Chirurgia rekonstrukcyjna w leczeniu odleżyn
Abstract
Background
There are several possible interventions for managing pressure ulcers (sometimes referred to as pressure injuries), ranging from pressure‐relieving measures, such as repositioning, to reconstructive surgery. The surgical approach is usually reserved for recalcitrant wounds (where the healing process has stalled, or the wound is not responding to treatment) or wounds with full‐thickness skin loss and exposure of deeper structures such as muscle fascia or bone. Reconstructive surgery commonly involves wound debridement followed by filling the wound with new tissue. Whilst this is an accepted means of ulcer management, the benefits and harms of different surgical approaches, compared with each other or with non‐surgical treatments, are unclear. This is an update of a Cochrane Review published in 2016.
Objectives
To assess the effects of different types of reconstructive surgery for treating pressure ulcers (category/stage II or above), compared with no surgery or alternative reconstructive surgical approaches, in any care setting.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was January 2022.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) that assessed reconstructive surgery in the treatment of pressure ulcers.
Data collection and analysis
Two review authors independently selected the studies, extracted study data, assessed the risk of bias and undertook GRADE assessments. We would have involved a third review author in case of disagreement.
Main results
We identified one RCT conducted in a hospital setting in the USA. It enrolled 20 participants aged between 20 and 70 years with stage IV ischial or sacral pressure ulcers (involving full‐thickness skin and tissue loss). The study compared two reconstructive techniques for stage IV pressure ulcers: conventional flap surgery and cone of pressure flap surgery, in which a large portion of the flap tip is de‐epithelialised and deeply inset to obliterate dead space. There were no clear data for any of our outcomes, although we extracted some information on complete wound healing, wound dehiscence, pressure ulcer recurrence and wound infection. We graded the evidence for these outcomes as very low‐certainty. The study provided no data for any other outcomes.
Authors' conclusions
Currently there is very little randomised evidence on the role of reconstructive surgery in pressure ulcer management, although it is considered a priority area. More rigorous and robust research is needed to explore this intervention.
PICOs
Streszczenie prostym językiem
Jakie są korzyści i zagrożenia związane z chirurgią rekonstrukcyjną w leczeniu odleżyn?
Najważniejsze informacje
– Nie mamy pewności co do korzyści i zagrożeń związanych z chirurgią rekonstrukcyjną (zwaną czasem chirurgią plastyczną) w leczeniu odleżyn.
– Znaleźliśmy jedno małe badanie (obejmujące 20 uczestników), w którym oceniano stosowanie chirurgii rekonstrukcyjnej w leczeniu głębokich, trudno gojących się odleżyn, jednak nie byliśmy w stanie wyciągnąć żadnych wniosków z przedstawionych wyników.
– Potrzebne są większe, dobrze zaprojektowane badania w celu zbadania tego priorytetowego obszaru.
Co to są odleżyny?
Odleżyny to uszkodzenia skóry i tkanek, powstające najczęściej u osób pozostających przez długi czas w jednej pozycji. Kiedy określone części ciała doznają ciągłego ucisku z zewnątrz, przepływ krwi w skórze i niżej położonych tkankach jest ograniczony. Może to doprowadzić do ich uszkodzenia, zwłaszcza w częściach ciała o zmniejszonej grubości tkanki tłuszczowej (np. dolna część pleców, pięty).
Ryzyko powstania odleżyn dotyczy w szczególności ludzi starszych oraz osób mających problemy z poruszaniem się, np. korzystających z wózka inwalidzkiego lub pozostających przez długi czas w szpitalu.
Jak leczyć odleżyny?
Odleżyny są poważnymi ranami i wiążą się z koniecznością podjęcia kosztownego leczenia, co sprawia że bardzo ważne jest stosowanie odpowiedniej profilaktyki. W przypadku wystąpienia odleżyn możliwe opcje terapeutyczne obejmują stosowanie opatrunków, antybiotyków oraz środków antyseptycznych.
Chirurgia rekonstrukcyjna jest z reguły zarezerwowana dla odleżyn głębokich i/lub trudnych do wyleczenia. Istnieje kilka typów zabiegów chirurgicznych, które można przeprowadzić. Większość z nich obejmuje usunięcie martwej tkanki znajdującej się w ranie, a następnie wykorzystanie tkanki tłuszczowej, tkanki mięśniowej i/lub skóry – pochodzących z innych części ciała pacjenta – w celu uzupełnienia ubytku.
Czego chcieliśmy się dowiedzieć?
Chcieliśmy ocenić korzyści i ryzyko przeprowadzania operacji rekonstrukcyjnych w leczeniu odleżyn w porównaniu z niewykonywaniem operacji; oraz korzyści i ryzyko różnych rodzajów operacji rekonstrukcyjnych w porównaniu ze sobą. Interesowały nas następujące wyniki:
– całkowite zagojenie się rany;
– ponowne otworzenie się ran lub pojawienie się nowych odleżyn w tym samym miejscu, co poprzednie;
– zużycie zasobów i koszty;
– jakość życia:
– zakażenie rany; oraz
– powstanie nowych odleżyn w innych miejscach niż poprzednie.
Co zrobiliśmy?
Przeszukaliśmy elektroniczne bazy danych i rejestry badań pod kątem badań z randomizacją (RCT), czyli badań klinicznych, które uczestników przydziela się losowo do różnych grup leczenia. Ten typ badań dostarcza najbardziej wiarygodnych danych na temat efektów leczenia. Uwzględniliśmy badania, w których badano efekty operacji rekonstrukcyjnej w leczeniu odleżyn, w porównaniu z niewykonywaniem takiej operacji. Włączyliśmy również badania, w których w leczeniu odleżyn porównywano różne rodzaje operacji rekonstrukcyjnych między sobą. Nie zastosowaliśmy żadnych ograniczeń dotyczących języka, daty publikacji czy miejsca przeprowadzenia badania. Na koniec oceniliśmy wiarygodność danych, opierając się na takich czynnikach jak metodyka i wielkość badań (tj. liczba badanych).
Czego się dowiedzieliśmy?
Znaleźliśmy jedno małe badanie, które przeprowadzono w USA obejmujące 20 uczestników przebywających w szpitalu. W tym badaniu analizowano dwie różne techniki chirurgii rekonstrukcyjnej w leczeniu odleżyn IV stopnia, które charakteryzują się pełną utratą grubości skóry oraz martwicą w obrębie tkanek. Badanie nie dostarczyło wystarczających informacji na temat gojenia się rany, ryzyka ponownego otwarcia się rany, nawrotu owrzodzenia lub zakażenia rany, abyśmy mogli ocenić skuteczność różnych technik chirurgicznych.
Jakie są ograniczenia danych naukowych?
Nie mamy pewności, jaki wpływ miały te dwie techniki chirurgiczne na gojenie się ran, ryzyko ponownego otwarcia się rany lub nawrót odleżyn, ponieważ badania nie zostało poprawnie przeprowadzone, źle raportowano wyniki i obejmowało niewielką liczbę uczestników.
Nie jesteśmy pewni co do oceny korzyści i szkód wynikających z zastosowania leczenia chirurgicznego lub różnych jego rodzajów w leczeniu odleżyn. Niezbędne są bardziej precyzyjne badania na ten temat, zwłaszcza że problem ten jest uważany za priorytetowy zarówno przez pacjentów, opiekunów, jak również lekarzy.
Jak aktualne są przedstawione dane naukowe?
Jest to aktualizacja poprzedniego przeglądu. Dane naukowe są aktualne do stycznia 2022 r.
Authors' conclusions
Summary of findings
| Cone of pressure flap compared with standard flap technique | ||||||
| Patient or population: people with stage IV pressure ulcers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard flap | Risk with COP flap | |||||
| Complete healing of surgical wound Follow‐up: 16 months | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study reported that minor complications, including delayed wound healing and wound dehiscence that did not require surgical intervention, were comparable between the groups. We could not calculate an effect estimate and are uncertain of the effect of the interventions on wound healing. | |
| Not calculable | Not calculable | |||||
| Wound dehiscence Follow‐up: 16 months | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study reported that minor complications, including delayed wound healing and wound dehiscence that did not require surgical intervention, were comparable between the groups. We could not calculate an effect estimate and are uncertain of the effect of the interventions on wound dehiscence. | |
| Not calculable | Not calculable | |||||
| Pressure ulcer recurrence | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study did not clearly report the proportion of participants in each group with pressure ulcer recurrence. We could not calculate an effect estimate and are uncertain of the effect of the interventions on ulcer recurrence. | |
| Not calculable | Not calculable | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COP: cone of pressure; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for high risk of bias in multiple domains, and two levels for imprecision due to low participant number and incomplete reporting. | ||||||
Background
Description of the condition
Pressure ulcers, also known as bedsores, decubitus ulcers or pressure injuries, are localised areas of ischaemic injury to the skin and underlying tissue caused by prolonged external mechanical forces such as pressure or shear beyond the normal physiological constraints (EPUAP‐NPUAP‐PPPIA 2014). These forces are greater in the presence of an underlying bony prominence such as the sacrum, ischium, trochanter or heel (Vanderwee 2007), which is where pressure ulcers tend to occur.
The populations at greatest risk include non‐ambulatory individuals, especially those with spinal cord injuries (Gefen 2014). Pressure ulcers also develop in people with prolonged impaired consciousness (e.g. during prolonged surgical procedures; Chen 2012; Primiano 2011), people in intensive care (Ranzani 2016), and people incapacitated through intoxication (Yanagawa 2011). Furthermore, acute and chronic comorbidities that limit mobility or tactile sensation increase risk, with older adults most vulnerable (Allman 1997; Bergstrom 1998; Berlowitz 1990; Berlowitz 1997; Brandeis 1994). Pressure ulcers commonly occur in people with systemic disease such as diabetes (Brem 2003). Incontinence can increase the risk of ulceration by producing a moist, contaminated environment for the skin injury (Brandeis 1994). Poor nutritional status can also impair the healing of these complex wounds (Allman 1997; Donini 2005), though there is currently limited evidence on the effectiveness of nutritional intake interventions for preventing or treating pressure ulcers (Langer 2014; Smith 2013).
Pressure ulcers vary in severity. One of the most widely recognised systems for categorising pressure ulcers is that of the National Pressure Injury Advisory Panel, which is summarised below (NPIAP 2016).
-
Category/Stage I: "Intact skin with a localized area of non‐blanchable erythema, which may appear differently in darkly pigmented skin. Presence of blanchable erythema or changes in sensation, temperature, or firmness may precede visual changes. Color changes do not include purple or maroon discolouration; these may indicate deep tissue pressure injury."
-
Category/Stage II: "Partial‐thickness loss of skin with exposed dermis. The wound bed is viable, pink or red, moist, and may also present as an intact or ruptured serum‐filled blister. Adipose (fat) is not visible and deeper tissues are not visible. Granulation tissue, slough and eschar are not present. These injuries commonly result from adverse microclimate and shear in the skin over the pelvis and shear in the heel. This stage should not be used to describe moisture associated skin damage (MASD) including incontinence associated dermatitis (IAD), intertriginous dermatitis (ITD), medical adhesive related skin injury (MARSI), or traumatic wounds (skin tears, burns, abrasions)."
-
Category/Stage III: "Full‐thickness loss of skin, in which adipose (fat) is visible in the ulcer and granulation tissue and epibole (rolled wound edges) are often present. Slough and/or eschar (dead tissue, dry scab) may be visible. The depth of tissue damage varies by anatomical location; areas of significant adiposity can develop deep wounds. Undermining and tunnelling may occur. Fascia, muscle, tendon, ligament, cartilage and/or bone are not exposed. If slough or eschar obscures the extent of tissue loss this is an Unstageable Pressure Injury."
-
Category/Stage IV: "Full‐thickness skin and tissue loss with exposed or directly palpable fascia, muscle, tendon, ligament, cartilage or bone in the ulcer. Slough and/or eschar may be visible. Epibole (rolled edges), undermining and/or tunnelling often occur. Depth varies by anatomical location. If slough or eschar obscures the extent of tissue loss this is an Unstageable Pressure Injury."
Prevalence estimates vary according to the population being assessed, the data collection methods used and decisions about whether to include stage I pressure ulcers (where there is no active wound, but people are 'at risk'). One large survey of hospitalised people undertaken in several European countries returned a pressure ulcer prevalence (stage II and above) of 10.5% (Vanderwee 2007). In 2009, an estimate for pressure ulcer prevalence (stage II and above) across acute care, long‐term care and rehabilitation settings in the USA was 9%, with the highest prevalence (26%) identified in long‐term acute care settings (VanGilder 2009). In the UK, national pressure ulcer data were until recently collected across community and acute settings as part of the National Health Service (NHS) Safety Thermometer initiative (Power 2012). The final report, published in March 2017, estimated that 5% of patients across these settings had pressure ulcers (NHS Safety Thermometer Report).
We note that all the prevalence figures quoted in the previous paragraph are for populations currently receiving medical care. One 2014 study estimated the point prevalence of pressure ulceration in the total adult population of Leeds, UK, using data from a cross‐sectional survey (Hall 2014). The study authors identified current cases of pressure ulcer ascertained across all care providers in the city, and extrapolated this figure to the total adult population of 751,485, giving a point prevalence of 0.31 per 1000 people (Hall 2014). In one UK urban area, the community prevalence of pressure ulcers estimated between February and April 2010 was 0.77 per 1000 adults (Stevenson 2013).
Pressure ulcers have a large impact on those affected: they can be painful and become infected or malodorous. After adjustment for age, sex and comorbidities, people with pressure ulcers have a lower health‐related quality of life than those without (Essex 2009). Pressure ulcer‐related treatment costs vary considerably: one systematic review published in 2015 found daily costs ranging from EUR 1.71 to 470.49 per person (Demarré 2015). The estimated financial cost of treating ulcers in the UK, based on 2015/16 prices, ranged from GBP 1400 for a stage I ulcer managed in the community, to over GBP 8500 for other stages of ulcer (Guest 2018). Research has shown that pressure ulcers increase length of hospital stay, readmission and mortality rates (Lyder 2012), and add considerably to the cost of an episode of hospital care (Chan 2013). One 2019 study conducted in the USA estimated that the average cost of hospital‐acquired pressure ulcers was USD 10,708 per person (Padula 2019). The total estimated cost was USD 26.8 billion at 2016 prices, and the 10.5% of people who developed stage III or IV ulcers accounted for over 58% of this sum (Padula 2019). The estimated cost to the Australian healthcare system of treating pressure ulceration was AUD 983 million per annum at 2012/13 prices (Nguyen 2015).
Conservative approaches to managing pressure ulcers, such as dressings, are often associated with a protracted investment of resources. In theory, if the aetiology of pressure sores is removed and nutrition optimised, most should heal (Bergstrom 1996; Bergstrom 1992). Clinicians tend to reserve surgical intervention for the most recalcitrant (treatment‐resistant) cases of stage III and IV ulcers after failure of conservative measures (Margara 2003). Surgical management usually involves debridement of unhealthy and necrotic tissue, underlying bursae (fibrotic capsule) or even bone, with or without immediate soft tissue cover (Conway 1956). Other than the type of surgical reconstruction, factors contributing to successful outcomes include the quality of local tissues, aetiology (causes of the ulcer), and the comorbidities, education status and motivation of the affected person (Kruger 2013).
Description of the intervention
This review focuses on the evidence on the surgical reconstruction of pressure ulcers, where surgical reconstruction is defined as any surgical procedure that leads to epithelial closure of the wound. When choosing among the diverse spectrum of possible surgical procedures, surgeons must consider the characteristics of the person they are treating and wound level factors. Many surgical procedures start with thorough debridement, which involves excision of the fibrotic capsule or bursa that forms around the chronic wound, to reveal healthy bleeding tissue. If the residual tissue is badly scarred, the skin is vulnerable to further breakdown. Any underlying dead or infected tissue or heterotrophic ossification (formation of ectopic bone) should be debrided.
Following surgical debridement, reconstructive surgical methods include the following (e.g. Maslauskas 2009).
-
Primary wound closure: direct advancement of the wound edges, either directly or in layers, to close the wound (Simman 2009).
-
Skin grafts: where a thin piece of skin is surgically removed from a donor area to replace skin in the defect or denuded area. Clinicians occasionally opt for skin grafts to treat pressure ulceration when all precipitating factors for pressure sore formation have been eliminated. Skin grafts facilitate quick wound cover and can accelerate wound healing (Srivastava 2009).
-
Local random pattern flaps: this involves surgically moving the local tissues around the wound, based on a random pattern of blood supply, into the wound defect (Nesbit 2015).
-
Regional flaps, including:
-
muscle or musculocutaneous flaps, which involves moving whole or part of a named muscle based on a defined blood supply, with or without a skin island, to provide cover to the wound (Liu 2013);
-
fascial or fasciocutaneous flaps, which involves moving a surgically defined fascial‐based island of tissue with its intact blood supply, with or without skin, to cover the wound (Robertson 2015); and
-
perforator flaps, which is a refinement of the previous musculocutaneous or fasciocutaneous flaps approach, whereby the surgeon identifies specific perforating blood vessels in the flap and dissects them to allow either greater movement or less muscle sacrifice, as well as separation of components to each flap (Koshima 1993).
-
-
Free flaps: this involves surgically detaching a defined island of tissue with an artery and vein and moving it to the site of the wound, which has local arteries or veins of similar size. The surgeon then anastomoses the vessels to re‐establish blood flow to the island of tissue (Lemaire 2008).
-
Tissue expansion: this involves a gradual increment and recruitment of tissue surrounding a pressure ulcer. A tissue expander is inserted into a subcutaneous pocket near the ulcer and slowly expanded at a defined rate with saline. Once the skin and soft tissues have enough volume to cover the pressure ulcer, the expander is removed, and the tissues are inset to cover the wound. Another method is to apply slow skin traction over the wound with an incremental traction dressing, which works on the same principle of gradual mechanical traction on skin, promoting tissue creep (Johnson 1993). Eventually the extra skin recruited can be used to close the wound (Wagh 2013).
Each of the above procedures can be performed alone or as part of a multistage procedure to increase the likelihood of the tissue surviving manipulation, reduce the overall surgical impact on the person and minimise infection and aggravating factors. This is particularly important as the skin quality around pressure ulcers is usually suboptimal (Maslauskas 2009).
How the intervention might work
Surgery is indicated when conservative measures have failed to accelerate the healing process in pressure ulceration, but only when all other parameters are optimised. Thus, surgical closure is often reserved for more complex pressure ulcers (usually stage III or IV but occasionally stage II), and the decision on whether to operate will depend on the probability of ulcer recurrence in each individual. The underpinning rationale for reconstructive surgery is that following the removal of devitalised tissue, the wound defect is filled with vascularised healthy tissue with adequate skin cover, which then forms a healed wound.
Why it is important to do this review
Much of the current literature around the treatment of pressure ulcers focuses on non‐surgical management. It is important to assess current evidence regarding the clinical effectiveness of surgery in suitable populations. The number of surgical options has increased with the introduction of novel approaches such as perforator flaps and free tissue transfer, although it is unclear how many people with pressure ulcers undergo reconstructive surgery in any country. The published UK National Institute for Health and Clinical Excellence (NICE) guidelines on the prevention and management of pressure ulcers makes no specific recommendations or suggestions regarding reconstructive surgery of these wounds (NICE 2014). One review of the evidence on all pressure ulcer treatments included four studies that investigated the role of reconstructive surgery, but as none were randomised controlled trials (RCTs), the review authors could only draw very limited conclusions (Smith 2013). In a James Lind Alliance Pressure Ulcer Partnership, patients, carers and health professionals prioritised the research question 'How effective are surgical operations to close pressure ulcers?' (Cullum 2016). The aim of this Cochrane Review is to present an overview of the current evidence base to help inform decision‐making in the treatment of pressure ulcers as well as to guide future research.
Objectives
To assess the effects of different types of reconstructive surgery for treating pressure ulcers (category/stage II or above), compared with no surgery or alternative reconstructive surgical approaches, in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished RCTs, including cluster‐RCTs, irrespective of language of report. We excluded cross‐over trials and studies using quasi‐randomisation.
Types of participants
We included studies that recruited adults with a diagnosis of a pressure ulcer (category/stage II or above) managed in any care setting. We excluded studies involving participants with category/stage I ulcers. We accepted study authors' definitions of stage II or above, unless it was clear that they included wounds with unbroken skin. We had planned to exclude studies with mixed wound populations (i.e. studies that did not restrict inclusion to pressure ulcers only and that may have included participants with other types of wounds such as venous leg or diabetic foot ulcers), but found no such studies. Although we would have included any stage II or above pressure ulcer, we had anticipated that we would identify studies of stage IV ulcers where the healing process had stalled or the wound was not responding to treatment.
Types of interventions
The primary intervention was reconstructive surgery for pressure ulceration, where reconstructive surgery is defined as any surgical procedure that leads to epithelial closure of the wound. We included any RCT in which the use of a specific surgical closure technique was the only systematic difference between treatment groups. We had anticipated that likely comparisons would include surgery versus no surgery and different types of surgery compared with each other. Because we had anticipated that reconstructive surgery would often include a stage of surgical wound debridement, we included this as a co‐intervention, extracted data and discussed them in the presentation of results. We had not planned to treat surgical debridement alone as a type of reconstructive surgery. Other co‐intervention details included postoperative protocols. Had we found evidence of a difference in use of co‐interventions between groups, we would not have considered the type of reconstructive surgery to be the only systematic difference between groups and so would have excluded these studies.
Types of outcome measures
Had we identified any studies that reported none of our predefined outcomes but that were otherwise eligible (i.e. correct study design, population and intervention/comparator), we would have contacted the study authors where possible to establish whether they had measured but not reported an outcome of interest.
We reported outcome measures at the latest available time point (assumed to be length of follow‐up if not specified) and at the time point specified in the methods as being of primary interest (if this was different from the latest available time point). For all outcomes, we classified outcome measures as follows.
-
Less than one week to eight weeks: short‐term.
-
From eight weeks to 16 weeks: medium‐term.
-
More than 16 weeks: long‐term.
Primary outcomes
-
Complete wound healing. We accepted study authors' definitions of wound healing. We had planned to record whether healing was defined immediately following surgery or not confirmed until after a specific timeframe following surgery, when the surgery was deemed to be successful. For this review, we regarded the following measures as providing the most relevant and rigorous measures of healing.
-
Time to complete wound healing. We had planned to record whether study authors had analysed this measure correctly, using techniques that account for data censoring and adjusting for prognostic covariates such as baseline size.
-
Proportion of ulcers healed (frequency of complete healing).
-
Where studies reported both of these measures, we had planned to present the data in an additional table of outcome data for reference, and to report time to healing.
-
Wound breakdown. We presented data on wound breakdown using the following two outcomes, presented separately.
-
Wound dehiscence. We had planned to assess this outcome as the proportion of wounds that dehisced along the wound edges that had been apposed and held together with sutures, staples, etc. in the reconstructive surgery. We had intended to record study authors' definitions of wound dehiscence.
-
Wound recurrence, defined as occurrence of a new pressure ulcer on the same site as a previous ulcer.
-
Secondary outcomes
-
Resource use, including measures such as number of dressing changes, nurse visits, length of hospital stay, readmission and reoperation/intervention
-
Health‐related quality of life, where reported with a validated scale such as the 36‐Item Short Form Health Survey (SF‐36) or the EuroQol 5‐Dimension questionnaire (EQ‐5D), or a validated disease‐specific questionnaire such as the Cardiff Wound Impact Schedule. We did not plan to include ad hoc measures of quality of life that were unvalidated or were not common to multiple trials
-
Wound infection, as defined by study authors
-
Costs applied to resource use
-
Incidence of secondary ulceration, where a second pressure ulcer formed in a different area during follow‐up.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials.
-
Cochrane Wounds Specialised Register (searched 26 January 2022);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) in the Cochrane Library (searched 26 January 2022);
-
MEDLINE Ovid including In‐Process & Other Non‐Indexed Citations (1946 to 26 January 2022);
-
Embase Ovid (1974 to 26 January 2022);
-
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 26 January 2022).
In MEDLINE Ovid, we combined the subject‐specific strategy with the sensitivity‐maximising version of the Cochrane highly sensitive search strategy for identifying randomised trials (2008 revision; Lefebvre 2021). We combined the Embase Ovid search with the Embase Ovid filter developed by Cochrane UK (Lefebvre 2021). We combined the CINAHL EBSCO Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 January 2022);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/clinical-trials-registry-platform; searched 26 January 2022).
Appendix 1 presents the search strategies for all the databases and registries.
For details of the search strategies used for the previous version of this review, see Wong 2016b.
Searching other resources
We searched the reference lists of included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports, to identify other potentially eligible trials or ancillary publications.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Wong 2016a), which were based on Chapter 5 of the Cochrane Handbook for Systematic Reviews of Interventions (Li 2022).
Selection of studies
Two review authors independently screened the titles and abstracts of the citations retrieved by the searches. After the initial assessment, we obtained full‐text copies of all studies considered potentially relevant. Two review authors independently checked the full papers for eligibility. We resolved disagreements by discussion and, where required, with the input of a third review author. We did not need to contact study authors to query any study details with regard to eligibility. We recorded all reasons for exclusion of studies for which we had obtained full copies. We completed a PRISMA flowchart to summarise this process (Liberati 2009).
We obtained all available publications of each study. Whilst we would only have included each study once in our review, we had planned to extract data from all reports.
Data extraction and management
We extracted and summarised details of the eligible study using a data extraction sheet. Two review authors extracted data independently and resolved disagreements by discussion. We would have consulted a third review author if required. We attempted to contact the study authors to obtain missing information. Had we included studies with more than two intervention arms, we would only have extracted data from intervention and control groups that met our eligibility criteria.
We had planned to extract the following data from each study for the prespecified interventions and outcomes in this review. We collected outcome data for relevant time points as described in Types of outcome measures.
-
Country of origin.
-
Type of wound and surgery.
-
Unit of randomisation (per participant): single wound or multiple wounds on the same participant.
-
Unit of analysis.
-
Trial design (e.g. parallel, cluster).
-
Care setting.
-
Number of participants randomised to each trial arm.
-
Eligibility criteria and key baseline participant data.
-
Details of treatment regimen received by each group.
-
Duration of treatment.
-
Details of any co‐interventions.
-
Primary and secondary outcome(s), with definitions.
-
Outcome data for primary and secondary outcomes (by group).
-
Duration of follow‐up.
-
Number of withdrawals (by group).
-
Publication status of study.
-
Source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed the included study using the Cochrane risk of bias tool (RoB 1), as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting and other issues. For this review, we had planned to record unit of analysis issues, for example, where trial authors had randomised clusters but analysed data at the individual level (Appendix 2). We assessed blinding and completeness of outcome data for each of the review outcomes separately. Because we had anticipated that blinding of participants and personnel would not be possible, the risk of detection bias assessment focused on whether the study had blinded outcome assessment: assessment of wound outcomes such as breakdown and healing can be subjective and is at high risk of detection bias when unblinded. We presented our risk of bias assessment results using two summary figures: one that summarises risk of bias for each item across all studies, and a second that shows a cross‐tabulation of each trial against each individual risk of bias item.
For trials using cluster randomisation, we had planned to consider recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials (Higgins 2022a; Appendix 3).
Measures of treatment effect
For dichotomous outcomes, we had planned to calculate risk ratio (RRs) with 95% confidence intervals (CIs). For continuously distributed outcome data, we had planned to use the mean difference (MD) with 95% CIs, where trials used the same or a similar assessment scale. For trials that used different assessment scales, we had planned to use standardised mean differences (SMDs) with 95% CIs. We had planned to consider mean or median time to healing without survival analysis as a valid outcome only where reports specified that all wounds had healed (i.e. where there was no censoring and the trial authors regarded time to healing as a continuous measure). We had planned to report time‐to‐event data (e.g. time to complete wound healing) as hazard ratios (HRs) where possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). For studies reporting time‐to‐event data (e.g. time to healing) without HRs, we had planned to estimate this effect measure using other reported outcomes, such as the numbers of events, by applying available statistical methods (Parmar 1998).
Unit of analysis issues
For studies that randomised at the participant level and measured outcomes at the wound level (e.g. wound healing), we had planned to treat the participant as the unit of analysis when the number of wounds assessed appeared equal to the number of participants (e.g. one wound per person).
Particular unit of analysis issues in wound care trials can occur:
-
when studies randomise at the participant level, use the allocated treatment on multiple wounds per participant, then analyse outcomes per wound; or
-
when studies undertake multiple assessments of an outcome over time per participant.
We would have treated studies using these approaches as cluster trials alongside more standard cluster designs, such as delivery of interventions at an organisational level.
Had we identified a correctly analysed cluster trial, we would have meta‐analysed the effect estimates and their standard errors using the generic inverse‐variance method in Review Manager 5 (RevMan 5; Review Manager 2020).
As part of the risk of bias assessment, we had planned to record where authors of cluster‐randomised trials had performed incorrect data analyses. If possible, we would have approximated the correct analyses based on guidance presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022b). We would have used information on:
-
the number of clusters randomised to each intervention group, or the mean size of each cluster;
-
the outcome data, ignoring the cluster design, for the total number of individuals (e.g. number or proportion of individuals with events, or means and standard deviations); and
-
an estimate of the intracluster (or intraclass) correlation coefficient (ICC).
Where we could not analyse study data correctly, we had planned to extract and present outcome data but not analyse them further.
Dealing with missing data
Data are often missing from trial reports. Excluding participants from the analysis after randomisation, or ignoring those lost to follow‐up, compromises the randomisation and potentially introduces bias into the trial. Where there were missing data, we requested them from the study authors.
Where data were unavailable for a proportion of the wounds healed, we would have assumed that the wounds of randomised participants not included in the results section had not healed (i.e. in the analysis, we would have included missing participants in the denominator but not the numerator).
For continuous variables (e.g. length of hospital stay and all secondary outcomes), we would have presented available data from the study reports or study authors, without imputing missing data. We had planned to calculate missing measures of variance wherever possible, or to contact study authors where not possible. Where these measures of variation were not available, we would have excluded the study from the relevant meta‐analyses.
Assessment of heterogeneity
Assessment of heterogeneity is a complex, multifaceted process. We had planned to consider clinical and methodological heterogeneity (i.e. the degree to which the included studies varied in terms of participants, interventions, outcomes and characteristics such as length of follow‐up) and supplement this assessment with information regarding statistical heterogeneity, assessed using the Chi² test (where a P level below 0.10 indicates statistically significant heterogeneity) in conjunction with the I² statistic (Higgins 2003). The I² statistic examines the percentage of total variation across RCTs that is due to heterogeneity rather than to chance (Higgins 2003). In general, I² values of 25% or less may represent a low level of heterogeneity (Higgins 2003), and values of 75% or more may indicate very high heterogeneity (Deeks 2022). However, these figures are only a guide, and statistical tests and metrics may miss important heterogeneity. For this reason, the overall assessment of heterogeneity would have considered these statistics in combination with the methodological and clinical assessment of heterogeneity. See Data synthesis for further information about how we would have handled heterogeneity in the data analyses.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs). Funnel plots enable a visual assessment of the presence of small‐study effects in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial's size or precision (Page 2022). We planned to present funnel plots for meta‐analyses comprising 10 or more RCTs using RevMan 5 (Review Manager 2020).
Data synthesis
We had planned to combine details of included studies in a narrative review according to type of comparator, taking into consideration the location or type of wound if appropriate, and then according to outcomes by time period. We had planned to consider clinical and methodological heterogeneity and undertake pooling when studies appeared sufficiently similar in terms of wound type, intervention type, duration of follow‐up and outcome type.
For meta‐analysis, our default approach would have been to use the random‐effects model. We had planned to only use a fixed‐effect approach when we considered clinical heterogeneity to be minimal and the statistical heterogeneity assessment gave a non‐significant Chi² P value and an I² value of 0% (Kontopantelis 2012). We prefer the more conservative random‐effects model because statistical assessments can miss potentially important between‐study heterogeneity (Kontopantelis 2013). Where clinical heterogeneity was thought to be acceptable or of interest but statistical heterogeneity was high, we had planned to consider meta‐analysis, attempting to interpret the causes behind the statistical heterogeneity (e.g. using meta‐regression; Thompson 1999).
We had planned to present data using forest plots where possible. For dichotomous outcomes, we had planned to present the summary estimates as RRs with 95% CIs. Where continuous outcomes were measured in the same way across studies, we had planned to present a pooled MD with 95% CI, and for outcomes measured with different measures, we would have used SMD estimates. For time‐to‐event data, we had planned to plot (and, if appropriate, pool) estimates of HRs and 95% CIs as presented in the study reports using the generic inverse variance method in RevMan 5 (Review Manager 2020).
We had planned to obtain pooled estimates of treatment effect using RevMan 5 (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
Where feasible, we would have explored the findings by ulcer stage and type of surgery.
Sensitivity analysis
Where possible, we would have performed sensitivity analyses to explore the effect on any pooled analysis of removing studies at high risk of bias in any domain.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in a summary of findings table. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined and the sum of the available data for the main outcomes (Schünemann 2022). Summary of findings tables also include an overall rating of the evidence related to each of the main outcomes based on the GRADE approach. This defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022). Due to the nature of the comparison, we did not downgrade the certainty of the evidence for high risk of performance bias alone (as blinding of surgeons appears impossible). We presented the following outcomes in the summary of findings table.
-
Complete wound healing.
-
Wound dehiscence.
-
Wound recurrence.
For other outcomes, we conducted a GRADE assessment and presented the results in narrative format in the Results section. In all cases, we followed the advice of the GRADE working group when preparing statements to justify our decisions (Santesso 2020).
Some elements of this methods section are based on the standard Cochrane Wounds protocol template.
Results
Description of studies
Results of the search
Searches for this update retrieved 1365 unique records following deduplication. We obtained 16 of these as potentially relevant to this review. Over the lifetime of the review, we have assessed 1962 records (49 as full‐text articles). One study, newly identified by this update, met the inclusion criteria (Gargano 2017). We adopted a comprehensive approach to checking other reviews and guidelines in the field of reconstructive surgery, as well as trials registers, but identified no additional records. We found no relevant ongoing studies or studies awaiting classification (Figure 1).
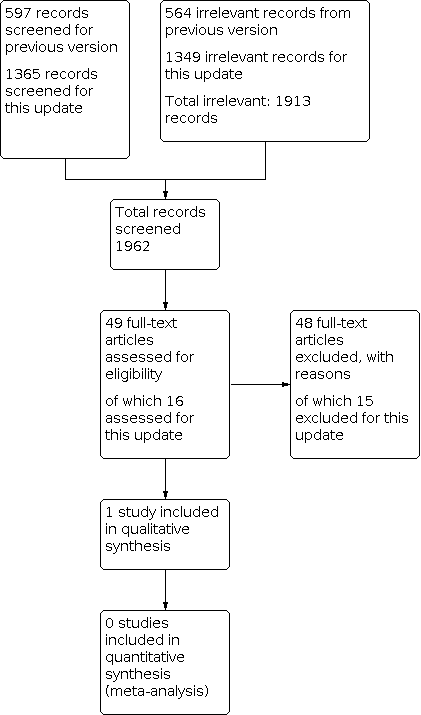
Study flow diagram.
Included studies
Participants
Gargano 2017 was undertaken in the USA and enroled 20 participants in hospital with stage IV ischial or sacral pressure ulcers, aged between 20 and 70 years.
Interventions
Gargano 2017 compared a conventional method of flap surgery to a novel cone of pressure method of flap surgery. In both groups, surgeons used rotation fasciocutaneous flaps (a posterior thigh flap for ischial ulcers and a gluteus flap for sacral ulcers). Participants in the conventional surgery group had the flap sutured at the superficial layers, such as the subcutaneous layer, the dermis and the epidermis. In the cone of pressure flap group, a large portion of the flap tip was de‐epithelialised and deeply inset to obliterate the dead space with externalised bolster sutures.
Excluded studies
We excluded 48 studies (15 in this update). The Characteristics of excluded studies table presents the reasons for exclusion. We excluded 21 studies because they did not assess reconstructive surgery as an intervention, 14 because they were not RCTs, and nine because they met neither of these two criteria. Two studies were systematic reviews, one was an animal study, and one was discontinued before recruiting any participants.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias in Gargano 2017. We contacted the study authors to request missing information regarding their methods, but received no reply.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Gargano 2017 provided no information on randomisation sequence generation or allocation concealment, so we judged the risk of selection bias as unclear.
Blinding
Gargano 2017 provided no information on blinding of participants, personnel or outcome assessors. We followed our prespecified approach (based on the nature of the intervention) and judged the risk of performance and detection bias as high for all outcomes.
Incomplete outcome data
Gargano 2017 reported some loss to follow‐up but did specify the number of participants or how this issue was managed. We therefore judged risk of attrition bias as unclear.
Selective reporting
We judged the risk of reporting bias in Gargano 2017 as high because it was unclear which outcomes the study authors planned to assess, and there were substantial limitations in the reporting of the outcomes.
Other potential sources of bias
Gargano 2017 had no clear additional sources of bias; we judged the study at unclear risk of other bias.
Effects of interventions
See: Summary of findings 1 Cone of pressure flap compared with standard flap technique
Conventional versus cone of pressure flap surgery (1 randomised controlled trial, 20 participants)
See summary of findings Table 1.
We attempted to contact the study authors to clarify reported data and obtain additional information, but we received no reply.
Complete wound healing and wound dehiscence (primary outcome)
Gargano 2017 did not report time to complete wound healing or the number of participants with complete healing. The authors explained that minor complications, including delayed wound healing and wound dehiscence that did not require surgical intervention, were comparable between the groups. There were no separate data for delayed wound healing, and we were unable to analyse these data further. We are uncertain of the effect of either method of flap surgery on complete wound healing or wound dehiscence because the evidence is of very low certainty. We downgraded the certainty of the evidence by two levels for high risk of bias in multiple domains and by two levels for imprecision due to low participant numbers and incomplete reporting.
Pressure ulcer recurrence (primary outcome)
Gargano 2017 reported wound recurrence rates of 9% (in the main text) and 12% (in the abstract) for the 11 participants in the cone of pressure flap group and 60% for the nine participants in the conventional flap coverage group at 16 months' follow‐up. Due to the discrepancy in the reported percentages in the cone of pressure flap group, and because we were uncertain how the denominators were impacted by losses to follow‐up, we could not analyse these data further. We are uncertain what the impact of the two methods of flap surgery is on recurrence because the evidence is of very low certainty. We downgraded the certainty of the evidence by two levels for high risk of bias in multiple domains and by two levels for imprecision due to low participant numbers and incomplete reporting.
Wound infection (secondary outcome)
In Gargano 2017, the study authors stated that three participants in the cone of pressure flap group and two participants in the conventional flap group had positive cultures (a surrogate outcome for wound infection). As we were uncertain what the denominator was due to unclear loss to follow‐up, we could not calculate an effect estimate. This is very low‐certainty evidence, downgraded by two levels for high risk of bias, by two levels for imprecision and by one level for indirectness.
Gargano 2017 did not report any of the other secondary outcomes (resource use, health‐related quality of life, costs, or incidence of secondary ulceration).
Discussion
Summary of main results
Despite an extensive search of numerous electronic databases, reviews, guidelines and clinical trials registers, we identified only one study that met the inclusion criteria for this review. We excluded most studies because they were either not RCTs, because they did not evaluate reconstructive surgery for the management of pressure ulcers, or for a combination of these reasons. We identified no ongoing RCTs but did exclude a record of a clinical trial that had been discontinued before recruiting any participants.
Overall completeness and applicability of evidence
We identified a single small RCT that compared two flap techniques in the management of stage IV pressure ulcers. This study provided very low‐certainty evidence for all outcomes reported. There were no studies evaluating any other comparisons. This area continues to lack both a robust evidence base and any randomised evidence for other comparisons of interventions or comparisons of surgery with alternative management.
Quality of the evidence
The certainty of the evidence is very low for all outcomes. The single small RCT was poorly reported, and we considered it at high risk of bias in several domains and at unclear risk for all other domains. There were insufficient outcome data to calculate effect estimates for any outcome. Due to the high risk of bias and the substantial imprecision resulting from the sample size of 20 participants, we considered the evidence to be very low certainty.
Potential biases in the review process
We employed a robust search strategy to locate as much relevant evidence as possible, applying no language restrictions. We located full articles for all potentially relevant papers and translated them where required. We found no relevant ongoing or unpublished studies in trials registers, though there may be additional unpublished data that we were unable to identify.
Agreements and disagreements with other studies or reviews
There is a lack of rigorous evidence regarding the benefits and harms of reconstructive surgery for people with pressure ulcers (Levine 2013). Some systematic reviews have regarded surgical reconstruction for pressure ulcers favourably, but these have included non‐randomised case series and retrospective studies (Smith 2013; Vathulya 2022; Zwanenburg 2021). Consequently, their usefulness in decision‐making is limited. NICE guidelines on the prevention and management of pressure ulcers do not refer to reconstructive surgery, which further reflects the lack of robust evidence in this area (NICE 2014).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Cone of pressure flap compared with standard flap technique | ||||||
| Patient or population: people with stage IV pressure ulcers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard flap | Risk with COP flap | |||||
| Complete healing of surgical wound Follow‐up: 16 months | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study reported that minor complications, including delayed wound healing and wound dehiscence that did not require surgical intervention, were comparable between the groups. We could not calculate an effect estimate and are uncertain of the effect of the interventions on wound healing. | |
| Not calculable | Not calculable | |||||
| Wound dehiscence Follow‐up: 16 months | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study reported that minor complications, including delayed wound healing and wound dehiscence that did not require surgical intervention, were comparable between the groups. We could not calculate an effect estimate and are uncertain of the effect of the interventions on wound dehiscence. | |
| Not calculable | Not calculable | |||||
| Pressure ulcer recurrence | Study population | Not calculable | 20 (1 RCT) | ⊕⊝⊝⊝ | The included study did not clearly report the proportion of participants in each group with pressure ulcer recurrence. We could not calculate an effect estimate and are uncertain of the effect of the interventions on ulcer recurrence. | |
| Not calculable | Not calculable | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COP: cone of pressure; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for high risk of bias in multiple domains, and two levels for imprecision due to low participant number and incomplete reporting. | ||||||

