Les programmes d'échange des aiguilles et seringues et la thérapie de substitution aux opiacés pour prévenir la transmission de l'hépatite C chez les utilisateurs de drogues injectables
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective cohort study; recruitment was done via RDS, street outreach and snowball sampling | |
| Participants | Country: Australia 449 PWID, defined as 'regularly' injecting illicit drugs in the last 6 months. Median age was 29.4 years, and 50% of participants reported injecting daily, but there was no information on the main drug being injected. | |
| Interventions | The intervention in this study was use of opioid substitution therapy (OST); OST was defined as use of OST in the previous month. The comparison group was no current OST use. Follow‐up: 196 person years Study duration: 5 years | |
| Outcomes | HCV seroconversion as measured by HCV antibody in serum | |
| Notes | Funding source is the Australian National Health and Medical Research Council | |
| Methods | Prospective cohort study; recruitment was done via street outreach, and snowball sampling | |
| Participants | Country: Canada 285 PWID | |
| Interventions | The interventions included in this study were needle syringe exchange programme (NSP) use in the previous 3 or 6 months, use of methadone maintenance in the previous 6 months. Further detail on the intensity of engagement with the intervention was gathered; researchers examined NSP use where 100% of needles/syringes used were obtained by NSP and a methadone dose of 0‐60 mg or 60+ mg, respectively. Comparisons were no NSP use in the previous 3 or 6 months or low NSP coverage (< 100%), no OST use in the previous 6 months, or < 59 mg of methadone Follow‐up: 589.3 person years Study duration: 7 years | |
| Outcomes | HCV seroconversion | |
| Notes | The funding source was the Canadian Institutes of Health Research, US National Institute on Drug Abuse and the Réseau SIDA et Maladies Infectieuses du Fonds de la Recherche en Sante du Quebec | |
| Methods | Prospective cohort study | |
| Participants | Country: Wales, UK 700 PWID, defined as injecting drugs in the previous 4 weeks. 29% were female and the mean age was 27.2 years. The main drug injected was not reported, but 42% had injected stimulants. | |
| Interventions | The intervention was either in opioid substitution treatment or not Follow‐up: 287.3 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion | |
| Notes | Funded by the Welsh Assembly Government | |
| Methods | Retrospective cohort study | |
| Participants | Country: Australia 1741 PWID; the mean age was 29.2 years and 42% were female; main drug was not reported | |
| Interventions | The intervention was defined as either continuous or interrupted methadone maintenance treatment; the comparison was no methadone maintenance Follow‐up: 85.4 person years Study duration: 4 years | |
| Outcomes | HCV seroconversion | |
| Notes | Individual funding was received from Research Fund of the Macfarlane Burnet Centre, Victorian Department of Health and Community Services Public Health Training Programme, the Commonwealth Department of Health and Family Service. | |
| Methods | Case‐control study | |
| Participants | Country: USA 46 PWID, where PWID status was defined as having injected drugs in the previous 6 months (cases). 24% of the sample were < 25 years, 45% were female; the main drug injected was not reported | |
| Interventions | The intervention under study was ever having used a needle syringe exchange programme and comparison was never having used a NSP Follow‐up: n/a Study duration: 2 years | |
| Outcomes | HCV seroconversion defined by presence of HCV antibodies | |
| Notes | Funded by the American Foundation for AIDS Research | |
| Methods | Prospective cohort study | |
| Participants | Country: USA 2462 PWID, defined as having injected drugs in the previous 12 months. 19% were < 25 years, 38% were female, 54% injected heroin and 59% injected daily | |
| Interventions | The intervention under study was either current sporadic or current regular needle syringe exchange programme use; the comparison was no use of the NSP. Follow‐up: 209 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion defined by presence of HCV antibodies (the timeframe for seroconversion was within the previous 12 months) | |
| Notes | Funded by the National Institute on Drug Abuse and Centre for Disease Control | |
| Methods | Prospective cohort study; recruitment was done via RDS and street outreach | |
| Participants | Country: USA 4663 PWID, defined as injecting drugs in the previous 6 or 12 months. 28% were less than 21 years old, 38% were female; main drug injected was not reported, but 49% injected daily | |
| Interventions | The intervention was participation (yes/no) in a needle syringe exchange programme (NSP) in either the previous 3 months or 6 months Follow‐up: n/a Study duration: 10 years | |
| Outcomes | HCV seroconversion measured by the presence of HCV antibodies | |
| Notes | Funding source not specified | |
| Methods | Cross‐sectional study. Recruitment of study participants was done via RDS | |
| Participants | Country: England, UK 299 PWID, defined as having injected drugs in the previous 4 weeks. 17% were < 25 years old, 23% were female, 94% injected opiates, 40% injected daily | |
| Interventions | The interventions were as follows:
Comparisons were no current use of OST, no or low NSP coverage Follow‐up: n/a Study duration: 6 months | |
| Outcomes | HCV seroconversion defined as HCV RNA positive and HCV antibody negative (dried blood spot testing); the window period for the outcome was 51–75 days (range) | |
| Notes | Funded by the National Treatment Agency for Substance Use and Health Protection Agency | |
| Methods | Cross‐sectional study; recruitment of study participants was done via RDS | |
| Participants | Country: England, UK 948PWID, defined as having injected drugs in the previous 4 weeks. Median age was 33 years, 48% injected heroin as their main drug, but 64% had injected crack/cocaine in the previous month, 19% were female and 53% injected daily | |
| Interventions | The interventions were as follows:
Comparisons were no current use of OST, no or low NSP coverage Follow‐up: 6 months | |
| Outcomes | HCV seroconversion defined as HCV RNA positive and HCV antibody negative (dried blood spot testing); the window period for the outcome was 51–75 days (range) Follow‐up: n/a Study duration: 6 months | |
| Notes | Funded by National Treatment Agency for Substance Use and the Health Protection Agency | |
| Methods | Prospective cohort study; recruitment was conducted via privileged access interviews and snowball sampling | |
| Participants | Country: England, UK 272 PWID, defined as having injected drugs in the previous 4 weeks. Median age was 27.6 years, 29% were female, 35% mainly injecting heroin, 84% injected daily | |
| Interventions | The intervention of interest was use of methadone maintenance treatment in the previous 6 months or longer, compared to no methadone in the same time period Follow‐up:116.7 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion | |
| Notes | Funded by the UK Department of Health | |
| Methods | Prospective cohort study; recuitment was conducted at drug treatment centres | |
| Participants | Country: France 321 PWID, defined as ever having injected drugs. Median age was 26.9 years, 17.6% were female, 28% injected opiates, 84% injected daily | |
| Interventions | The intervention under study was having received OST in the 3 months prior to study enrollment; the comparison was no OST in the 3 months prior to study enrollment Follow‐up: 178.4 person years Study duration: 1 year | |
| Outcomes | Seroconversion measured as the presence of HCV antibodies in oral fluid and serum on positive tests; the window period for the outcome was the midpoint between previous negative oral fluid test and first positive serum test | |
| Notes | Funded by the Agence Nationale de Recherche su le SIDA, Institute de Veille Sanitaire, Programme Hospitalier de Recherce Clinique, Direction Departementale de l'Action Sanitaire et Sociale du Nord, Academie Nationale de Medecine | |
| Methods | Prospective cohort study; recruitment was conducted in community settings and in low‐threshold drug treatment settings | |
| Participants | Country: Australia 294 PWID, defined as injection in the previous 6 months. Median age was 24 years, 32% were female, 69% injected heroin | |
| Interventions | The intervention under study was having received OST in the previous 6 months; the comparison was no OST in the previous 6 months Follow‐up: 212.86 person years Study duration: 3 years | |
| Outcomes | Seroconversion as measured by anti‐HCV serology at baseline using 1‐2 third‐generation enzyme‐linked immunosorbent assays. PCR testing to detect HCV RNA on all final HCV antibody negative specimens | |
| Notes | Funded by the Australian National Health and Medical Research Council | |
| Methods | Prospective cohort study; recruitment was conducted through community‐based outreach | |
| Participants | Country: USA 471 PWID, defined as having injected within the preceding 11 years. Median age was 34 years, 18.3% were female, 65% injected heroin and cocaine, 92% had injected in the previous year at baseline | |
| Interventions | The intervention under study was being in methadone treatment in the previous 6 months; the comparison was no methadone treatment in the previous 6 months Follow‐up: 166.5 person years Study duration: 20 years | |
| Outcomes | HCV seroconversion, measured through serum samples | |
| Notes | Funded by the National Institute on Drug Abuse | |
| Methods | Prospective cohort study; recruitment included snowball sampling | |
| Participants | Country: Canada 3741 PWID, defined as having injected drugs in the previous 4 weeks. 30% were female, 34% injected opiates and the mean age was 34 years among methadone users and 23 years among non‐methadone users | |
| Interventions | The interventions under study were:
Comparison was no use of MMT within the same time periods Follow‐up: 2108.4 person years Study duration: 16 years | |
| Outcomes | HCV seroconversion defined by presence of HCV antibodies | |
| Notes | Funded by the US National Institutes on Drug Abuse | |
| Methods | Prospective cohort study; recruitment occurred through street outreach | |
| Participants | Country: USA 552 PWID, defined as people who have injected drugs in the previous month and less than 30 years old. 42.5% were < 22 years, 22% were female and 61% injected heroin/heroin mixed in the previous month | |
| Interventions | The intervention under study was use of a NSP in the previous 3 months and the comparison was no use of NSP Follow‐up: 681.3 person years Study duration: 15 years | |
| Outcomes | HCV seroconversion defined by presence of HCV antibodies or HCV RNA | |
| Notes | Funded by the National Institute on Drug Abuse | |
| Methods | Cross‐sectional study; participants were recruited at NSPs | |
| Participants | Country: Scotland, UK 7954 PWID, defined as ever having injected drugs (but 80% had injected in previous 6 months). Mean age is 34 years, 27.5% are female, 55.3% inject daily and 17% injected stimulants | |
| Interventions | The interventions were defined as:
The comparisons were no OST or no/low NSP use Follow‐up: 602.7 person years Study duration: 4 years | |
| Outcomes | The outcome was HCV seroconversion defined as being HCV antibody negative and HCV RNA positive | |
| Notes | Funded by the Scottish Government | |
| Methods | Prospective cohort study; recruitment included snowball sampling | |
| Participants | Country: Canada 1345 PWID, defined as having injected drugs in the previous 4 weeks. 30% were female, the median age was 34 years, 63% injected opiates and 54% injected stimulants, 54% injected daily | |
| Interventions | The intervention under study was
The comparison was NSP attendance or no methadone in the previous 6 months Follow‐up: 207.9 person years Study duration: 3 years | |
| Outcomes | HCV seroconversion measured by HCV antibody positivity | |
| Notes | Funding source was not specified | |
| Methods | Case‐control study; recruitment methods employed a convenience sample of service attenders | |
| Participants | Country: Italy 746 PWID, defined as being a heroin user. 21% were < 28 years, 3% were female, 100% injected opiates and 32% also injected stimulants, 69% injected daily | |
| Interventions | The intervention under study was being in methadone maintenance treatment in the previous 6 months, the comparison was no methadone maintenance in the same time period Follow‐up: 73.4 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion, measured by HCV antibody positivity in serum samples | |
| Notes | Funded by the Progretto AIDS, Ministero della Sanita‐Instituto Superiore di Sanita | |
| Methods | Serial cross‐sectional survey; recruitment methods employed service attenders at drug treatment programmes | |
| Participants | Country: Canada 1380 PWID, defined as having injected in the previous 6 months. Mean age was 31.8 years, 27% were female, 19% injected opiates and 75% injected stimulants | |
| Interventions | The intervention under study was using an NSP in the previous 6 months, and the comparison was no use of the NSP Follow‐up: 267 person years Study duration: 6 years | |
| Outcomes | HCV RNA positive on anti‐HCV negative (oral fluid). HCV seroconversions were attributed to the midpoint between the previous negative and first positive test results | |
| Notes | Funded by the Health Canada, Ministere de la Sante et des Services Sociaux du Quebec | |
| Methods | Prospective cohort study; recruitment occurred via community outreach and snowball sampling | |
| Participants | Country: China 379 PWID, defined as having injected drugs in the previous 3 months. 44% were < 28 years and 100% injected opiates. There was no information on sex or frequency of injecting. | |
| Interventions | The intervention of interest was lifetime experience of methadone maintenance treatment (yes or no). Follow‐up: 258 person years Study duration: 3 years | |
| Outcomes | HCV antibody positivity in serum samples (incidence density); the time of seroconversion was the midpoint between the previous negative and first positive HCV antibody test result | |
| Notes | Funded by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, China Comprehensive Integrated Programmes for Research on AIDS, the National Institute of Allergy and Infectious Diseases and the National Institutes of Health | |
| Methods | Prospective cohort study; recruitment via community outreach and snowball sampling | |
| Participants | Country: Canada 377 PWID, defined as having injected in the previous 4 weeks. Median age was 23 years, 53% were female, 18% injected opiates, 10% injected stimulants, 18% injected daily | |
| Interventions | The intervention of interest was being in methadone maintenance treatment (yes or no) at the time of survey; comparison was no current use of methadone maintenance Follow‐up:338.6 person years Study duration: 6 years | |
| Outcomes | HCV antibody positivity in serum samples (incidence density); the time of seroconversion was the midpoint between the previous negative and first positive HCV antibody test result | |
| Notes | Funded by the Institute for Aboriginal Peoples Health and the Canadian Institutes for Health Research | |
| Methods | Prospective cohort study; recruitment from a drug treatment setting | |
| Participants | Country: USA 716 PWID, defined as having injected drugs in the previous 4 weeks. 5.4% were < 25 years, 49% were female, 23% injected stimulants and 25% injected daily | |
| Interventions | The interventions under study were:
The comparison was no MMT. Follow‐up: 80 person years Study duration: 4 years | |
| Outcomes | HCV seroconversion, as demonstrated by the presence of HCV antibodies in serum | |
| Notes | Funded by the Centers for Disease Control and Prevention | |
| Methods | Prospective cohort study; recruitment via street outreach, targeted advertising, and peer referrals | |
| Participants | Country: USA 702 PWID, defined as having injected in the previous 6 months. 53% were aged 18‐22 years, 49% were female, 23% injected stimulants and 39% injected daily | |
| Interventions | The intervention of interest was use of an NSP in the previous 6 months and the comparison was no use of the NSP Follow‐up: 327.2 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion as demonstrated by the presence of HCV antibodies in serum; time of seroconversion was taken to be the midpoint between the previous negative and first positive HCV antibody test result | |
| Notes | Funding source was not specified | |
| Methods | Prospective cohort study; recruitment via street outreach | |
| Participants | Country: USA 992 PWID, defined as having injected in the previous 4 weeks and aged < 30 years. 16% were aged 15‐18 years, 32% were female, 60% injected opiates and 33.2% injected stimulants | |
| Interventions | The interventions of interest included:
The comparison was no opiate agonist therapy in the same time frame Follow‐up: 680 person years Study duration: 13 years | |
| Outcomes | HCV seroconversion. Incidence was calculated using behavior or characteristic at the previous period that participant was seronegative for HCV (uninfected during follow‐up) or the first HCV‐seropositive risk period (incident infections). Incident acute HCV infections were: a new test result positive for HCV RNA and/or anti‐HCV after a previously documented test result negative for anti‐HCV; or a positive HCV RNA test result concomitant with a negative anti‐HCV test result. | |
| Notes | Funded by the National Institute on Drug Abuse, National Institute of Health, National Institute on Alcohol and Alcoholism | |
| Methods | Prospective cohort study; recruitment was street‐based and employed targeted sampling and chain‐referral methods | |
| Participants | Country: Spain 513 PWID; PWID were required to have used heroin at least 12 days and at least 1 day in the past 3 months. 40% were < 25 years, 27% were female, 31% injected stimulants. There was no information on daily injecting. | |
| Interventions | The intervention of interest was methadone maintenance; further details of the intervention (e.g. intensity or duration of engagement in the intervention) was not specified, the comparison was no use of methadone maintenance. Follow‐up: 105.4 peron years Study duration: 3 years | |
| Outcomes | HCV seroconversion, defined by HCV antibody positivity by dried blood spot testing | |
| Notes | Funded by the Foundation for AIDS Prevention and Research | |
| Methods | Retrospective cohort study; recruitment at drug treatment services | |
| Participants | Country: Australia 1078 PWID, 61.5% were < 20 years, 55.9% were female, 19% injected opiates, 27.9% injected stimulants | |
| Interventions | The intervention under study was ever having received methadone; the comparison was no methadone Follow‐up:148.2 person years Study duration: 2 years | |
| Outcomes | HCV seroconversion | |
| Notes | Funded by the Australian National Council on AIDS and Related Diseases | |
| Methods | Prospective cohort study; enrollment occurred through 'open' recruitment | |
| Participants | Country: Netherlands 168 PWID, defined as those who had ever injected drugs. Median age was 31.4 years, 33% were female, 33% injected opiates and 51% injected stimulants, 51.7% injected daily | |
| Interventions | The interventions of interest were measured as follows:
The comparsion was no methadone in the past 6 months, and/or no use of NSP or no injection Follow‐up: 598.56 person years Study duration: 22 years | |
| Outcomes | HCV seroconversion | |
| Notes | Funded by the Netherlands National Institute for Public Health and the Environment | |
| Methods | Prospective cohort study; recruitment via snowball sampling, social networks, RDS, and targeted outreach sampling | |
| Participants | Country: Australia 166 PWID, defined as those who had injected drugs in the previous 12 months. Median age was 27 years, 25% were female. Participants mainly injecting opioids, but frequency of injecting was not reported | |
| Interventions | The intervention assessed was having accessed a needle syringe exchange programme or opioid substitution treatment in the previous 6 months, the comparison was no use of the NSP or OST in the same time frame. Follow‐up: 215.2 person years. Study duration: 3 years | |
| Outcomes | HCV seroconversion defined as being negative for HCV antibodies and positive for HCV RNA | |
| Notes | Funded by the National Health and Medical Research Council | |
HCV: hepatitis C virus; NSP: needle syringe programme; OST: opioid substitution therapy; PCR: polymerase chain reaction; PWID: people who inject drugs; RDS: respondent‐driven sampling.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No outcome of interest | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest; no comparison of interest: all participants on OST | |
| No outcome of interest | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest; simulation study | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest. No comparison of interest: all participants on OST | |
| No outcome of interest | |
| No outcome of interest | |
| No comparison of interest: all participants on OST | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest; no intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest; no intervention of interest: no OST or NSP | |
| No outcome of interest; no intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| Editorial | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest; No intervention of interest: no OST or NSP | |
| No outcome of interest; no intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest | |
| No comparison of interest: all participants on OST | |
| No outcome of interest; simulation study | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest; simulation study | |
| No intervention of interest: no OST or NSP | |
| No comparison of interest | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| Overview | |
| No intervention of interest: no OST or NSP | |
| No comparison of interest: all on OST | |
| No outcome of interest; simulation model | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest. No comparison of interest: all participants on OST | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No comparison of interest: all participants on OST | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No comparison of interest: all participants on OST | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest. No comparison of interest: all participants on OST | |
| No outcome of interest | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No intervention of interest (NSP shuts down for some of the follow‐up) | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No outcome of interest. No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No intervention of interest: no OST or NSP | |
| No intervention of interest: doesn't specify OST, only that it is drug treatment | |
| No comparison of interest: all participants on OST | |
| No intervention of interest: no OST or NSP | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No outcome of interest | |
| No comparison of interest: all participants on OST | |
| No comparison of interest: all participants on OST | |
| No outcome of interest |
NSP: needle syringe programme; OST: opioid substitution therapy.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective cohort |
| Participants | 313 HCV‐seronegative PWID (injection in the previous month) were enrolled with at least one follow‐up visit. 22% were female, 43% were under 30 years old and 58% injected cocaine |
| Interventions | Opioid agonist therapy (1‐59 mg, methadone or suboxone, ≥ 60 mg methadone) and injection material coverage (100% safe sources vs no) |
| Outcomes | Seroconversion to HCV antibody positive |
| Notes | The study was conducted in Montreal, Canada. No funding source is specified. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in Chinese. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in Chinese. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in Chinese. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in Chinese. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | The text is in French, and there is little information in the abstract. |
| Methods | Prospective cohort recruited between 2011 and 2015 |
| Participants | People who inject drugs, defined as regular injectors (at least one a month in the 6 months prior to recruitment), a total of 502 participants, approximately 36% were female and mean age 30 was years |
| Interventions | Current opoid substitution therapy prescription; NSP as usual source of syringe acquisition in the past month, measure of injections covered by sterile syringe (syringes acquired divided by syringes distributed divided by past week injecting frequency) |
| Outcomes | HCV RNA positive among negative samples |
| Notes | Data drawn from the Melbourne injecting drug use cohort study (MIX). Funding provided by the Colonial Foundation Trust and the National Reserch Council |
| Methods | Longitudinal incidence study, participants recruited in community settings through peer referrals in places where drugs are used |
| Participants | People who inject drugs defined as injection at least once in the previous 3 months and residing in Delhi. A total of 2292 PWID recruited of whom all were male; median age was 29 years |
| Interventions | Accessed NSP in the previous 3 months |
| Outcomes | anti HCV negative and HCV RNA positive |
| Notes | Funding received from the Canadian Government (Department of Foreign Affairs, Trade and Development Canada). No incidence data reported, but need to contact authors for measures |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in German. |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | There is no abstract, and the text is in Japanese. |
HCV: hepatitis C virus; NSP: needle syringe programme; PWID: people who inject drugs.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses by region Show forest plot | 12 | 6361 | Risk Ratio (Random, 95% CI) | 0.50 [0.40, 0.63] |
| Analysis 1.1 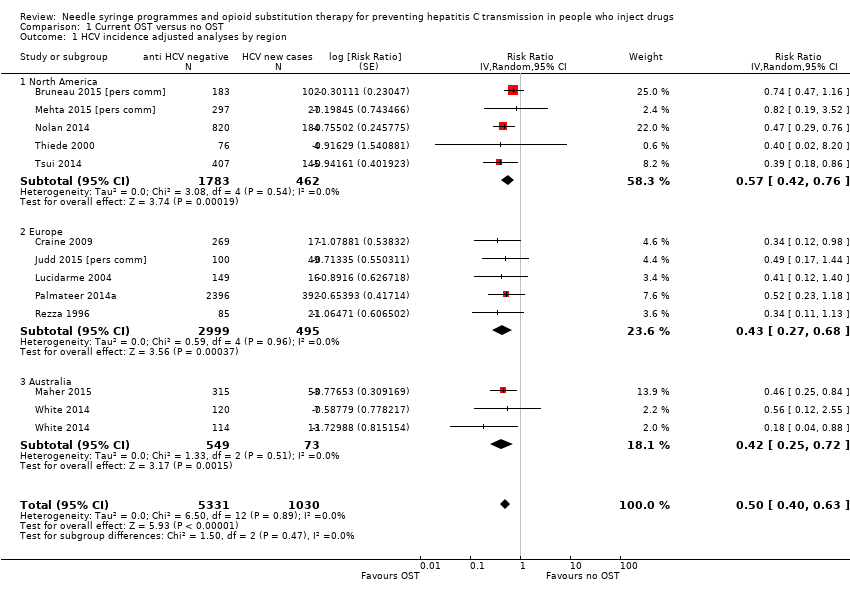 Comparison 1 Current OST versus no OST, Outcome 1 HCV incidence adjusted analyses by region. | ||||
| 1.1 North America | 5 | 2245 | Risk Ratio (Random, 95% CI) | 0.57 [0.42, 0.76] |
| 1.2 Europe | 5 | 3494 | Risk Ratio (Random, 95% CI) | 0.43 [0.27, 0.68] |
| 1.3 Australia | 2 | 622 | Risk Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 2 HCV incidence adjusted analysis by study design Show forest plot | 12 | 6361 | Risk Ratio (Random, 95% CI) | 0.50 [0.40, 0.63] |
| Analysis 1.2 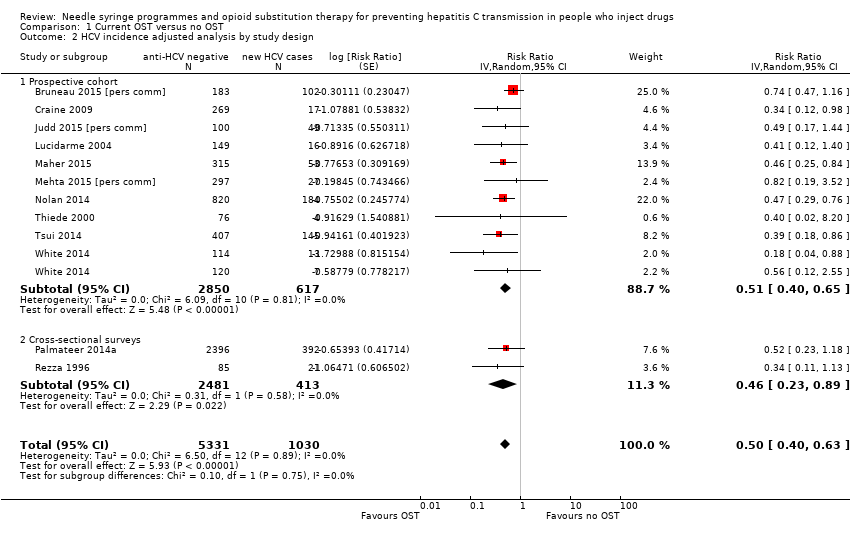 Comparison 1 Current OST versus no OST, Outcome 2 HCV incidence adjusted analysis by study design. | ||||
| 2.1 Prospective cohort | 10 | 3467 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.65] |
| 2.2 Cross‐sectional surveys | 2 | 2894 | Risk Ratio (Random, 95% CI) | 0.46 [0.23, 0.89] |
| 3 HCV incidence unadjusted analyses by different modes of OST provision Show forest plot | 9 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.3 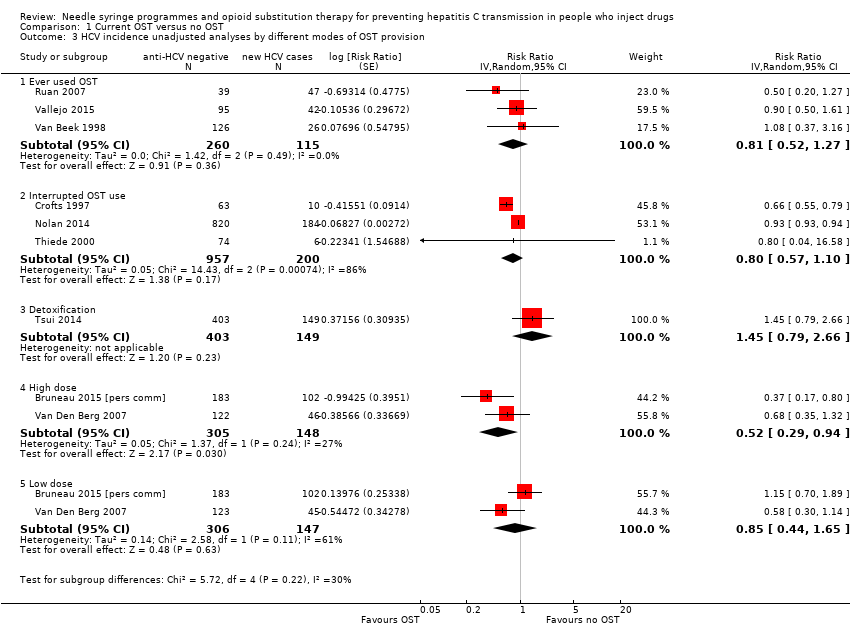 Comparison 1 Current OST versus no OST, Outcome 3 HCV incidence unadjusted analyses by different modes of OST provision. | ||||
| 3.1 Ever used OST | 3 | 375 | Risk Ratio (Random, 95% CI) | 0.81 [0.52, 1.27] |
| 3.2 Interrupted OST use | 3 | 1157 | Risk Ratio (Random, 95% CI) | 0.80 [0.57, 1.10] |
| 3.3 Detoxification | 1 | 552 | Risk Ratio (Random, 95% CI) | 1.45 [0.79, 2.66] |
| 3.4 High dose | 2 | 453 | Risk Ratio (Random, 95% CI) | 0.52 [0.29, 0.94] |
| 3.5 Low dose | 2 | 453 | Risk Ratio (Random, 95% CI) | 0.85 [0.44, 1.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 8 | 5235 | Risk Ratio (Random, 95% CI) | 0.42 [0.31, 0.58] |
| Analysis 2.1  Comparison 2 Sensitivity analysis: OST versus no OST, adjusted analyses excluding unpublished datasets, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 9 | 5782 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.64] |
| Analysis 3.1  Comparison 3 Sensitivity analysis: OST versus no OST, adjusted analyses excluding studies at critical risk of bias, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 10 | 3467 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.65] |
| Analysis 4.1  Comparison 4 Sensitivity analysis: OST versus no OST, adjusted analyses excluding cross‐sectional studies, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 16 | 9499 | Risk Ratio (Random, 95% CI) | 0.57 [0.45, 0.73] |
| Analysis 5.1  Comparison 5 OST versus no OST, unadjusted analysis, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses by region Show forest plot | 5 | 3530 | Risk Ratio (Random, 95% CI) | 0.79 [0.39, 1.61] |
| Analysis 6.1  Comparison 6 High NSP coverage versus no/low NSP coverage, Outcome 1 HCV incidence adjusted analyses by region. | ||||
| 1.1 North America | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.25 [0.63, 2.46] |
| 1.2 Europe | 2 | 2903 | Risk Ratio (Random, 95% CI) | 0.24 [0.09, 0.62] |
| 2 HCV incidence adjusted analyses by study design Show forest plot | 5 | 3530 | Risk Ratio (Random, 95% CI) | 0.95 [0.50, 1.82] |
| Analysis 6.2  Comparison 6 High NSP coverage versus no/low NSP coverage, Outcome 2 HCV incidence adjusted analyses by study design. | ||||
| 2.1 Prospective cohorts | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.44 [1.01, 2.05] |
| 2.2 Cross‐sectional surveys | 2 | 2903 | Risk Ratio (Random, 95% CI) | 0.24 [0.09, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 4 | 3245 | Risk Ratio (Random, 95% CI) | 0.77 [0.28, 2.13] |
| Analysis 7.1  Comparison 7 Sensitivity analysis: high NSP versus low/no NSP, excluding unpublished data, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.25 [0.63, 2.46] |
| Analysis 8.1  Comparison 8 Sensitivity analysis: high NSP versus low/no NSP, excluding cross‐sectional surveys, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 7 | 6455 | Risk Ratio (Random, 95% CI) | 0.78 [0.39, 1.55] |
| Analysis 9.1  Comparison 9 High NSP coverage versus low/no coverage, unadjusted estimates, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence, adjusted analyses Show forest plot | 6 | 2765 | Risk Ratio (Random, 95% CI) | 1.43 [0.82, 2.49] |
| Analysis 10.1  Comparison 10 Low NSP coverage versus no coverage, Outcome 1 HCV incidence, adjusted analyses. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 9 | 3242 | Risk Ratio (Random, 95% CI) | 1.41 [0.95, 2.09] |
| Analysis 11.1  Comparison 11 Low NSP coverage versus no NSP, unadjusted analysis, Outcome 1 HCV incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses Show forest plot | 3 | 6197 | Risk Ratio (Random, 95% CI) | 0.45 [0.22, 0.94] |
| Analysis 12.1  Comparison 12 Combined OST and high/low NSP versus no OST and low/no NSP, Outcome 1 HCV incidence adjusted analyses. | ||||
| 1.1 High NSP coverage | 3 | 3241 | Risk Ratio (Random, 95% CI) | 0.26 [0.07, 0.89] |
| 1.2 Low NSP coverage | 2 | 2956 | Risk Ratio (Random, 95% CI) | 0.87 [0.44, 1.68] |
| 2 HCV incidence unadjusted analyses Show forest plot | 4 | 6427 | Risk Ratio (Random, 95% CI) | 0.47 [0.27, 0.80] |
| Analysis 12.2  Comparison 12 Combined OST and high/low NSP versus no OST and low/no NSP, Outcome 2 HCV incidence unadjusted analyses. | ||||
| 2.1 Combined OST and high NSP versus no OST and low/no NSP | 4 | 3356 | Risk Ratio (Random, 95% CI) | 0.29 [0.13, 0.65] |
| 2.2 Combined OST and low NSP versus no OST and low/no NSP | 3 | 3071 | Risk Ratio (Random, 95% CI) | 0.76 [0.44, 1.33] |

Study flow diagram.
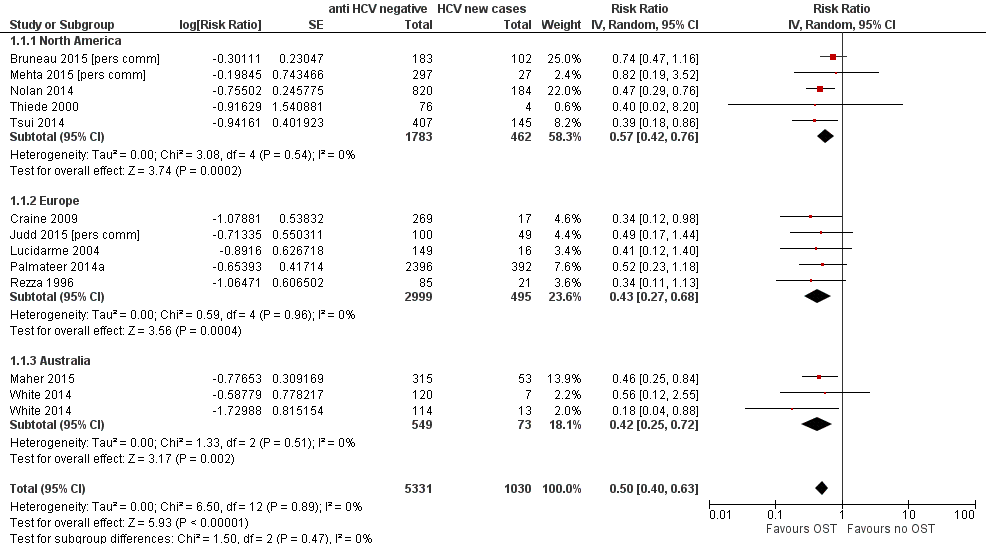
Forest plot of comparison: 1 Current OST versus no OST, outcome: 1.1 HCV incidence adjusted analyses by region.

Funnel plot of comparison: 1 Current OST versus no OST, outcome: 1.1 HCV incidence adjusted analyses by region.

Forest plot of comparison: 2 High NSP coverage versus no/low NSP coverage, outcome: 2.1 HCV incidence adjusted analyses by region.
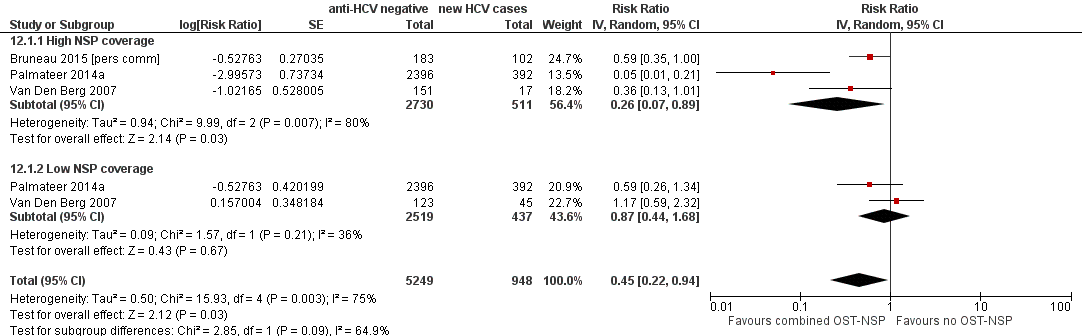
Forest plot of comparison: 4 Combined OST and high/low NSP versus no OST and low/no NSP, outcome: 4.1 HCV incidence adjusted analyses.

Comparison 1 Current OST versus no OST, Outcome 1 HCV incidence adjusted analyses by region.

Comparison 1 Current OST versus no OST, Outcome 2 HCV incidence adjusted analysis by study design.
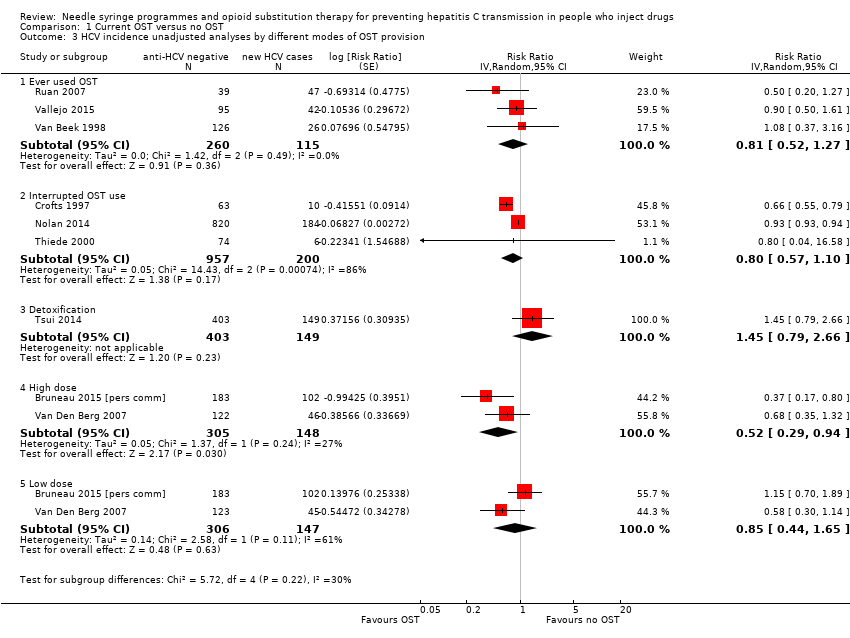
Comparison 1 Current OST versus no OST, Outcome 3 HCV incidence unadjusted analyses by different modes of OST provision.
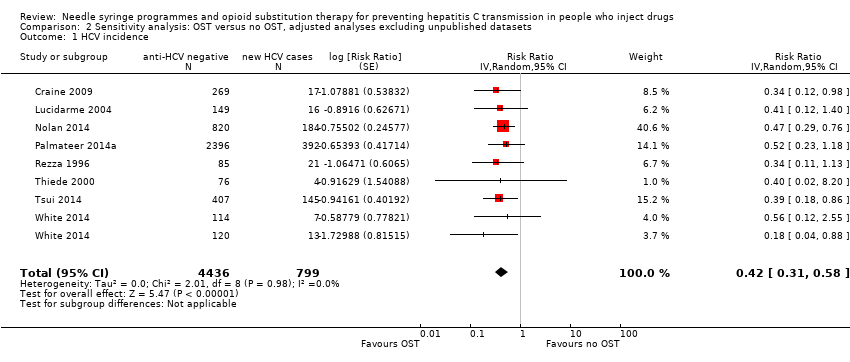
Comparison 2 Sensitivity analysis: OST versus no OST, adjusted analyses excluding unpublished datasets, Outcome 1 HCV incidence.

Comparison 3 Sensitivity analysis: OST versus no OST, adjusted analyses excluding studies at critical risk of bias, Outcome 1 HCV incidence.

Comparison 4 Sensitivity analysis: OST versus no OST, adjusted analyses excluding cross‐sectional studies, Outcome 1 HCV incidence.

Comparison 5 OST versus no OST, unadjusted analysis, Outcome 1 HCV incidence.

Comparison 6 High NSP coverage versus no/low NSP coverage, Outcome 1 HCV incidence adjusted analyses by region.

Comparison 6 High NSP coverage versus no/low NSP coverage, Outcome 2 HCV incidence adjusted analyses by study design.
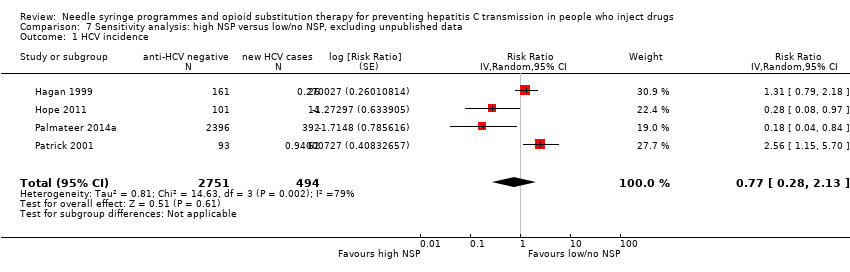
Comparison 7 Sensitivity analysis: high NSP versus low/no NSP, excluding unpublished data, Outcome 1 HCV incidence.

Comparison 8 Sensitivity analysis: high NSP versus low/no NSP, excluding cross‐sectional surveys, Outcome 1 HCV incidence.

Comparison 9 High NSP coverage versus low/no coverage, unadjusted estimates, Outcome 1 HCV incidence.

Comparison 10 Low NSP coverage versus no coverage, Outcome 1 HCV incidence, adjusted analyses.

Comparison 11 Low NSP coverage versus no NSP, unadjusted analysis, Outcome 1 HCV incidence.

Comparison 12 Combined OST and high/low NSP versus no OST and low/no NSP, Outcome 1 HCV incidence adjusted analyses.
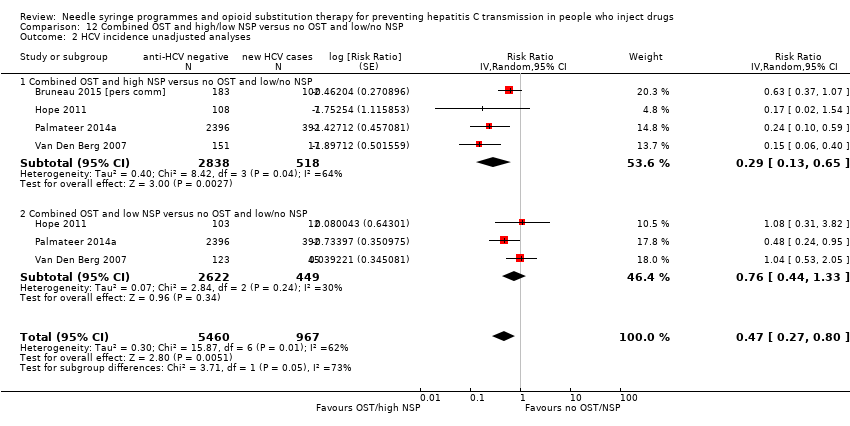
Comparison 12 Combined OST and high/low NSP versus no OST and low/no NSP, Outcome 2 HCV incidence unadjusted analyses.
| Current OST versus no OST | |||||
| Patient or population: people who inject drugs | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No OST | Current OST | ||||
| HCV incidence adjusted analyses | — | — | RR 0.50 (0.40 to 0.63) | 6361 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level due to overall moderate risk of bias in 2 studies, overall serious risk of bias in 6 studies, 2 studies at overall critical risk of bias in 2 studies; not enough information to make judgment in 2 studies. | |||||
| High NSP coverage versus no/low NSP coverage | |||||
| Patient or population: people who inject drugs | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No/low NSP coverage | High NSP coverage | ||||
| HCV incidence adjusted analyses | — | — | RR: 0.79 (0.39 to 1.61) | 3530 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level due to serious overall risk of bias in all the studies. | |||||
| Combined OST and highNSP versus no OST and low/no NSP | |||||
| Patient or population: people who inject drugs | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No OST and low/no NSP | Combined OST and high NSP | ||||
| HCV incidence adjusted analyses | — | — | RR: 0.26 (0.07 to 0.89) | 3241 | ⊕⊕⊕⊝ Lowa,b |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level due to serious overall risk of bias in all studies. | |||||
| Study | Confounding | Selection bias | Measurement of interventions | Departures from intended interventions | Missing data | Measurement of outcomes | Selection of reported result | Overall risk of bias |
| Critical | Critical | Serious | No info | Critical | Low | No info | Critical | |
| Moderate | Serious | Moderate | No info | No info | Low | Low | Serious | |
| Serious | Serious | Serious | No info | Serious | Low | Low | Serious | |
| Critical | Serious | Low | No info | Serious | Serious | Low | Critical | |
| Serious | Serious | Serious | No info | Low | Low | Low | Serious | |
| Moderate | Serious | Low | No info | Low | Low | Low | Serious | |
| Serious | Serious | Moderate | No info | No info | Low | Low | Serious | |
| Moderate | Moderate | Serious | No info | Low | Low | Low | Serious | |
| Moderate | Moderate | Serious | No info | No info | Low | Low | Serious | |
| Moderate | Critical | Critical | No info | Critical | Low | Low | Critical | |
| Moderate | Serious | Serious | No info | Serious | Low | Low | Serious | |
| Moderate | Serious | Serious | No info | No info | Low | Low | Serious | |
| Moderate | No info | No info | No info | No info | Low | Low | No info | |
| Serious | Serious | Moderate | No info | Low | Low | Low | Serious | |
| Moderate | No info | No info | No info | No info | Low | Low | No info | |
| Serious | Serious | Moderate | No info | Serious | Low | Low | Serious | |
| Serious | Moderate | Serious | No info | Serious | Low | Low | Serious | |
| Serious | Low | Serious | No info | Critical | Low | Low | Critical | |
| Serious | Serious | Serious | No info | Critical | Low | Low | Critical | |
| Critical | Critical | Serious | No info | Serious | Low | Low | Critical | |
| Serious | Serious | Moderate | No info | Low | Low | Low | Serious | |
| Moderate | Moderate | Low | No info | Low | Low | Low | Moderate | |
| Serious | Serious | Serious | No info | Moderate | Low | Low | Serious | |
| Moderate | Moderate | Low | No info | Moderate | Low | Low | Moderate | |
| Serious | Serious | Low | No info | Serious | Low | Low | Serious | |
| Critical | Serious | Serious | No info | Critical | Low | Low | Critical | |
| Serious | Serious | Moderate | No info | Serious | Low | Low | Serious | |
| Moderate | Serious | Moderate | No info | No info | Low | Low | Serious |
| Variable | Studies | Univariable rate ratio (95% CI) | Ratio of rate ratios (95% CI) | P value | Tau2 |
| Geographic region | |||||
| Europe | 8 | 0.51 (0.37‐0.70) | 1.0 (ref) | — | — |
| Australia | 5 | 0.55 (0.28‐1.11) | 1.12 (0.52‐2.41) | — | — |
| North America | 6 | 0.69 (0.44‐1.08) | 1.42 (0.73‐2.78) | 0.53 | 0.10 |
| Site of recruitment | |||||
| Service attenders | 12 | 0.67 (0.49‐0.92) | 1.0 (ref) | — | — |
| Community | 7 | 0.49 (0.33‐0.73) | 0.73 (0.42‐1.27) | 0.256 | 0.06 |
| Study design | |||||
| Cross‐sectional | 4 | 0.51 (0.31‐0.85) | 1.0 | — | — |
| Prospective cohort | 15 | 0.58 (0.43‐0.77) | 1.12 (0.48‐2.61) | 0.784 | 0.10 |
| Females | 17 | — | 1.59 (1.13‐2.29) | 0.01 | 0.04 |
| Prison experience | 11 | — | 1.057 (0.61‐1.79) | 0.821 | 0.43 |
| Experience of homelessness | 12 | — | 1.08 (0.83‐1.40) | 0.521 | 0.23 |
| Injection of stimulants | 12 | — | 0.89 (0.65‐1.22) | 0.373 | 0.17 |
| Daily injection | 7 | — | 0.88 (0.64‐1.22) | 0.373 | 0.17 |
| CI: confidence interval; HCV: hepatitis C virus; OST: opioid substitution therapy. | |||||
| Variable | Studies | Univariable rate ratio (95%CI) | Ratio of rate ratios (95%CI) | P value | Tau2 |
| Geographic region | |||||
| Europe | 5 | 0.44 (0.24‐0.80) | 1.0 (Ref) | — | — |
| North America | 3 | 1.58 (0.57‐4.42) | 3.73 (0.95‐14.7) | 0.057 | 0.41 |
| Recruitment site | |||||
| Service attenders | 3 | 0.67 (0.28‐1.59) | 1.0 (Ref) | — | — |
| Community | 5 | 0.82 (0.29‐2.32) | 0.76(0.12‐4.88) | 0.74 | 0.89 |
| Study design | |||||
| Cross‐sectional survey | 3 | 0.34 (0.16‐0.75) | 1.0 (Ref) | — | — |
| Prospective cohort | 4 | 1.26 (0.55‐2.93) | 3.53 (0.78‐15.86) | 0.087 | 0.48 |
| Females | 7 | — | 2.97(0.38‐23.1) | 0.24 | 0.87 |
| Prison experience | 3 | — | NA | — | — |
| Experience of homelessness | 6 | — | 1.01 (0.38‐2.67) | 0.976 | 1.53 |
| Injection of stimulants | 7 | — | 1.08 (0.47‐2.51) | 0.827 | 1.15 |
| Daily injection | 5 | — | 3.66 (0.22‐61.3) | 0.239 | 1.15 |
| CI: confidence interval; HCV: hepatitis C virus; NSP: needle syringe programmes. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses by region Show forest plot | 12 | 6361 | Risk Ratio (Random, 95% CI) | 0.50 [0.40, 0.63] |
| 1.1 North America | 5 | 2245 | Risk Ratio (Random, 95% CI) | 0.57 [0.42, 0.76] |
| 1.2 Europe | 5 | 3494 | Risk Ratio (Random, 95% CI) | 0.43 [0.27, 0.68] |
| 1.3 Australia | 2 | 622 | Risk Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 2 HCV incidence adjusted analysis by study design Show forest plot | 12 | 6361 | Risk Ratio (Random, 95% CI) | 0.50 [0.40, 0.63] |
| 2.1 Prospective cohort | 10 | 3467 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.65] |
| 2.2 Cross‐sectional surveys | 2 | 2894 | Risk Ratio (Random, 95% CI) | 0.46 [0.23, 0.89] |
| 3 HCV incidence unadjusted analyses by different modes of OST provision Show forest plot | 9 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Ever used OST | 3 | 375 | Risk Ratio (Random, 95% CI) | 0.81 [0.52, 1.27] |
| 3.2 Interrupted OST use | 3 | 1157 | Risk Ratio (Random, 95% CI) | 0.80 [0.57, 1.10] |
| 3.3 Detoxification | 1 | 552 | Risk Ratio (Random, 95% CI) | 1.45 [0.79, 2.66] |
| 3.4 High dose | 2 | 453 | Risk Ratio (Random, 95% CI) | 0.52 [0.29, 0.94] |
| 3.5 Low dose | 2 | 453 | Risk Ratio (Random, 95% CI) | 0.85 [0.44, 1.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 8 | 5235 | Risk Ratio (Random, 95% CI) | 0.42 [0.31, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 9 | 5782 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 10 | 3467 | Risk Ratio (Random, 95% CI) | 0.51 [0.40, 0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 16 | 9499 | Risk Ratio (Random, 95% CI) | 0.57 [0.45, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses by region Show forest plot | 5 | 3530 | Risk Ratio (Random, 95% CI) | 0.79 [0.39, 1.61] |
| 1.1 North America | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.25 [0.63, 2.46] |
| 1.2 Europe | 2 | 2903 | Risk Ratio (Random, 95% CI) | 0.24 [0.09, 0.62] |
| 2 HCV incidence adjusted analyses by study design Show forest plot | 5 | 3530 | Risk Ratio (Random, 95% CI) | 0.95 [0.50, 1.82] |
| 2.1 Prospective cohorts | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.44 [1.01, 2.05] |
| 2.2 Cross‐sectional surveys | 2 | 2903 | Risk Ratio (Random, 95% CI) | 0.24 [0.09, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 4 | 3245 | Risk Ratio (Random, 95% CI) | 0.77 [0.28, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 3 | 627 | Risk Ratio (Random, 95% CI) | 1.25 [0.63, 2.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 7 | 6455 | Risk Ratio (Random, 95% CI) | 0.78 [0.39, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence, adjusted analyses Show forest plot | 6 | 2765 | Risk Ratio (Random, 95% CI) | 1.43 [0.82, 2.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence Show forest plot | 9 | 3242 | Risk Ratio (Random, 95% CI) | 1.41 [0.95, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HCV incidence adjusted analyses Show forest plot | 3 | 6197 | Risk Ratio (Random, 95% CI) | 0.45 [0.22, 0.94] |
| 1.1 High NSP coverage | 3 | 3241 | Risk Ratio (Random, 95% CI) | 0.26 [0.07, 0.89] |
| 1.2 Low NSP coverage | 2 | 2956 | Risk Ratio (Random, 95% CI) | 0.87 [0.44, 1.68] |
| 2 HCV incidence unadjusted analyses Show forest plot | 4 | 6427 | Risk Ratio (Random, 95% CI) | 0.47 [0.27, 0.80] |
| 2.1 Combined OST and high NSP versus no OST and low/no NSP | 4 | 3356 | Risk Ratio (Random, 95% CI) | 0.29 [0.13, 0.65] |
| 2.2 Combined OST and low NSP versus no OST and low/no NSP | 3 | 3071 | Risk Ratio (Random, 95% CI) | 0.76 [0.44, 1.33] |

