Le yoga versus soins non‐standard pour le traitement de la schizophrénie
Résumé scientifique
Contexte
Le yoga est une pratique spirituelle ancienne originaire d'Inde et qui est actuellement acceptée dans le monde occidental comme une forme de relaxation et d'exercice physique. On s'est intéressé au yoga pour ses éventuelles vertus thérapeutiques pour les personnes souffrant de schizophrénie.
Objectifs
Évaluation systématique des effets du yoga versus soins non‐standard pour les personnes souffrant de schizophrénie.
Stratégie de recherche documentaire
L'Information spécialisée du registre du groupe Cochrane sur la schizophrénie a effectué une recherche dans son propre registre d'essais (dernière recherche réalisée le 30 mars 2017), qui est basé sur des recherches régulières dans MEDLINE, PubMed, Embase, CINAHL, BIOSIS, AMED, PsycINFO, et les registres d'essais cliniques. Nous avons effectué des recherches dans les références bibliographiques de toutes les études incluses. Il n'existe aucune restriction de langue, de date, de type de document ou de statut de publication pour l'inclusion des données dans le registre.
Critères de sélection
Tous les essais contrôlés randomisés (ECR) portant sur des patients atteints de schizophrénie et comparant le yoga avec les soins non‐standard. Nous avons inclus les essais qui répondaient à nos critères de sélection et rapportaient des données utilisables.
Recueil et analyse des données
L'équipe de la revue a sélectionné les études, évalué la qualité et extrait les données de manière indépendante. Pour les résultats binaires, nous avons calculé le risque relatif (RR) et son intervalle de confiance à 95 % (IC), avec une analyse en intention de traiter. Pour les données continues, nous avons estimé la différence moyenne (DM) entre les groupes et ses IC à 95 %. Nous avons utilisé un modèle à effets fixes pour les analyses. Nous avons examiné l'hétérogénéité des données (technique I2), évalué le risque de biais des études incluses et créé un tableau « Résumé des résultats » pour sept critères de jugement principaux d'intérêt en utilisant le système GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Résultats principaux
Nous avons pu inclure six études (586 participants). Les soins non‐standard étaient constitués uniquement d'un autre type d'exercice physique. Tous les résultats étaient à court terme (moins de six mois). Il y avait une nette différence dans les résultats de l'abandon précoce de l'étude (6 ECR, n = 586, RR de 0,64, IC entre 0,49 et 0,83, des preuves de qualité moyenne) en faveur du groupe du yoga. Il n'y avait aucune différence claire entre les groupes pour les autres critères de jugement. Ces études portaient sur l'état mental (amélioration sur l'échelle Positive and Negative Syndrome Scale, 1 ECR, n = 84, RR de 0,81, IC entre 0,62 et 1,07, preuves de faible qualité), le fonctionnement social (amélioration sur l'échelle Social Occupational Functioning Scale, 1 ECR, n = 84, RR de 0,90, IC entre 0,78 et 1,04, preuves de faible qualité), la qualité de vie liée à la santé mentale (le changement moyen 36‐Item Short Form Survey (SF‐36) en termes de qualité de vie sub‐scale, 1 ECR, n = 69, DM de ‐5,30, IC entre ‐17,78 et 7,18, preuves de faible qualité), la santé physique, (le changement moyen sur WHOQOL‐BREF physical‐health sub‐scale, 1 ECR, n = 69, DM de 9,22, IC entre ‐0,42 et 18,86, preuves de faible qualité). Une seule étude a rapporté des effets indésirables, ne trouvant aucune incidence d'événements indésirables dans aucun des deux groupes de traitement. Il y avait un nombre considérable de résultats manquants, qui incluaient les rechutes, les modifications cognitives, le coût des soins, l'effet sur les soins standard, les interventions de service, le handicap et les activités de la vie quotidienne.
Conclusions des auteurs
Nous avons trouvé des différences minimes entre le yoga et les soins non‐standard qui consistaient en un autre exercice de comparaison, qui pourrait être qualifié grossièrement d'exercices d'aérobie. Les critères de jugement ont été largement basés sur des études uniques avec des tailles d'échantillons limitées et un suivi à court terme. Dans l'ensemble, de nombreux critères de jugement n'étaient pas rapportés et les preuves présentées dans cette revue sont de qualité faible à modérée, donc trop faibles pour indiquer que le yoga est supérieur ou inférieur à des soins non‐standard pour la prise en charge des patients atteints de schizophrénie.
PICO
Résumé simplifié
Le yoga versus soins non‐standard pour le traitement de la schizophrénie
Problématique de l'étude
Le yoga est‐il un traitement adjuvant efficace par rapport à d'autres traitements complémentaires pour les personnes atteintes de schizophrénie ?
Contexte
Le yoga provient de l'Inde antique et implique des postures physiques et des exercices de respiration pour favoriser l'équilibre entre l'esprit et le corps. Le yoga est désormais largement adopté en tant que méthode de relaxation et en tant que discipline sportive, améliorant la force, la souplesse, la coordination, l'endurance, et le contrôle de la respiration et de la concentration. Il a aussi été démontré que le yoga permet de réduire le stress d'apporter à la fois santé et sentiment de bien‐être. Le yoga a été utilisé en tant que traitement complémentaire pour de nombreux problèmes de santé, y compris l'amélioration du contrôle de la pression artérielle, ainsi que les problèmes de santé mentale, tels que la dépression et les troubles anxieux.
Certaines recherches suggèrent que le yoga pourrait également être bénéfique en tant que traitement adjuvant pour réduire les symptômes complexes de la schizophrénie, trouble mental grave (tels que le fait d'entendre des voix, voir des choses, un manque d'intérêt pour les personnes et les activités, la fatigue, la perte des émotions et des symptômes de sevrage) et à améliorer la qualité de vie des personnes atteintes de schizophrénie. L'efficacité du yoga par rapport à d'autres traitements complémentaires disponibles (thérapies non‐médicamenteuses et non parlantes) n'est pas assez étudiée.
Recherche de preuves
Nous avons effectué des recherches électroniques de tests (la dernière recherche a été effectuée en mars 2017) pour des essais randomisés portant sur des patients atteints de schizophrénie pratiquant le yoga ou ayant un autre traitement adjuvant. Mille trente quatre dossiers ont été trouvés et vérifiés par les auteurs de la revue.
Eléments de preuve découverts
Six essais portant sur 586 participants remplissaient les critères requis pour être inclus dans cette revue et ont fourni des données utilisables. Les autres traitements adjuvants consistaient à pratiquer d'autres formes d'exercices. Il existe peu de preuves actuellement disponibles, elles sont de faible qualité, et suggèrent que le yoga n'est pas plus efficace que d'autres traitements complémentaires pour le traitement de la schizophrénie.
Conclusion
Les preuves actuelles issues d'essais contrôlés randomisés montrent que le yoga n'est pas plus efficace que d'autres traitements d'appoint pour le traitement de la schizophrénie, mais les seuls traitements disponibles comparés au yoga étaient d'autres formes d'exercice. Les preuves ne sont pas concluantes compte tenu du faible nombre d'études disponibles, et seul un suivi à court terme a été rapporté. De plus nombreux essais, à plus long terme et avec un plus large effectif, qui comparent le yoga à d'autres alternatives sont donc nécessaires.
Authors' conclusions
Summary of findings
| YOGA versus NON‐STANDARD CARE for schizophrenia | ||||||
| Patient or population: people with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YOGA versus NON‐STANDARD CARE | |||||
| Mental state: clinically important change Follow‐up: 4 months | Low1 | RR 0.81 | 84 | ⊕⊕⊝⊝ | ||
| 800 per 1000 | 648 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 729 per 1000 | |||||
| High1 | ||||||
| 1000 per 1000 | 810 per 1000 | |||||
| Global state: relapse | No trial reported this outcome. | |||||
| Social functioning: clinically important change | Low1 | RR 0.90 | 84 | ⊕⊕⊝⊝ | ||
| 700 per 1000 | 630 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 810 per 1000 | |||||
| Adverse effects ‐ any | See comment | See comment | Not estimable | 85 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The study reported no adverse effects. |
| Quality of life: clinically important change SF‐36 average change score mental health * | The mean quality of life: average change ‐ mental health in the intervention (yoga) groups was | 69 | ⊕⊕⊝⊝ | * no trial reported binary data; we chose 1 of 2 QOL measures as proxy measure | ||
| Physical health: clinically important change WHQOL‐BREF ‐ average change score * | The mean physical health: average change in the intervention (yoga) groups was | 41 | ⊕⊕⊝⊝ | * no trial reported binary data; we chose physical health dimension of QOL measure as proxy measure. | ||
| Costs: direct and indirect costs of care | No trial reported this outcome. | |||||
| Leaving the study early: short term | Low1 | RR 0.64 | 586 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 120 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 240 per 1000 | |||||
| High1 | ||||||
| 600 per 1000 | 360 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence CI: confidence interval; RR: risk ratio | ||||||
| 1 Moderate risk approximates to that of non‐standard care in trial(s). 3 Imprecision: Downgraded one level due to small sample size 7 Imprecision: Downgraded one level as based on one study with no reported adverse events. 8 Risk of bias: Downgraded one level as a number of participants withdrew from one trial and it was not clear to which group they were randomised. | ||||||
Background
Description of the condition
Schizophrenia is a relatively common mental disorder with a lifetime prevalence of 0.3% to 0.6% and an incidence of 10.2 to 22.0 per 100,000 (McGrath 2008). Schizophrenia is characterised by a constellation of symptoms that can present in a wide variety of ways depending on the individual. Symptoms can broadly be divided into 'positive' and 'negative' symptoms. Positive symptoms are delusions, hallucinations, disorganised speech, and disorganised behaviour. Negative symptoms are anhedonia (lack of pleasure), alogia (reduced speech), and affective flattening, or a lack of emotional responsiveness (Tandon 2013). Additionally, while they are not included in the current International Classification of Diseases (ICD‐10) or Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) diagnostic systems as diagnostic criteria, characteristic cognitive deficits are widely recognised in schizophrenia and are the target of considerable clinical and research attention (Carbon 2014).
Schizophrenia has been identified as a serious public health concern, ranking 11th in the causes of years lived with disability worldwide (Global Burden of Disease Study 2013). The mainstay of treatment is antipsychotic medication. A recent review highlighted that antipsychotic medication is associated with an increased risk for several physical diseases, including obesity, dyslipidaemia, diabetes mellitus, thyroid disorders, hyponatraemia, cardiovascular, respiratory tract, gastrointestinal, haematological, musculoskeletal, and renal diseases, as well as movement and seizure disorders (Correll 2015). Although antipsychotic medication is effective in reducing positive symptoms, usually within the early stages of treatment (Leucht 2013), it is of less benefit for negative symptoms and cognitive deficits (Fusar‐Poli 2015; Nielsen 2015). Unfortunately, it is these symptoms that cause the most disability (Vancampfort 2011; Vancampfort 2012). The side‐effect profile and inherent limitations of antipsychotics, as well as patient preference to avoid this route where possible, have resulted in additional non‐pharmacological interventions being utilised as either an adjunct or alternative to medication therapy (Kern 2009). Low‐cost treatments that decrease negative symptoms, reduce cognitive deficits, and promote mental and physical quality of life and functional recovery are warranted.
Description of the intervention
Yoga originates from India as an ancient Hindu practice incorporating physical postures with breathing exercises seeking to bring about a balance between the mental and physical state (Bussing 2012; Ross 2012; Sherman 2012). The principles behind its practice were first described by Patanjali, and were believed to allow the mind and the body to be prepared for spiritual development (Ross 2012). Today in the Western world, yoga has been widely adopted as both a method of relaxation and exercise. Hatha yoga is the most widely adopted practice used in the Western world (Collins 1998). Yoga's use of postures (asanas) improves strength, flexibility, co‐ordination, and endurance, and its use of breathing exercises (pranayama) improves respiratory control and concentration. Mantra yoga is a well‐known and widely practiced form of Hindu yoga that focuses on the use of chants to achieve mental and spiritual transformation (Sherman 2012).
With its increasing popularity, research into the effect of yoga on both physical and mental health has identified key benefits of yoga. It has been shown to both reduce stress and improve cognitive function in 'healthy' individuals and to be useful as a complementary therapy for many health conditions, including blood pressure control and mental health conditions such as depression and anxiety disorders (Bangalore 2012; Bussing 2012).
The benefits of yoga for other mental health conditions lead to research into the role of yoga as a complementary therapy for the management of schizophrenia. A systematic review of randomised controlled trials (RCTs) indicated that yoga could also be of benefit as an add‐on treatment to reduce both positive and negative symptoms of schizophrenia and improve the health‐related quality of life of people with schizophrenia (Vancampfort 2012a), although this review included only three trials. A further review echoed the possible increases in quality of life but highlighted that long‐term benefits are not known and did not report the safety of the intervention (Cramer 2013).
Non‐standard care could consist of other exercise (not including yoga), talking therapy, expressive therapies, or a combination of the above.
Exercise is "a subset of physical activity that is planned, structured, and repetitive and has as a final or an intermediate objective the improvement or maintenance of physical fitness" (Caspersen 1985). Exercise is intentional and has an aim of increasing one or more of the components of physical fitness (that is, cardiorespiratory fitness, muscular strength and endurance, body composition, flexibility, and neuromotor fitness), physical activity, or to improve a physical indicator such as blood pressure. As yoga is technically defined as an 'exercise', 'other exercise' will consist of any other activity that falls under the umbrella term of exercise, encompassing broad categories of skill‐related fitness, health‐related fitness, and body‐mind fitness, as well as physical activities that are not specifically fitness focused.
Expressive therapies include broad categories of art therapy, dance therapy, drama therapy, music therapy, and writing therapy. These represent different approaches, but the uniting principle is that these forms of therapy take place within a patient‐therapist relationship.
In art therapy, the person is directed to use a range of art materials to make images, and the focus is on the relationship between the image, the creator, and the therapist (Crawford 2007). Dance therapy is also sometimes referred to as dance‐movement therapy and dance has been used as a healing ritual since earliest human history, although there is no special therapeutic dance (Payne 2006; Ren 2013). Drama therapists use games, storytelling, and role‐play (Crawford 2007). Music therapy is often perceived as a psychotherapeutic method in the sense that it addresses intra‐ and interpsychic, as well as social processes by using musical interaction as a means of communication, expression, and transformation (Mössler 2011). Writing therapy uses the act of writing and processing the written word as a therapeutic tool.
Talking therapies can be considered to consist of, although not limited to, talking treatments, counselling, psychological therapies or treatments, and psychotherapies. Cognitive‐behavioural therapy (CBT) is one well‐recognised form of talking therapy. In CBT, links are made between the person’s feelings and patterns of thinking that underpin their distress. The person is encouraged to take an active part in the therapy by using the following techniques (Jones 2012).
-
Challenging habitual patterns of thinking.
-
Examining the evidence for and against the distressing beliefs.
-
Using reasoning abilities and personal experience to develop rational and personally acceptable alternative explanations and interpretations and to test these alternative explanations in real‐world situations. In addition, the beneficial effects of enhancing coping strategies and general problem‐solving skills are highlighted (Alford 1994; Tarrier 1993).
As CBT has latterly developed into a catch‐all term for a variety of interventions, we will incorporate the criteria developed by Jones 2012 in this review.
How the intervention might work
Yoga has been identified as having a role in regulating the autonomic nervous system (Varambally 2012a), decreasing sympathetic tone, creating a reaction the opposite to the fight‐or‐flight reaction. There is a subsequent effect on the limbic system and hypothalamic pituitary axis leading to a reduction in blood cortisol levels. This leads to a regulation of heart rate and blood pressure, which has obvious cardiovascular benefits (Damodaran 2002). Yoga also focuses on relaxed breathing, and this internal concentration is thought to reduce stress by minimising mental focus on external stressors or threats (Bangalore 2012). This decrease in cortisol levels is also believed to lead to better control of blood glucose, cholesterol, and total lipids. Since antipsychotic medication for the treatment of schizophrenia is associated with dyslipidaemia, diabetes, and obesity (Mitchell 2013), yoga may be a useful adjuvant to antipsychotic medication therapy to minimise these effects (Bangalore 2012). The improvement in the physical health of these patients could have a direct improvement in their mental health. Yoga is also identified as having a role in improving sleep (Collins 1998). There is also thought to be a role of oxytocin, a hormone related to improved mood, analogues of which have been suggested as possible treatment of schizophrenia (Bangalore 2012; Feifel 2011). Research has found that plasma levels of oxytocin are higher in people after practice of yoga (Varambally 2012a).
Mechanisms explaining the beneficial effects of exercise in people with schizophrenia have not yet been fully elucidated. At present, the plausible mechanisms for change in positive and negative symptoms through exercise fall into one of two broad testable hypotheses: (1) biochemical changes such as increased levels of neurotransmitters (for example endorphins, dopamine, or serotonin), which could be tested in schizophrenia‐like animal models, and (2) psychological changes such as social support, sense of autonomy, improved perceptions of competence, enhanced body image, self‐efficacy, and distraction. Cardio‐metabolic and neurochemical pathways between skeletal muscle, the spinal cord, and the brain offer plausible, testable mechanisms that might help explain the effects of exercise on brain health in people with schizophrenia. Previous research has demonstrated that changes in hippocampal volume and cortical thickening (or less thinning) following aerobic exercise are correlated with improvements in aerobic fitness measured by change in maximum oxygen consumption (Vancampfort 2014). The underlying mechanisms of brain volume increases resulting from improved aerobic fitness are still unknown, but recently it was shown that an increased production of brain‐derived neurotrophic growth factors probably plays a role (Kimhy 2015). More interventional and longitudinal exploration of the underlying mechanisms for brain health improvements in people with schizophrenia following exercise is needed. Future research could investigate whether, for example, exercise reduces the inflammatory status of the brain by increasing levels of the anti‐inflammatory cytokine interleukin‐10.
As expressive therapy consists of broad categories of art therapy, dance therapy, drama therapy, music therapy, and writing therapy, the effects of these treatments are diverse and not fully known. It is unclear whether the healing aspect of therapy is the process of the actual expressive therapy, the relationship that develops between the therapist and the patient, or most likely, a complex fusion of the two. Generally, research into the physiological and biochemical effects of these therapies in schizophrenia is in its infancy. From a social and emotional perspective, music therapy, for example, can have particular motivating, relationship‐building, and emotionally expressive qualities that may help those who do not respond to verbal therapy (Rolvsjord 2001; Solli 2008), while dance therapy can stimulate and release feelings, enable communication, and enhance non‐verbal contact. In addition, the non‐critical therapeutic setting can decrease anxiety (Ren 2013).
Talking therapies are a diverse set of treatments that can be considered under the following broad categories; cognitive‐behavioural, humanistic, insight‐oriented, postmodernist, systemic, and other, and are therefore associated with a broad range of effects, some of which are not fully understood. CBT, for instance, aims to offset distressing emotional experiences or dysfunctional behaviour by changing the way in which the individual interprets and evaluates the experience or cognates on its consequence and meaning (Jones 2012). Normalisation techniques as well as behavioural techniques are used to reduce distress and improve functioning (Naeem 2015). It has been proposed that CBT might also focus on the following (Birchwood 2006).
-
Distress reduction or the reduction of depression and problem behaviour associated with beliefs about psychotic symptomatology.
-
Emotional and interpersonal difficulties in individuals at high risk of developing psychosis.
-
Relapse prodromes to prevent relapse in psychosis.
-
Comorbid depression and social anxiety, including the person’s appraisal of the diagnosis and its stigmatising consequences.
-
General stress reactivity, thereby increasing resilience to life stress and preventing psychotic relapse.
-
Increasing self‐esteem and social confidence in people with psychosis.
Why it is important to do this review
It was originally envisaged that one Cochrane review entitled 'Yoga for schizophrenia' would adequately map this area, but on closer evaluation it became apparent that the yoga comparison included several distinct strands. We therefore made a pragmatic decision to logically group comparisons into a series of independent reviews (Table 1) conducted by the same core group of authors and synthesised into an overview entitled 'Yoga for schizophrenia, an overview of Cochrane systematic reviews'.
| Review title | Status |
| Yoga versus standard care for schizophrenia | Full review: Broderick 2015 |
| Yoga versus non‐standard care for schizophrenia | This review. |
| Yoga as part of a package of care versus standard care | Protocol: Broderick 2016b |
| Yoga as part of a package of care versus non‐standard care |
Due to a growing demand from people with schizophrenia for alternative or adjunct treatment to their medication and a prevalence of poor antipsychotic compliance (Elkins 2005; Van Os 2009), adjunctive options are increasingly important. Yoga and other approaches to non‐standard care (other exercise, expressive therapies, and talking therapies) are non‐pharmacologically based add‐on or adjunctive treatments in the management of schizophrenia. In resource‐constraint times the question arises: is yoga or other non‐standard care most efficacious for people with schizophrenia? This review will provide the most comprehensive answer possible to this question and may expedite the integration of yoga or other non‐standard care options into clinical practice.
Objectives
To systematically assess the effects of yoga versus non‐standard care for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We considered all relevant randomised controlled trials (RCTs). We included RCTs meeting our inclusion criteria and reporting useable data. We would have considered trials that were described as 'double blind' ‐ in which randomisation is implied ‐ and would have included or excluded such trials once we carried out a sensitivity analysis (see Sensitivity analysis). We excluded quasi‐randomised studies, such as those that allocate intervention by alternate days of the week. Where people were given additional treatments as well as yoga as a package of care, we only included data if the adjunct treatment was evenly distributed between groups and it was only the yoga intervention that was randomised.
Types of participants
We considered people with a diagnosis of schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder, and delusional disorder, regardless of their gender, age, or severity of illness, whose diagnosis was made by any means. We were interested in ensuring that information was as relevant to the current care of people with schizophrenia as possible, and so proposed, if possible, to clearly highlight the current clinical state (acute, early postacute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Yoga therapy*
Yoga, however defined by the study, incorporating any of the major subtypes such as Mantra, Laya, Hatha, and Raja. Definitions of yoga also include breathing exercises and/or meditation and/or body postures.
2. Non‐standard care*
It is accepted that non‐standard care could be considered an ambiguous term. We proposed to use the following breakdown, but recognised that this may not be entirely inclusive.
2.1 Other exercise (not including yoga)
This included broad categories of exercise focused on health‐related fitness, skills‐related fitness, mind‐and‐body fitness, and other physical activity not necessarily focused on fitness. We proposed to keep each of the above categories separate, as they represent quite different approaches.
2.2 Talking therapy
This included broad categories of cognitive‐behavioural, humanistic, insight‐oriented, postmodernist, systemic, and other. We proposed to keep each of the above categories separate, as they represent quite different approaches.
2.3 Expressive therapies
This include broad categories of art therapy, dance therapy, drama therapy, music therapy, and writing therapy. We propose to keep each of the above categories separate, as they represent quite different approaches.
2.4 Combination of above
Should at least five trials in any of these areas of non‐standard care become available, we will conduct an independent review.
* Both yoga and non‐standard care interventions are in addition to standard care , where standard care is the care a person would normally receive (see Differences between protocol and review).
Types of outcome measures
We aimed to divide all outcomes into short term (less than six months), medium term (seven to 12 months), and long term (over one year).
We endeavoured to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale, as defined within the trials) before any others. Thereafter, we listed other binary outcomes and then those that are continuous. Of note, to ensure uniformity with the portfolio of yoga reviews under construction, the following outcomes are consistent with reviews outlined in Table 1
Primary outcomes
1. Mental state
1.1 Clinically important change in mental state (as defined by individual studies)
1.2 Average endpoint score on mental state scales
1.3 Average change scores on mental state scales
2. Global state
2.1 Relapse
2.2 Clinically important change in global state (as defined by individual studies)
2.3 Any change in global state (as defined by individual studies)
2.4 Average endpoint or change scores from global state scales
3. Social functioning
3.1 Clinically important change in social functioning (as defined by individual studies)
3.2 Average endpoint score on social functioning scales
3.3 Average change scores on social functioning scales
4. Adverse effects
4.1 Any clinically important adverse effect
Secondary outcomes
5. Quality of life
5.1 Clinically important change in quality of life functioning (as defined by individual studies)
5.2 Average endpoint score on quality of life scales
5.3 Average change scores on quality of life scales
6. Cognitive functioning
6.1 Clinically important change in cognitive functioning (as defined by individual studies)
6.2 Average endpoint score on cognitive functioning scales
6.3 Average change scores on cognitive functioning scales
7. Leaving the study early
7.1 Any reason
7.2 Due to adverse effects of intervention
7.3 Due to lack of engagement with intervention
7.4 Due to death (suicide, natural causes, other)
8. Costs of care
8.1 Direct costs of care
8.2 Indirect costs of care
9. Effect on standard care
9.1 Reduction in reported adverse effects of standard care
9.2 Change in the level of standard care required to manage condition
10. Physical health
10.1 Clinically important change in physical health (as defined by individual studies)
10.2 Any change in physical health (as defined by individual studies)
11. Service use
11.1 Acute hospital admissions
11.2 Length of hospital stay
12. Disability
12.1 Clinically important change in disability (as defined by individual studies)
13. Daily living
13.1 Clinically important change in daily living skills (as defined by individual studies)
13.2 Average endpoint score daily living scales
13.3 Average change scores on daily living scales
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011) and to export data from this review to create summary of findings Table for the main comparison. This table provides outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision making. We aimed to select the following main outcomes for inclusion in the 'Summary of findings' table.
-
Mental state: clinically important change (as defined by individual studies)
-
Global state: relapse
-
Social functioning: clinically important change (as defined by individual studies)
-
Adverse effects: any clinically important effect
-
Quality of life: clinically important change (as defined by individual studies)
-
Physical health: clinically important change (as defined by individual studies)
-
Costs of care: indirect or direct costs of care
If data were not available for these pre‐specified outcomes but were available for ones that were similar, we presented the closest outcome to the pre‐specified one in the table but took this into account when grading the finding.
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group’s Register of Trials
The Information Scientist searched the Cochrane Schizophrenia Group’s Trials Register (latest 30 March 2017) using the following search strategy:
*Yoga* in Intervention of STUDY
In such a study‐based register, searching the major concept retrieves all the synonym keywords and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including MEDLINE, PubMed, Embase, AMED, BIOSIS, CINAHL, PsycINFO, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module for full details). There was no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Three review authors (JB, AW, and NC) independently inspected citations from the searches and identified relevant abstracts. We compared findings to ensure reliability. If disputes had arisen, we would have acquired the full‐text report for more detailed scrutiny. JB and AW obtained and inspected full‐text reports of the abstracts meeting the review criteria. CEA (see Acknowledgements) re‐inspected all identified reports in order to ensure reliable selection. If it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Review authors JB, AW, and NC independently extracted data from all included studies and then compared results of extracted data from all of the studies. Any disagreements were discussed with DV, decisions documented and, if necessary, we contacted authors of studies for clarification. With remaining problems, CEA helped to clarify issues, and we documented these final decisions. We extracted data presented only in graphs and figures whenever possible, but included data in the review only if two review authors independently had the same result. If studies had been multicentre, we would have extracted data relevant to each component centre separately. We reported total end‐scale measures, as opposed to sub‐scale measures, where possible. There were two exceptions: the Positive and Negative Syndrome Scale (PANSS), for which we reported positive syndrome scores and negative syndrome scores, as well as total scores. We also reported quality of life sub‐scale measures if total end‐score values were not reported. We attempted to contact authors through an open‐ended request to obtain missing information or for clarification whenever necessary.
2. Management
2.1 Forms
We extracted data onto standardised, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument were described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument had not been written or modified by one of the trialists for that particular trial.
The instrument should be a global assessment of an area of functioning and not sub‐scores which are not, in themselves, validated or shown to be reliable. However there are exceptions; we included sub‐scores from mental state scales measuring positive and negative symptoms of schizophrenia.
Ideally, the measuring instrument should either be i.) a self‐report or ii.) completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we noted in the Description of studies section if this was the case or not.
2.3 Endpoint versus change data
There are advantages to both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only used change data if the former were not available. We combined endpoint and change data in the analysis, as we preferred to use mean differences (MD) rather than standardised mean differences (SMD) throughout (Deeks 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
(Please note, we planned to enter all relevant data from studies of more than 200 participants in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies. We would have entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not.)
For endpoint data from studies of less than 200 participants:
a) When a scale started from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it would have strongly suggested a skew, and we would have presented these data as 'other data'. If this ratio was higher than 1 but below 2, there was suggestion of skew. We entered these data and tested whether their inclusion or exclusion would change the results substantially. Finally, if the ratio was larger than 2, we included these data, because skew is less likely (Altman 1996; Higgins 2011).
b) If a scale started from a positive value (such as the PANSS (Kay 1986), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew was present if 2 SD > (S ‐ S min), where S is the mean score and 'S min' was the minimum score.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that could be reported in different metrics, such as days in hospital (mean days per year, per week, or per month) to a common metric (for example, mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. We did this by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale, Overall 1962, or the PANSS, Kay 1986, this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the yoga intervention. Where adhering to this made it impossible to avoid outcome titles with clumsy double‐negatives (for example 'Not un‐improved'), we reported data where the left of the line indicates an unfavourable outcome. We noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors JB, AW, and NC worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011a). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
If the raters had disagreed, we would have made the final rating by consensus with the involvement of another member of the review group. Where details of randomisation and other characteristics of trials were inadequate, we contacted the study authors to obtain further information. We would have reported non‐concurrence in quality assessment, but if disputes had arisen as to which category a trial was to be allocated, again, we would have resolved this by discussion.
We noted the level of risk of bias in both the text of the Risk of bias in included studies table within the Characteristics of included studies and summary of findings Table for the main comparison for the main comparison.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio and its 95% confidence interval (CI). It has been shown that risk ratio is more intuitive than odds ratios and that clinicians tend to interpret odds ratios as risk ratio (Boissel 1999; Deeks 2000). The number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) statistics with their CIs are intuitively attractive to clinicians but are problematic both in their accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the summary of findings Table for the main comparison, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the mean difference between groups. We preferred not to calculate effect size measures (standardised mean difference). However, if scales of very considerable similarity were used, we calculated the effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster‐randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data pose problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, CIs unduly narrow, and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If we had included cluster‐randomised trials and if clustering had not been accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, if such studies are included, we will seek to contact first authors of studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and adjust for this using accepted methods (Gulliford 1999). If clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster‐randomised study, but adjusted for the clustering effect.
We sought statistical advice and were advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC (Design effect=1 + (m ‐ 1)*ICC) (Donner 2002). If the ICC was not reported, we will assume it to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. This occurs if an effect (for example, pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we had planned to use only the data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we will simply added these combined within the two‐by‐two table. If data were continuous, we combined data following the formula in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We determined that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses, (except for the outcome 'leaving the study early'). If, however, more than 50% of data in one arm of a study were lost, but the total loss was less than 50%, we would have marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). We assumed those participants leaving the study early to have the same rates of negative outcome as those who completed, with the exception of the outcomes of death and adverse effects. For these outcomes, we used the rate of those who stayed in the study, in that particular arm of the trial for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from participants who completed the study to that point were compared to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from participants who completed the study to that point were reported, we used these data.
3.2 Standard deviations
If in future updates standard deviations (SDs) are not reported, we will first try to obtain the missing values from the authors. If these are not available, where there are missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals are available for group means, and either P value or t value are available for differences in mean, we can calculate them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When only the SE is reported, SDs can be calculated by the formula SD=SE * square root (n). The Cochrane Handbook for Systematic Reviews of Interventions present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges, or other statistics (Higgins 2011). If these formulae do not apply, we will calculate the SDs according to a validated imputation method that is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless will examine the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data were used in the trial, if less than 50% of the data were assumed, we presented and used these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, in order to judge clinical heterogeneity. We simply inspected all studies for clearly outlying participants or situations that we had not predicted would arise. If such situations or participant groups had arisen, we would have fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, in order to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted would arise. If such methodological outliers had been present, we would have fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i.) magnitude and direction of effects and ii.) strength of evidence for heterogeneity (for example a P value from Chi2 test, or a confidence interval for I2). We planned to interpret an I2 estimate greater than or equal to around 50%, accompanied by a statistically significant Chi2 statistic, as evidence of substantial levels of heterogeneity (see Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Deeks 2011). If we had found substantial levels of heterogeneity in the primary outcome, we would have explored reasons for the heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systematic reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not intend to use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. Only six studies were included, therefore we did not prepare funnel plots. In future updates of the review, where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for the use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seemed to be true to us, and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. However, there is a disadvantage to the random‐effects model, in that it puts added weight onto small studies, which are often the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not anticipate a need for any subgroup analysis.
1.2 Clinical state, stage, or problem
We proposed to undertake this review as part of a family of similar reviews that will provide an overview of the effects of yoga for people with schizophrenia in general. In addition, we aimed to report data on subgroups of people in the same clinical state, stage, and with similar problems.
2. Investigation of heterogeneity
Where inconsistency was high, we reported this. Firstly, we investigated whether data had been entered correctly. Secondly, if data were correct, we visually inspected the graph and we removed outlying studies to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present these data. If not, we would not pool the data, and we would discuss these issues. We know of no supporting research for this 10% cut‐off, but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
If in future updates of this review unanticipated clinical or methodological heterogeneity is obvious, we will simply state hypotheses regarding these. We do not anticipate undertaking sensitivity analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we would have included these studies, and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we would have employed all relevant data from these studies.
2. Assumptions for lost binary data
Where we made assumptions regarding participants lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and when we used data only from participants who completed the study to that point. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
Had we needed to make assumptions regarding missing SDs data (see Dealing with missing data), we would have compared the findings of the primary outcomes when we used our assumption/s and when we used data only from participants who completed the study to that point. We would have undertaken a sensitivity analysis to test how prone results were to change when completer‐only data were compared to imputed data using the above assumption. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
3. Risk of bias
For the primary outcome, we analysed the effects of excluding trials that we judged to be at high risk of bias across relevant domains (see Assessment of reporting biases) for the meta‐analysis of the primary outcome. If excluding trials at high risk of bias had substantially altered the direction of effect or the precision of the effect estimates, then we would have included relevant data from these trials in the analysis.
4. Imputed values
We had intended to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials. No cluster‐randomised trials were included in this version of the review.
If we had noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not have pooled data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed‐effect and random‐effects
We synthesised data using a fixed‐effect model, however we also synthesised data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the result. If the significance of results had changed we would have noted this in the text.
Results
Description of studies
Please see Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies
Results of the search
In the search we undertook for this review, we found 1034 papers that were potentially relevant. We did not identify any duplicates. After removing 727 articles that were clearly irrelevant, we inspected 307 abstracts. From these, we selected 35 reports to further assess for inclusion. We excluded 18 reports (17 studies). 17 reports (7 studies) were then included in the qualitative anaylsis. One of these studies was added to 'ongoing studies' and six studies were included. The PRISMA table shows results of our search (see Figure 1).

37Study flow diagram for 2015 searches
Included studies
1. Allocation
No studies were double‐blind due to the nature of the intervention. In an effort to minimise bias, the majority of studies (5/6) stated that the outcomes assessor was blind to group allocation. Details regarding blinding were unclear in one study (Manjunath 2013). All studies were parallel studies and were stated to be randomised. Only one study (Lin 2015) reported outcomes immediately after intervention, with the remaining studies reporting outcomes after a follow‐up period of self‐practice (Behere 2011; Bhatia 2017; Duraiswamy 2007; Manjunath 2013; Varambally 2012).
2. Length of trials
The overall duration of all trials included was 'short term', consisting of 4.5 months in total: Manjunath 2013 (two weeks supervised, then four months of self‐practice); four months in two studies (Duraiswamy 2007), three weeks of supervised practice with a three month follow‐up, Varambally 2012: one month with three months of self‐practice at home). Lin 2015 was 12 weeks in duration. This trial, however, included a long‐term follow‐up at 18 months, but we were unable to use the 18‐month follow‐up data due dropout levels of > 50%. The longest trial we included was Bhatia 2017, which consisted of a 21‐day programme with self‐practice and follow‐up at three and six months.
3. Participants
A total of 586 people participated in the six studies. The largest study included data for 104 participants in the yoga arm and 90 in the non‐standard care arm (Bhatia 2017). Other studies consisting of > 100 participants were Varambally 2012 and Lin 2015. Both of these trials, however, included standard care arms which were not considered as part of this review. Both men and women were included in all studies with the exception of Lin 2015 which exclusively included females. All studies consistently employed the same diagnostic criteria (DSM‐IV) and all included people with schizophrenia. In Lin 2015 it was reported that 61 (53.5%) were diagnosed with schizophrenia, 13 (11.4%) had schizoaffective disorder, and 39 (34.2%) had schizophreniform, brief psychotic disorders and psychosis not otherwise specified. No report referred to the clinical state of participants in terms of the percentage of acute, early post‐acute, partial remission, or remission and similarly no report focused on people with particular problems, for example negative symptoms or treatment illnesses. All studies reported the mean specific length of illness which ranged from 36.8 months (3.1 years) to 129.7 months (10.8 years).
4. Setting
Manjunath 2013 took place in an inpatient setting. Four of the trials were set in outpatient departments (Behere 2011; Bhatia 2017; Lin 2015; Varambally 2012), and one in both an inpatient and outpatient setting (Duraiswamy 2007). Five studies took place in India, three within the same institute (National Institute of Mental Health and Neurosciences, Bangalore) (Behere 2011; Duraiswamy 2007; Varambally 2012); the two remaining Indian studies within large psychiatric institutes (Bhatia 2017; Manjunath 2013). Lin 2015 took place in China.
5. Interventions
The only available non‐standard care comparator to yoga was 'other exercise'. No studies compared yoga to other non‐standard care categories of talking therapies, expressive therapies or combinations of these therapies.
Two studies were two‐arm trials which compared yoga to non‐standard care which consisted of other exercise (Duraiswamy 2007; Manjunath 2013). Four studies were three‐arm trials which compared yoga, standard care and other exercise (Behere 2011;Bhatia 2017, Lin 2015, Varambally 2012).
5.1 Yoga
The yoga discipline was mentioned in one study only (Lin 2015), which referred to Hatha yoga. In the majority of remaining studies, (Behere 2011;Duraiswamy 2007; Manjunath 2013; Varambally 2012), the yoga practice was developed by the same school (Swami Vivekananda Yoga Anusandhana Samsthana) and the majority of these studies took place in the same centre (NIMHANS in Bangalore, India). The author affiliations for 4/6 of the listed study authors of this study was the NIMHANS, so considerable consistency was evidenced between trials.
The majority of studies described in this review (4/6) implemented the same yoga intervention which consisted of 'loosening exercises' for 10 minutes, yoga postures (asanas) for 20 minutes, breathing exercises for 18 minutes and relaxation for three minutes, which was approximately 45 minutes in total. In the Bhatia 2017 study the time in each individual component of the yoga programme was not detailed. In the study by Lin 2015, yoga consisted of 10 minutes breathing control, 10 minutes warm‐up, 30 minutes yoga postures and 10 minutes relaxation (60 minutes in total). Meditation was not included in any studies.
We excluded any combinations of yoga and other adjunctive practices such as counselling in an attempt to make the 'purest' comparison of yoga versus non‐standard care. By its nature however, yoga is a heterogeneous practice, intuitively adapted based on factors such as the energy needs of the group as well as the training of the yoga instructor. Even though most yoga practice and exercise practice was consistent with a duration of approximately 45 minutes to 60 minutes in total, exposure varied from 10 sessions to a maximum of 35. This difference in exposure and longer unsupervised period in the absence of adherence data means optimum yoga or exercise 'dosage' to effect results remains unclear.
5.2 Non‐standard care
The non‐standard care comparison consisted of exercise only. For the majority of studies 5/6 (Behere 2011; Bhatia 2017, Duraiswamy 2007; Manjunath 2013; Varambally 2012), the same 'physical exercise' regimen adopted from the National Fitness Corps (Ministry of Education 1965) was carried out, although some adaptation of this appears to be evident in the Bhatia 2017 study as 15 minutes of brisk walking was conducted at the outset. The total duration was one hour, and consisted of brisk walking (10 minutes), jogging (five minutes), exercise in standing (20 minutes), and sitting posture exercises (20 minutes), with two‐minute breaks with a non‐specific instruction of ‘just relax now’ between different exercises.
In the Lin 2015 study, the exercise element consisted of 12 weeks of treadmill walking for 15 to 20 minutes and stationary cycling for 25 to 30 minutes. During the exercise session, each participant's heart rate was continuously monitored using a portable recorder, the heart rate was maintained in the range of 45% to 49% of the VO2 max value. Even though the gold‐standard exercise testing is to be commended, it is questionable whether this low training intensity range could effect any physiological changes in participants, especially as progression of this intensity over the 12 weeks of the programme was not detailed. Furthermore, a 4% heart rate range would seem quite narrow, and may have been difficult to comply with in practice.
In the other five studies described within this review, there was consistency in the exercise and yoga regimens. The 'other exercise' arm were non‐specific programmes consisting mostly of general mobility exercises which returned to the 'position attention' that were devised from the National Fitness Corps in India dating back to a 1960s handbook. It would be questionable whether these exercise programmes would stand up to scientific scrutiny as basic exercise physiology principles were not really applied. There was no pre‐programme fitness test and exercise was not individually prescribed or described in terms of intensity or type.
5.3 Frequency and duration
The yoga and exercise programmes described in the included studies were heterogeneous in terms of frequency and duration. The frequency of yoga/exercise sessions provided varied from sessions daily over two weeks (at least 10 sessions) (Manjunath 2013), to three times weekly over 12 weeks (Lin 2015), to 25 sessions over one month (Varambally 2012), or five days a week for three weeks (Duraiswamy 2007), and on consecutive days (excluding Sundays and bank holidays) for 21 days in the study by Bhatia 2017. Two studies specified yoga was delivered over one month, but frequency was not specified (Behere 2011). The number of sessions provided therefore ranged from 10 (Manjunath 2013) to 36 sessions (Lin 2015).
We included data from only one study which assessed follow‐up straight after completion of the yoga intervention (Lin 2015). All other studies included a follow‐up period of four weeks (Manjunath 2013), two months (Behere 2011), three months (Duraiswamy 2007; Varambally 2012), and over five months Bhatia 2017 of ''self‐practice''.
5.4 Delivery
The yoga intervention was delivered by trained or certified yoga instructors in all studies. All studies employed supervised group yoga or exercise sessions with therapist:participant ratios of up to 1:5 (Bhatia 2017; Manjunath 2013), 1:5 to 10 (Lin 2015), or unclear/non‐specified (Behere 2011;Duraiswamy 2007; Varambally 2012).
The exercise intervention was delivered by the yoga instructor in a number of studies (Behere 2011; Manjunath 2013). Duraiswamy 2007 stated that a "therapist trained to teach both YT and PT taught the subjects in their allocated treatment groups". It was not specified if an exercise or yoga therapist delivered the intervention in two studies (Lin 2015; Varambally 2012). An instructor trained in physical education conducted the exercise component in the Bhatia 2017 study.
5.5 Compliance
It was specified that participants were expected to adhere to 70% to 75% of supervised sessions in two studies (Lin 2015; Varambally 2012). Expected adherence to the yoga or non‐standard care arms was not detailed in the remaining studies. In the Duraiswamy 2007 study, it was described that during the three months of self‐practice, the therapist reviewed the 'adherence and the correctness of yoga or physical exercises' once a month, and in addition, participants were also reminded through telephone and letters about practising the exercises, although the frequency of telephone and letter reminders was not detailed. Three studies (Behere 2011; Manjunath 2013; Varambally 2012) referred to relative/caregiver engagement with monitoring frequency of self‐practice at home. Monthly follow‐up appointments were also provided in the Varambally 2012 study and a relative who accompanied the patient during the one‐month training was ''requested to encourage'' the patient to practice at home. In this study, a log book was supplied to verify compliance with practice at home and participants were requested to bring this log book to their follow‐up appointment. It was reported that ''negligible'' numbers brought the books at follow‐up, so the authors were unable to evaluate adherence to the home‐practice. Again, participants were expected to self‐practice yoga or exercise at home in the study by Behere 2011. Only caregiver engagement in monitoring the yoga sessions however, not exercise, was described. A compliance log was completed by participants in the Bhatia 2017 study, which was collected at the three‐ and six‐month follow‐up. In this study a 'yoga training booklet' was given to participants, presumably to the exercise group as well. This may have influenced the randomisation process by the exercise group carrying out yoga practice during follow‐up but details regarding this practice were unclear.
Most studies did not describe any feasibility outcomes with the exception of Lin 2015, which specified adherence to the prescribed supervised yoga classes of 51% and exercise of 58% and Bhatia 2017 who reported compliance logs were returned by 87% in the yoga group and 85% in the exercise group at six months.
6. Outcomes
The following outcomes for which we could obtain usable data are listed below, followed by a summary of data that could not be used in this review as well as missing outcomes.
6.1 Outcome scales
6.1.1 Global State
i. Clinical Global Impression Severity (CGIS) (Guy 1976)START HERE
This seven‐point scale requires the clinician to rate the severity of the patient's illness at the time of assessment, relative to the clinician's past experience with patients who have the same diagnosis. Considering total clinical experience, a patient is assessed on severity of mental illness at the time of rating one, normal, not at all ill; two, borderline mentally ill; three, mildly ill; four, moderately ill; five, markedly ill; six, severely ill; or seven, extremely ill.
6.1.2 Mental state
i. Positive and Negative Syndrome Scale (PANSS) (Kay 1986)
This 30‐item scale assesses severity of psychotic symptomology in general. It consists of three sub‐scales; positive symptoms, negative symptoms general psychopathology, and a total score. Scoring ranges from one to seven, with a low score indicating a lesser severity of symptoms (1=absent, 2=minimal, 3=mild, 4=moderate, 5=moderate severe, 6=severe, 7=extreme). Positive symptoms, negative symptoms and total score were included in this review. One study (Varambally 2012) reported PANSS results using a binary measure (improved, not improved), while three other studies (Duraiswamy 2007;Lin 2015; Manjunath 2013) reported average scores at endpoint.
ii. Calgary Depression Scale (CDS) (Addington 1994)
This nine‐point scale measures depression in schizophrenia rated from zero to three (0=symptom is absent). The total score includes the following nine items; depression, hopelessness, self‐depreciation, guilty ideas of reference, pathological guilt, morning depression, waking early, suicide, and observed depression.
iii The Hamilton Depression Rating Scale (HDRS) (Hamilton 1960)
This multiple item questionnaire is used to provide an indication of depression, and as a guide to evaluate recovery. This is a 17‐item questionnaire which has a recall period of one week. Each item on the questionnaire is scored on a three‐ or five‐point scale, depending on the item, and the total score is compared to the corresponding descriptor. A total score of zero to seven is considered to be normal. Scores of 20 or higher indicate moderate, severe, or very severe depression.
6.1.3 Social functioning
i. Social Occupational Functioning Scale (SOFS) (Saraswat 2006)
This scale assesses various aspects of social functioning and incorporates 14 domains (bathing and grooming, clothing and dressing, eating feeding and diet, neatness and maintenance activities, conversational skills, social appropriateness/politeness, social engagement, money management, orientation/mobility, instrumental social skills, recreation/leisure, work, respect for property, independence/responsibility) with each being graded on a five‐point Likert scale (1=no impairment, 2=mild impairment, 3=moderate impairment, 4=severe impairment, 5=extreme impairment), with a high score indicating greater severity of social impairment. The sum of individual domains gives an overall score.One study reported this scale dichotomously (Varambally 2012), while another presented average endpoint scores (Behere 2011).
ii. TRENDS Accuracy Score (Behere 2008)
This scale assesses emotional recognition abilities. This tool consists of 80 images (52 static (still) and 28 dynamic (video clip) images) of six basic emotions ‐ happy, sad, fear, anger, surprise, disgust and a neutral expression emoted by four actors. A higher score indicates a higher number of correctly identified emotions out of a maximum of 80.
6.1.3 Quality of life
i. WHOQOL‐BREF (Skevington 2004)
This scale which assesses an individual's quality of life consists of 26 questions based on four domains; physical health, psychological, social relationships and environment. No total or composite score is generated. Each question is rated from one to five, raw scores are converted to transformed scores with domain scores a maximum possible score of 100, a higher score indicating a higher quality of life.
ii. SF‐36 (Ware 1993)
This 36‐point questionnaire evaluates quality of life, and consists of a eight‐point scale profile of scores and a summary of physical and mental measures. The summary scores of physical and mental health are the weighted sums of the eight dimensions of physical health (physical functioning, physical role, pain and general health) and mental health (energy, social functioning, emotional role and emotional well‐being). Higher scores indicate better physical or mental health.
6.2 Missing outcomes
Overall, this review was subject to a considerable number of missing outcomes. Even though all primary outcomes were reported, a number of secondary outcomes were not reported. Two studies presented a number of scales which investigated dimensions of cognition functioning (Bhatia 2017; Lin 2015), but no total end scores were provided and were therefore not included in this review. No studies reported data on key outcomes of costs of care, service use, disability or activities of daily living.
Lin 2015 reported long‐term follow‐up data (18‐month time point), but closer examination of a dissertation pertaining to this study revealed that unfortunately at some time point between the 12‐week and 18‐month time point, the control group received a 'compensated' 12‐week yoga or exercise programme which systematically negated the control group condition. In addition, there was > 50% attrition. For these reasons we were unfortunately unable to include these long‐term follow‐up data in the review.
Excluded studies
Over 1000 potential studies were generated from this search. Seven hundred and twenty‐seven studies were excluded by the Cochrane Schizophrenia Group's Information Specialist, and a further 280 reports were considered not relevant by review authors as they did not meet review criteria. A further 27 reports were examined in detail. Seventeen studies (18 reports) were subsequently excluded. Six studies were excluded as they included yoga versus standard care comparisons only (Hu 2014; Ikai 2013; Ikai 2014; Jayaram 2013; Lin 2006; Visceglia 2011). Four studies were excluded are they included yoga as a package of care, not yoga alone (Isuru 2015; Paikkatt 2012; SLCTR‐2013‐008; Xie 2006), or otherwise did not compare yoga with non‐standard care (Mahal 1976; Ramu 1999). One study investigated the effects of yoga on caregivers, not on patients with a diagnosis of schizophrenia themselves (Varambally 2013). One study was excluded as it was a cross‐over trial of yoga and exercise, and data were not available for the group to which participants were randomised to first (Vancampfort 2011). Three further studies were excluded due to methodological reasons; one study employed quasi‐randomisation (Kavak 2016) and Wu 2014 randomly selected participants. Bhatia 2012 was also excluded due to lack of randomisation. Details of excluded studies are detailed in the Characteristics of excluded studies.
Awaiting assessment
No studies are currently awaiting assessment.
Ongoing studies
Limited details were available for the ongoing trial numbered JPRN‐UMIN000013746 as only a clinical trial register was accessible.
Risk of bias in included studies
See also 'Risk of bias' tables in Characteristics of included studies and Figure 2 and Figure 3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
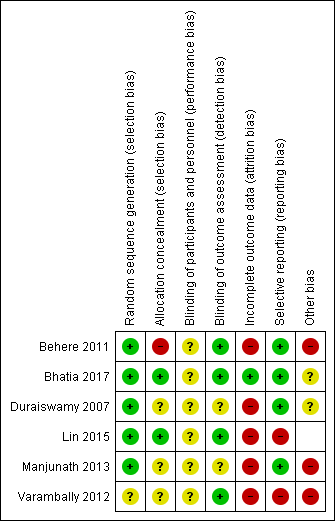
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were randomised controlled trials. The majority of studies had adequate randomisation methods described and were rated as low risk of bias. Three (Behere 2011; Bhatia 2017; Duraiswamy 2007; Lin 2015) utilised computer‐generated random numbers, with only Lin 2015 using block randomisation with a block size of 12. Only Manjunath 2013 utilised a randomisation table. One study was rated as unclear risk of bias as an investigator (uninvolved in the treatment or assessment) generated random numbers ‐ detail was lacking whether this sequence‐generation was adequate (Varambally 2012).
We only rated Lin 2015 and Bhatia 2017 as low risk for adequate concealment as it was reported in both trials that a randomisation list was kept concealed from research staff involved in recruitment, assessment and the study intervention. Varambally 2012a reported that "subjects' allocation was kept concealed", although no concealment strategy was described, therefore this study was rated as unclear risk. Duraiswamy 2007 and Manjunath 2013 were rated as unclear risk as no explicit detail on concealment approach was reported . One study was rated as high risk due to a inadequate allocation concealment as group randomisation was performed by one of the study authors (Behere 2011). H Maxwell: Bhatia 2017 also low risk
Blinding
None of the studies were able to use a double‐blind technique due to the nature of the intervention, since it would not be possible for yoga/control participants or practitioners delivering the yoga intervention to be blind to group allocation. The risk of bias implications were therefore unclear.
Four studies provided explicit detail on blinding of the outcome assessor and were rated as low risk of bias for blinding of outcome assessment (Behere 2011; Bhatia 2017; Lin 2015; Varambally 2012). The remaining studies were rated as unclear risk of bias due to inadequate detail on blinding of outcome assessor.
Incomplete outcome data
Five studies were rated as a high risk of attrition bias as no studies included all randomised participants in the final analysis. Varambally 2012 was noted to have systematic differences between the yoga and exercise groups. Behere 2011 was rated as high risk due to the relatively high proportion of participants randomised who were not included in the analysis (27.5%). In the Lin 2015 study, attrition bias was deemed to be high as 94 participants were randomised but data were only provided for 63 (33%). Bhatia 2017 was rated as low risk due to intention‐to‐teat analysis employed and reasons for attrition were listed.
Selective reporting
Two studies were rated as high risk of bias with regard to selective reporting as no data were reported for one or more outcomes listed (Lin 2015; Varambally 2012). All remaining studies reported data for all outcomes listed and were therefore rated as low risk of bias.
Other potential sources of bias
A number of studies appeared to have other potential sources of bias. Three studies (Behere 2011; Manjunath 2013; Varambally 2012) were ranked as high risk of other bias as one of the authors may have been invested in the yoga intervention due to their affiliation with Swami Vivekananda Yoga Anusandhana Samsthana. Yoga for these studies was developed from this school, which must be borne in mind when interpreting the results of this review. Bhatia 2017 supplied a yoga training booklet 'to participants' after completion of 21 day programme. Presumably booklet was supplied to exercise group also which may have systematically influenced effect of randomisation.
Effects of interventions
See: Summary of findings for the main comparison YOGA versus NON‐STANDARD CARE for schizophrenia
Effects of interventions
1. Comparison 1: YOGA versus NON‐STANDARD CARE
We evaluated one single comparison in this review: yoga versus non‐standard‐care. Six studies compared yoga to non‐standard care for schizophrenia (Behere 2011; Bhatia 2017; Duraiswamy 2007; Lin 2015; Manjunath 2013; Varambally 2012a;), from which it was possible to extract numerical data. Lin 2015 included long‐term follow‐up data, but, prior to the 18‐month time point, the control group was offered a "compensatory" exercise or yoga intervention, which systematically negated the control condition, thereby precluding inclusion of these data. In addition, there was > 50% attrition by this time point. Consequently, no useable data were available for medium‐ or long‐term outcomes, so all outcomes listed were short term (less than six months). For all trials, the non‐standard care intervention was 'other exercise'.
1.1 Mental state: 1. Clinically important change (PANSS ‐ not improved) short term
1.1.1 Overall
One trial with a total of 84 people reported the number of participants who had no overall improvement in mental state by short‐tem follow‐up. There was no evidence of a difference between 'yoga' and 'non‐standard care' for this outcome (risk ratio (RR) 0.81 confidence interval (CI) 0.62 to 1.07, low quality evidence); Analysis 1.1.
1.1.2 Specific ‐ negative symptoms
A single trial with a total of 84 people provided data specific improvement in negative symptoms. There was a clear difference between 'yoga' and 'non‐standard care' (RR 0.72 CI 0.57 to 0.90) in favour of the yoga group; Analysis 1.1.
1.1.3 Specific ‐ positive symptoms
A single trial with a total of 84 people provided data specific improvement in positive symptoms. For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.08 CI 0.84 to 1.38); Analysis 1.1.
1.2 Mental state: 2. Average endpoint score (various scales) short term
1.2.1 Overall (PANSS)
Three trials, which included a total of 170 participants reported overall PANSS endpoint scores. We found evidence of a clear difference between 'yoga' and 'non‐standard care' (mean difference (MD) ‐4.69 CI ‐8.35 to ‐1.03) in favour of the yoga group. For this outcome heterogeneity was high (Chi2 =5.03; df=2.0; P=0.08; I2 =60%); Analysis 1.2. When Lin 2015 was removed I2 homogeneity was restored (I2 16%), with minimal change to results.
1.2.2 Specific ‐ depressive symptoms (CDS)
One trial (total n=69) reported data. There was no difference between 'yoga' and 'non‐standard care' for this outcome (MD 0.10 CI ‐1.01 to 1.21); Analysis 1.2.
1.2.3 Specific ‐ depressive symptoms (HDRS)
There was a single trial in this subgroup, with a total of 60 people. For this outcome 'yoga' was not superior or inferior to 'non‐standard care' (MD ‐1.41 CI ‐2.40 to ‐0.42); Analysis 1.2.
1.2.4 specific ‐ negative symptoms (PANSS)
Four trials with a total of 214 people reported data for this outcome. There was no difference between 'yoga' and 'non‐standard care' (MD ‐1.15 CI ‐2.30 to 0.01). For this outcome heterogeneity was high (Chi2 =8.88; df=3.0; P=0.03; I2 =66%); Analysis 1.2. When the outlying trial Duraiswamy 2007, was removed the I2 decreased somewhat to 41%.
1.2.5 specific ‐ positive symptoms (PANSS)
Four trials with a total of 214 people reported data for this outcome. We found no evidence of a difference between 'yoga' and 'non‐standard care' within this subgroup (MD ‐0.36 CI ‐1.35 to 0.63); Analysis 1.2. For this outcome heterogeneity was moderate (Chi2=4.77; df=3.0; P=0.19; I2 =37%).
1.3 Global state: Average endpoint score (CGIS, low score=good) short term
One trial (n=60) reported data for this outcome. There was a clear effect, favouring yoga for this outcome (MD ‐0.85 CI ‐1.21 to ‐0.49); Analysis 1.3.
1.4 Social functioning: Clinically important change (SOFS ‐ not improved) short term
We identified one study relevant to this outcome (total n=84). 'Yoga' was not superior or inferior to 'non‐standard care' (RR 0.90 CI 0.78 to 1.04, low quality evidence); Analysis 1.4.
1.5 Social functioning: Average endpoint score (two scales) short term
1.5.1 Average score at endpoint (SOFS, high score=good)
One study was relevant to this outcome (n=44). We found no difference between 'yoga' and 'non‐standard care' (MD 3.5 95% CI ‐0.7 to 8.10); Analysis 1.5
1.5.2 Emotional recognition: Average endpoint score (TRACS, high=good)
A single trial with 44 people provided data. There was no evidence of a difference between 'yoga' and 'non‐standard care' for this measure (MD 1.8 95% CI ‐5.54 to 9.14); Analysis 1.5
1.6 Quality of life: Average endpoint scores (various scales) short term
1.6.1 mental health (SF‐36, average summary score, high score=good)
One trial with a total of 69 people reported data for this outcome. We found no evidence of a difference between 'yoga' and 'non‐standard care' (MD ‐5.30 CI ‐17.78 to 7.18); Analysis 1.6.
1.6.2 physical health (WHOQOL‐BREF, high=good)
One trial with a total of 41 people reported data for this outcome. There was no difference between 'yoga' and 'non‐standard care' within this subgroup (MD 9.22 CI ‐0.42 to 18.86, low quality evidence); Analysis 1.6.
1.6.3 physical health (SF‐36, average summary score, high score=good)
A single trial with a total of 69 people provided data. There was no clear difference between 'yoga' and 'non‐standard care' (MD ‐3.60 CI ‐11.98 to 4.78); Analysis 1.6.
1.6.4 psychological health (WHOQOL‐BREF, high=good)
A single trial with a total of 41 people reported data for this outcome. There was evidence that 'non‐standard care' had better scores than 'yoga' (MD 17.70 CI 6.50 to 28.90, low quality evidence); Analysis 1.6.
1.6.5 Social well‐being (WHOQOL‐BREF, high=good)
A single trial with a total of 41 people reported data for this outcome. There was evidence that participants in the 'non‐standard care' group had better scores at the short‐term follow‐up than those in the 'yoga' group (MD 20.75 CI 7.42 to 34.08); Analysis 1.6.
1.7 Adverse effects ‐ short term
1.7.1 any serious
The result was not estimable as no serious adverse effects were reported for the yoga or non‐standard care group; Analysis 1.7.
1.7.2 others
The result was not estimable, as no serious adverse effects were reported for the yoga or non‐standard care group; Analysis 1.7.
1.8 Leaving the study early ‐ short term
We identified six studies relevant to this outcome, with a total of 586 people. For this outcome evidence that fewer people left from the yoga group at short term than those in the non standard care group (RR 0.64 CI 0.49 to 0.83, medium‐quality evidence); (Analysis 1.8).
Discussion
Summary of main results
1. YOGA versus NON‐STANDARD CARE for schizophrenia
It is accepted that drug management is the mainstay of treatment for schizophrenia, but given that limitations of pharmacotherapy are increasingly apparent, other adjunctive treatments to ameliorate the side‐effect profile and improve disease‐related factors deserve careful consideration. One such adjunctive therapy is yoga, of which there is a body of literature, albeit limited. Increasingly apparent in resource‐constraint times and settings, the relative merits of adjunctive therapy treatments, relevant to this review, yoga versus other non‐pharmacological, non‐standard care therapy, need to be scrutinised, but no clear difference emerged and the limited scope of comparisons in this review limits the possibility of definitely answering this question.
The only available non‐standard care comparator to yoga was another form of exercise, which appears limited given the multitude of available adjunctive interventions within the broad areas of expressive therapies, talking therapy, or combinations of these therapies. In our protocol for this review we had chosen seven key outcomes to summarise the effects of yoga in comparison to other non‐standard care packages.
1.1 Mental state
Data were derived from only one small study (n=84) that was rated as low quality. At four months, there was some suggestion of an improvement in negative symptoms favouring those allocated to yoga, but the strength of effect was low so an argument could be made for further investment of effort in relevant trials for more robust analysis. Overall mental state data were equivocal.
1.2 Relapse
Unfortunately, no data for this important outcome were reported in the trials. Most trials were short and did not have 'relapse' as a priority for an outcome ‐ and if the outcome had been a priority, the studies may also have been too short term to robustly assess this outcome. Notwithstanding this, no trial even mentioned relapse and this would seem to be an omission, given the gravitas and deterioration associated with relapse in this illness.
1.3 Social functioning
In Varambally 2012, we found a binary outcome for social functioning (not improved, derived from Social Occupational Functioning Scale (SOFS) data). Again, this did not reach conventional levels of statistical significance, but again, did give some encouragement to those who might want to design a larger, longer study.
1.4 Quality of life: overall improvement (as defined by studies)
Total scores for the outcome of quality of life were lacking, we therefore took a pragmatic approach of including sub‐score outcomes which had been presented continuously. We identified a commonly used proxy measure for binary quality of life (average score on SF‐36) but data were inclusive and, we thought, of low quality in themselves. It is encouraging that trialists are building in such measures to their work.
1.5 Change in physical health
The same applies to physical health measures. There was no definitive measure of 'physical health' so the physical health sub‐score of a quality of life measure was included. We were disappointed that the trials really did not measure this directly when yoga ‐ and in this case also the non‐standard care intervention ‐ are so focused on both mental and physical well=being. Low‐grade data were inconclusive but this really should be part of the outcome set in any future studies.
1.6 Adverse effects
No adverse effects were reported to have occurred in one study of 85 people. Although expected to be minor and mostly musculoskeletal in nature, adverse effects can occur with these therapies and it is important that future trials do have clear adverse effect/event reporting.
1.7 Costs of care: direct and indirect
Reporting of the economic burden (costs of care) is not common in trials of people with schizophrenia and these yoga trials were no exception. This would seem to be an important outcome to be consistently omitted from these studies.
1.8 Leaving the study early
This outcome was added in the absence of relapse data (please see Differences between protocol and review). Overall, the attrition rate from the yoga interventions was 22.4%, while the corresponding rate for the non‐standard care component was 35.3%, which manifested as a clear difference between comparator arms. The attrition rate for the non‐standard care studies noted here is higher than the rate of 26.7% for physical activity interventions in schizophrenia quoted in a recent meta‐analysis (Vancampfort 2016). The majority of studies in this review included a brief, non‐individualised supervised intervention period and a longer unsupervised follow‐up period, during which time participants were expected to continue practice of yoga or exercise, which was the only available non‐standard care comparator. Individually prescribed and supervised programmes throughout the duration of an intervention appear to be associated with significantly lower dropout rates (Vancampfort 2016) in schizophrenia. Given that the most frequently quoted barrier to physical activity participation cited by health professionals among people with schizophrenia is lack of motivation (Soundy 2014), alternative study designs incorporating more supervision and support may be associated with lower attrition levels. Commonly, people with schizophrenia have difficulties in adopting and maintaining a healthy lifestyle, incorporating autonomous motivation, therefore it is likely to be more efficacious to offer a flexible programme with a choice of evidence‐based interventions underpinned by sound psychological behavioural‐change theory.
It is also possible that given the cultural setting of these studies, yoga may have been a more of an acceptable practice to continue at home rather than the exercise programme described in these studies. Lack of adherence data makes it difficult to compare interventions as the dosage of either regimen was incalculable. Furthermore, the exercise regimens described in these reviews were delivered by yoga instructors in most of these studies. The exercise credentials of instructors was unclear. Notably, a recent review (Stubbs 2016), which investigated dropout from randomised controlled trials of exercise interventions for people with depression, reported that programmes supervised by physiotherapists and exercise physiologists resulted in lower attrition rates. This highlights the importance of delivery of exercise interventions by professionals with specific training in exercise prescription.
Overall completeness and applicability of evidence
1. Completeness
1.1 One comparison
Evidence was relevant but overall data were too sparse and narrowly focused to really address the objectives of this review. The search strategy identified six trials, involving 586 people comparing yoga with non‐standard care. We realise 'non‐standard care' could be considered quite a nebulous term. In this review we considered expressive, talking therapies, other exercise (not including yoga) or combinations of these therapies as 'non‐standard care'. A further limitation of this review is that we do not have any comparisons other than another type of exercise to comment upon. Within 'exercise', many types of interventions exist with broad categories of physical activity (not specifically fitness focused), skill‐related fitness, health‐related fitness and body‐mind fitness. For example, yoga versus Tai'chi would have been a legitimate comparison for which no data exist.
1.2 Limited duration of follow‐up, missing outcomes
Within this review, a further limitation, is the absence of medium‐ or long‐term outcomes. One study did include 18‐month data, but a 'compensatory programme' and high attrition rate by this time point unfortunately precluded our ability to include this long‐term data. It is therefore unknown whether small changes seen in a limited number of outcomes were maintained. Further studies reporting medium‐ and particularly long‐term follow‐up are necessary given the (often) chronic nature of schizophrenia, although the difficulty of long‐term follow‐up in exercise studies in general is acknowledged. Even within short‐term outcomes, no data were provided for key outcomes of change in cognition, costs of care, effect on activities of daily living, disability, or service use.
1.3 Confusion of measures
Data extraction for outcomes of interest was also significantly limited by more than one measurement tool being used for the same outcome as well as lack of agreement in data presentation even for the same scale measurement. This suggests that trialists were working largely independent of each other with little regard for providing data for use beyond their final report. Other factors were the lack of clinically meaningful results with minimal presentation of binary data and lack of intention‐to‐treat analysis in the majority of studies included in this review. There is a place, perhaps especially in areas where there are few trials, for careful consideration of what has gone before, agreement of what needs to be measured, and wide access to the final data sets in a way which is consistent across studies (COMET).
For these reasons the central question underlying the review, whether yoga confers any advantage over non‐standard care can not be answered with any degree of confidence.
2. Applicability
The majority of studies included a homogeneous schizophrenia‐only population. There was also consistency in terms of diagnostic criteria with DSM‐IV used in all studies. If there had been conclusive data on the effects of yoga, there may have been an issue in the applicability of those findings on groups of people with less rigorously defined illnesses, even those labelled as 'schizophrenia'. Settings did vary between studies and included a mix of inpatients and outpatients, with only one study including an exclusive inpatient cohort. The likely 'real‐world' setting of the majority of studies in this review is to be commended, which may be reflective of the majority of normal living experiences for people with schizophrenia. Notably perhaps, all studies were based in India (n=5) and China (n=1). Yoga may be a more accepted mainstream practice in India where yoga originated so wider applicability to other cultures of care is not clear. Resources to obtain yoga training may be more available in lower‐ or middle‐income countries, so the implication of these, albeit weak, results may find greater resonance in some non 'western' settings. While the broadly 'aerobic' exercise regimen described in the majority of studies was limited by its non‐specific nature and lack of adherence to exercise‐physiology principles, it nonetheless required no equipment, which may be important in resource‐limited settings. In addition, this type of exercise regimen appears to be prevalent in some Asian countries so social and contextual factors may be important to consider when deciphering the implications of this review.
Studies varied in duration from six weeks to six months, with the majority of four months duration. Bearing in mind the non‐curative nature of schizophrenia, limited conclusions can be drawn about the long‐term safety and efficacy of these treatments and future long‐term trials are needed.
Quality of the evidence
The quality of available data limits our confidence in the small positive changes in favour of the yoga group as a significant number of limitations pertained to study methodology in the majority of studies included in this review. One of the fundamental pre‐requisites of randomised trial methodology is random sequence allocation. This was unclear, however, in one study raising concern about selection bias. It is accepted due to the nature of the intervention, study participants and yoga and exercise practitioners could not be blinded to the intervention. A low risk of detection bias was reported as outcome assessors appeared to be blinded to treatment allocation, although their blinding was not reported to be tested. Attrition bias was problematic in all studies as intention‐to‐treat analysis was not employed and large losses to follow‐up were noted, up to 40% in the non‐standard care arm of one study. Another source of bias which merits consideration was the exercise intervention, which was delivered by yoga therapists in a number of studies. Furthermore, a number of study authors were affiliated to yoga schools which may influence trial equipoise (Figure 3).Using GRADE standards we had to rate quality of current evidence as 'low' to 'moderate' (Please see summary of findings Table for the main comparison).
Potential biases in the review process
The search for studies aimed to be as extensive as possible, and every attempt was made to identify and include all relevant studies and was not just limited to those published in English language. There remains a possibility that there may be other unpublished trials of intervention which the review authors do not currently have access to. This is possible, but we think it unlikely that we have missed any large authoritative study.
Agreements and disagreements with other studies or reviews
The current review only found a clear difference between yoga and non‐standard care for the outcome of 'leaving the study early' in favour of the yoga group, otherwise remaining outcomes were comparable. A previous systematic review covered a similar topic and the results appear to be broadly similar to the findings of this review (Vancampfort 2012a). A further review found no benefits for yoga over exercise for the following outcomes; positive symptoms, negative symptoms, quality of life, cognitive function or social function (Cramer 2013). In a more recent review (Dauwan 2016), yoga showed a significant effect in reducing general symptoms, whereas aerobic exercise was non‐significant, but comparable results were found for reduction of negative symptoms.

37Study flow diagram for 2015 searches

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 1 Mental state: 1. Clinically important change (PANSS ‐ not improved) short term.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 2 Mental state: 2. Average endpoint score (various scales) short term.
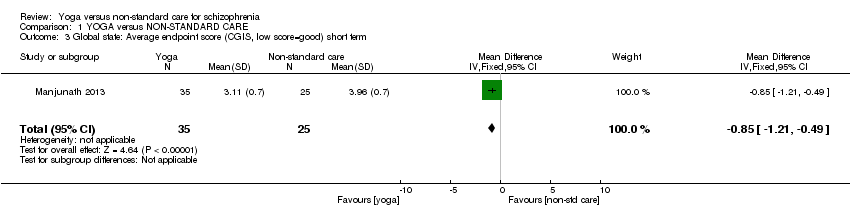
Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 3 Global state: Average endpoint score (CGIS, low score=good) short term.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 4 Social functioning: 1. Clinically important change (SOFS ‐ not improved) short term.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 5 Social functioning: 2. Average score at endpoint (two scales).
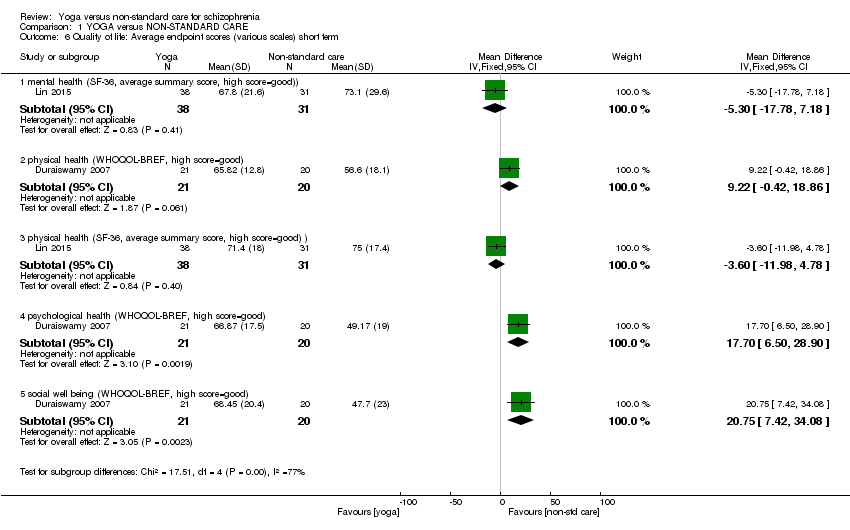
Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 6 Quality of life: Average endpoint scores (various scales) short term.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 7 Adverse effects.

Comparison 1 YOGA versus NON‐STANDARD CARE, Outcome 8 Leaving the study early ‐ short term.
| Intervention | plus | Control | Participants | Reference tag | Relevant Cochrane review |
| Yoga | nil | standard care | people with schizophrenia | Behere 2011; Ikai 2013; Ikai 2014; Jayaram 2013; Lin 2006;Lin 2015;Varambally 2012a; Visceglia 2011 | Yoga versus standard care for schizophrenia |
| counselling | standard care | ||||
| motivational and feedback session | |||||
| nil | caregivers of people with schizophrenia | ||||
| Yoga | non‐standard care | people with schizophrenia | Yoga as part of a package of care versus non‐standard care | ||
| Chlorpromazine | nil | placebo | Chlorpromazine versus placebo for schizophrenia | ||
| 'Tagara' (local drug with anti‐psychotic properties) and 'Brahmyadiyoga' (a herbal compound) | nil | Chlorpromazine versus herbal compounds for schizophrenia | |||
| chlorpromazine | |||||
| * This particular study used yoga combined with relaxation exercises, breathing exercises, body movement exercises, basic acting exercises, the Alexander technique, theatre games, exercise ''to build self confidence'', creative work using props, use of music to enhance creativity and moods. | |||||
| Methods | Allocation: randomised (clearly described). |
| Participants | Diagnosis: people with a clinical diagnosis of schizophrenia. |
| Interventions | 1. Yoga: the yoga intervention should be clearly described and consist of the following components; (i) shithileekarana vyayama (loosening exercises) for approximately 10 minutes (ii) yoga postures (asanas) for approximately 20 minutes (iii) breathing exercises and relaxation techniques for approximately 20 minutes using a manualised protocol, yoga programme for 12 weeks, 3 times weekly, follow‐up at 6 months and 1 year, yoga delivered by a trained yoga instructor, meditation not included. 2. Standard care control*. All groups stable pharmacotherapy. |
| Outcomes | Mental state (binary outcomes). Relapses (binary outcomes). QOL (binary outcomes). Disability (binary outcomes). Activities of daily living (binary outcomes). Costs: cost of services, cost of care. Adverse events related to yoga (number and type of injuries). Service outcomes: days in hospital, time attending outpatient psychiatric clinic. |
| Notes | Adherence should be logged with patients expected to adhere to 70% to 75% of scheduled sessions. * Regarding design of a future study, readers are directed to the first yoga review in this series (Broderick 2015) ‐ as a comprehensive yoga versus standard care study has not yet been published, this would be the initial priority. When this is conducted, many legitimate active comparators to yoga could be suggested; such as, but not limited to the following; yoga versus talking therapy, yoga versus expressive therapies, yoga versus other forms of exercise such as Tai'chi. |
| Intervention | plus | Active Comparator Broad Group | Specific interventions* |
| Yoga | nil | expressive therapy | art therapy |
| drama therapy | |||
| music therapy | |||
| dance therapy | |||
| writing therapy | |||
| talking therapies | cognitive behavioural therapy | ||
| dialectic behaviour therapy | |||
| humanistic therapies | |||
| exercise | aerobic‐based interventions | ||
| combined aerobic and resistance programme | |||
| resistance‐based interventions | |||
| Tai'chi | |||
| qi'gong | |||
| *This list of specific interventions is not exhaustive and merely provides some examples of legitimate active comparators to yoga which are not currently available. These interventions could also be considered alternatively. For instance, exercise consists of heterogeneous interventions, it is accepted there could be other equally justifiable intervention titles such as 'supervised' exercise, 'non‐supervised exercise', or 'group‐based exercise', 'individual exercise', or exercise could be considered in terms of intensity such as 'high intensity', 'moderate intensity' and 'low intensity'. Note the specific intervention should be delivered by suitably qualified personnel. | |||
| YOGA versus NON‐STANDARD CARE for schizophrenia | ||||||
| Patient or population: people with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YOGA versus NON‐STANDARD CARE | |||||
| Mental state: clinically important change Follow‐up: 4 months | Low1 | RR 0.81 | 84 | ⊕⊕⊝⊝ | ||
| 800 per 1000 | 648 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 729 per 1000 | |||||
| High1 | ||||||
| 1000 per 1000 | 810 per 1000 | |||||
| Global state: relapse | No trial reported this outcome. | |||||
| Social functioning: clinically important change | Low1 | RR 0.90 | 84 | ⊕⊕⊝⊝ | ||
| 700 per 1000 | 630 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 810 per 1000 | |||||
| Adverse effects ‐ any | See comment | See comment | Not estimable | 85 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences. The study reported no adverse effects. |
| Quality of life: clinically important change SF‐36 average change score mental health * | The mean quality of life: average change ‐ mental health in the intervention (yoga) groups was | 69 | ⊕⊕⊝⊝ | * no trial reported binary data; we chose 1 of 2 QOL measures as proxy measure | ||
| Physical health: clinically important change WHQOL‐BREF ‐ average change score * | The mean physical health: average change in the intervention (yoga) groups was | 41 | ⊕⊕⊝⊝ | * no trial reported binary data; we chose physical health dimension of QOL measure as proxy measure. | ||
| Costs: direct and indirect costs of care | No trial reported this outcome. | |||||
| Leaving the study early: short term | Low1 | RR 0.64 | 586 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 120 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 240 per 1000 | |||||
| High1 | ||||||
| 600 per 1000 | 360 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence CI: confidence interval; RR: risk ratio | ||||||
| 1 Moderate risk approximates to that of non‐standard care in trial(s). 3 Imprecision: Downgraded one level due to small sample size 7 Imprecision: Downgraded one level as based on one study with no reported adverse events. 8 Risk of bias: Downgraded one level as a number of participants withdrew from one trial and it was not clear to which group they were randomised. | ||||||
| Review title | Status |
| Yoga versus standard care for schizophrenia | Full review: Broderick 2015 |
| Yoga versus non‐standard care for schizophrenia | This review. |
| Yoga as part of a package of care versus standard care | Protocol: Broderick 2016b |
| Yoga as part of a package of care versus non‐standard care |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: 1. Clinically important change (PANSS ‐ not improved) short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 overall | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.07] |
| 1.2 specific ‐ negative symptoms | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.90] |
| 1.3 specific ‐ positive symptoms | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 2 Mental state: 2. Average endpoint score (various scales) short term Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 overall (PANSS) | 3 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐4.69 [‐8.35, ‐1.03] |
| 2.2 specific ‐ depressive symptoms (CDS) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.01, 1.21] |
| 2.3 specific ‐ depressive symptoms (HDRS) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.40, ‐0.42] |
| 2.4 specific ‐ negative symptoms (PANSS) | 4 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.30, 0.01] |
| 2.5 specific ‐ positive symptoms (PANSS) | 4 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.35, 0.63] |
| 3 Global state: Average endpoint score (CGIS, low score=good) short term Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.21, ‐0.49] |
| 4 Social functioning: 1. Clinically important change (SOFS ‐ not improved) short term Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 5 Social functioning: 2. Average score at endpoint (two scales) Show forest plot | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐0.57, 6.97] |
| 5.1 SOFS, high score=good | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐0.70, 8.10] |
| 5.2 Emotional recognition, TRACS, high score=good | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐5.54, 9.14] |
| 6 Quality of life: Average endpoint scores (various scales) short term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 mental health (SF‐36, average summary score, high score=good)) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐17.78, 7.18] |
| 6.2 physical health (WHOQOL‐BREF, high score=good) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 9.22 [‐0.42, 18.86] |
| 6.3 physical health (SF‐36, average summary score, high score=good) ) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐11.98, 4.78] |
| 6.4 psychological health (WHOQOL‐BREF, high score=good) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 17.70 [6.50, 28.90] |
| 6.5 social well being (WHOQOL‐BREF, high score=good) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 20.75 [7.42, 34.08] |
| 7 Adverse effects Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 any serious | 1 | 85 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.2 others | 1 | 85 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8 Leaving the study early ‐ short term Show forest plot | 6 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.51, 0.86] |

