanli (ST36); The auxiliary points (selected 2‐3from following): Shenshu (BL23); Kunlun (BL60); Guanyuan (CV4); Qihai (CV6); Huantiao (GB30); Taixi (K3); Taichong (LIV3); Pishu (PL20) | |
| The main points: Ganshu (BL18); Pishu (BL20); Shenshu (BL23); Yishu; Feishu (BL58); Zusanli (ST36); Sanyinjiao (SP6), Taibai (SP3); Zutonggu; Qihai (CV6); Guanyuan (CV4); Fenglong(ST40) and Yanglingquan (GB34); The auxiliary points: Jianyu (LI15); Quchi (LI11); Shousanli (LI10); Hegu (LI4); Biguan (ST31); Futu (ST32); Liangqiu (ST34); Xiangu (ST43) and Neiting (ST 44); Added for blood stasis points: Geshu (BL17) and Xuehai (SP10); Added for severe numbness of the hands and feet points: Bafeng(EX‐LE10) and Baxie (EX‐UE9). |
| Outcomes | Scales | Description of scales | Relevant Studies |
| Participant‐reported pain intensity | Visual Analogue Scale (VAS) | The VAS is a visual analogue scale for pain intensity, in which 0 means no pain and 10 (or 100) means the worst pain ever experienced. | |
| Quality of life | Short Form (36) Health Survey (SF‐36) | The SF‐36 is a 36‐item, patient‐reported survey of patient health and consists of 8 scaled scores, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0‐100 scale on the assumption that each question carries equal weight. The lower the score, the more disability. The 8 sections are: vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning and mental health. Summary scores for the SF‐12, version 2 (SF‐12v2) health status measure are based on scoring coefficients derived for version 1 of the SF‐36. The higher score is better. | |
| Functional Assessment | The FACT/GOG‐Ntx questionnaire is used to investigate patients' daily activities and evaluate the degree of neuropathy. The questionnaire includes 7 questions about physical well‐being, 7 questions about social/family well‐being, 6 questions about emotional well‐being, 7 questions about functional well‐being and 9 questions about additional concerns. Where in each question, 0 = not at all and 4 = very much, lower is better. |
| Acupuncture versus sham acupuncture | ||||||||||
| Outcome | Specific measurement | Study | Manual acupuncture group | Sham acupuncture group | Effect measure | Statistical test | ||||
| Mean | SD | Total | Mean | SD | Total | MD (95%CI) | P value | |||
| Pain intensity | VASa | 5.8 | 2.6 | 24 | 6.2 | 2.3 | 21 | ‐0.40 (‐1.83 to 1.03) | 0.58 | |
| Quality of life | SF‐36b: physical health score | 31.9 | 9.2 | 24 | 32.1 | 9.8 | 21 | ‐0.20 (‐5.78 to 5.38) | 0.94 | |
| SF‐36: mental health score | 39.2 | 14 | 24 | 35.7 | 12.6 | 21 | 3.50 (‐4.17 to 11.27) | 0.38 | ||
| SF‐36: bodily pain score | 37.7 | 27.4 | 24 | 27.7 | 16.9 | 21 | 10.00 (‐3.13 to 23.13) | 0.14 | ||
| Acupuncture + other active therapies versus other active therapies | ||||||||||
| Outcome | Specific measurement | Study | Acupuncture + other active therapies group | Other active therapies group | Effect measure | Statistical test | ||||
| Mean | SD | Total | Mean | SD | Total | MD (95%CI) | P value | |||
| Pain intensity | VAS | 3.23 | 0.17 | 52 | 4.25 | 0.197 | 52 | ‐1.02 (‐1.09 to ‐0.95) | < 0.00001 | |
| Quality of life | FACT/the GOG‐Ntxc | 32.98 | 0.542 | 52 | 35.17 | 0.518 | 52 | ‐2.19 (‐2.39 to ‐1.99) | < 0.00001 | |
| MD: mean difference; SD: standard deviation | ||||||||||
| Acupuncture versus sham acupuncture | ||||||||
| Outcome | Study | Manual acupuncture group | Sham acupuncture group | Effect measure | Statistical test | |||
| Events | Total | Events | Total | RR (95%CI) | NNTB | P value | ||
| Withdraw from trial due to any cause | 4 | 28 | 10 | 31 | 0.44 (0.16 to 1.25) | NNTB = 6 | 0.53 | |
| Adverse events: any cases | 1 | 28 | 2 | 31 | 0.55 (0.05 to 5.78) | NNTB = 34 | 0.62 | |
| Acupuncture + other active therapies versus other active therapies | ||||||||
| Outcome | Study | Acupuncture + other active therapies group | Other active therapies group | Effect measure | Statistical test | |||
| Events | Total | Events | Total | RR (95%CI) | NNT | P value | ||
| Any pain‐related outcomes: no clinical response | 4 | 30 | 10 | 30 | 0.40 (0.14 to 1.14) | NNTB = 5 | 0.09 | |
| Withdraw from trial due to any cause | 3 | 52 | 3 | 52 | 1.00 (0.21 to 4.73) | NA | 1.00 | |
| NA: not applicable; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pain‐related outcomes: no clinical response ‐ defined by original study Show forest plot | 3 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.51] |

