Intervenciones para la prevención y el tratamiento de la enfermedad hepática avanzada en la fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012056.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 30 marzo 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
| Roles and responsibilities | |
| Task | Who will undertake the task? |
| Protocol stage: draft the protocol | SK, SM, NNT, IVM, AWT |
| Review stage: select which trials to include (2 + 1 arbiter) | SK, NNT, AWT |
| Review stage: extract data from trials (2 people) | SK, SM |
| Review stage: enter data into RevMan | SK, IVM, NNT |
| Review stage: carry out the analysis | SK, SM, NNT, AWT |
| Review stage: interpret the analysis | SK, SM, IVM, AWT |
| Review stage: draft the final review | SK, NNT, IVM, AWT |
| Update stage: update the review | SK, NNT, IVM, AWT |
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors: none known.
Acknowledgements
We would like to take this opportunity to express our gratitude to Mrs Nikki Jahnke, Managing Editor at the Cochrane Cystic Fibrosis and Genetic Disorders (CFGD) Group who supported us throughout this work. We are thankful for her inspiring guidance, and friendly advice on writing this review. We thank Dr. Soe Moe, Community Medicine Department, Melaka Manipal Medical College for her contribution in previous version of the review. We also would also like to express our warm thanks to Professor Jaspal Singh Sahota, Chief Executive of Melaka Manipal Medical College and Professor Adinegara Lutfi Abas, Dean of Faculty of Medicine, Melaka Manipal Medical College, for their support in writing this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Mar 30 | Interventions for preventing and managing advanced liver disease in cystic fibrosis | Review | Senthil K Palaniappan, Nan Nitra Than, Aung Win Thein, Indra van Mourik | |
| 2017 Aug 29 | Interventions for preventing and managing advanced liver disease in cystic fibrosis | Review | Senthil K Palaniappan, Nan Nitra Than, Aung Win Thein, Soe Moe, Indra van Mourik | |
| 2016 Jan 27 | Interventions for managing advanced liver disease in cystic fibrosis | Protocol | Senthil K Palaniappan, Nan Nitra Than, Soe Moe, Indra van Mourik, Aung Win Thein | |
Differences between protocol and review
None
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Cystic Fibrosis [*complications, genetics];
- Cystic Fibrosis Transmembrane Conductance Regulator [genetics];
- Gastrointestinal Hemorrhage [prevention & control];
- Hypertension, Portal [prevention & control];
- Liver Diseases [etiology, prevention & control, *therapy];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
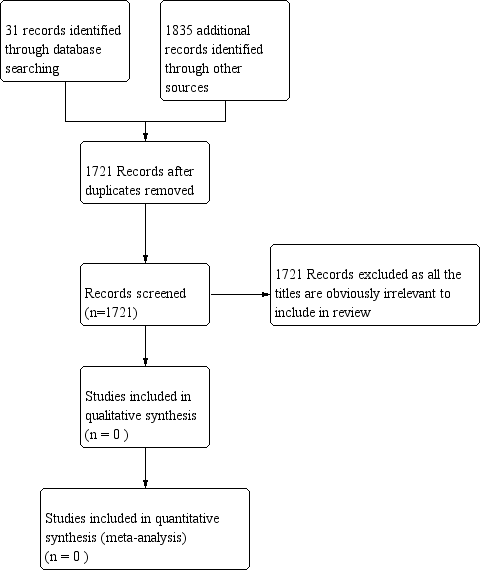
Figure 1 PRISMA study flow diagram

