| Grommets versus active monitoring for recurrent acute otitis media in children |
| Patients: children with recurrent acute otitis media
Setting: secondary and tertiary care
Intervention: grommets
Control: active monitoring |
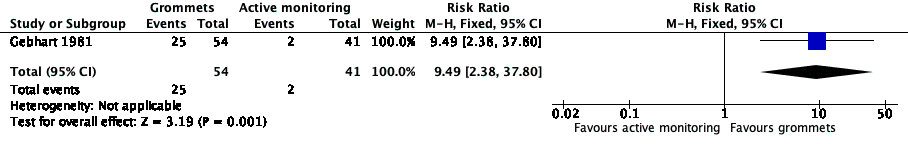
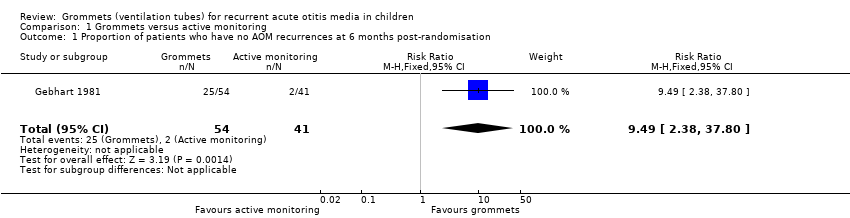
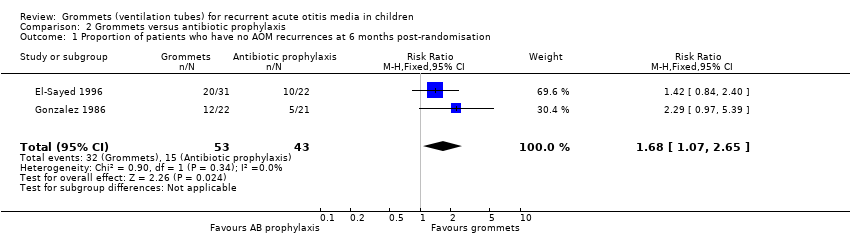
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 9.49
(2.38 to 37.80) | 95
(1 RCT) | ⊕⊕⊝⊝
low1 | The NNTB based on the study population risk was 1/ (463‐49)* 1000 = 2.41 |
| 49 per 1000 | 463 per 1000
(116 to 1000) |
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 0 (0/54) | n/a | 54 (1 RCT) | ⊕⊕⊝⊝
low1 | — |
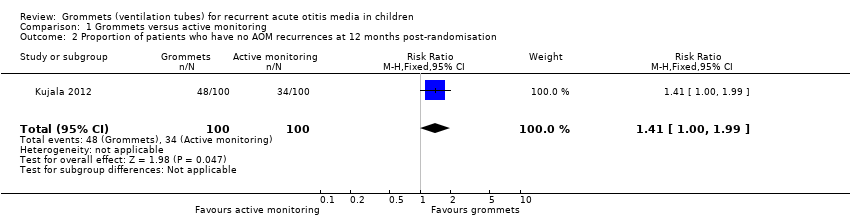
| Proportion of patients who have no AOM recurrences at 12 months post‐randomisation | Study population | RR 1.41
(1.00 to 1.99) | 200
(1 RCT) | ⊕⊕⊝⊝
low1 | The NNTB based on the study population risk was 1/ (479‐340)* 1000 = 7.19 |
| 340 per 1000 | 479 per 1000
(340 to 677) |
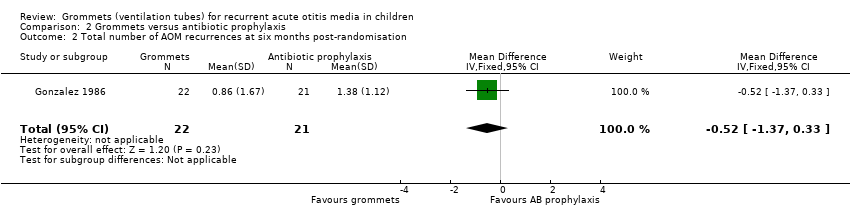
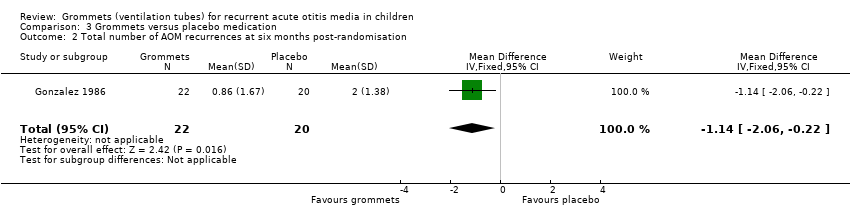
| Total number of AOM recurrences at 6 months post‐randomisation | 89 AOM recurrences in 41 children; mean number of AOM recurrences per child: 2.17 | 36 AOM recurrences in 54 children; mean number of AOM recurrences per child: 0.67 | MD ‐1.50, 95% CI ‐1.99 to ‐1.01 | 95 (1 RCT) | ⊕⊕⊝⊝
low1 | — |
| Total number of AOM recurrences at 12 months post‐randomisation | 119 AOM recurrences in 100 children; incidence rate 1.70 | 92 AOM recurrences in 100 children; incidence rate 1.15 | Incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93 | 200
(1 RCT) | ⊕⊕⊝⊝
low1 | — |
| Disease‐specific health‐related quality of life of the child at 4 and 12 months post‐randomisation using the OM‐6 questionnaire | "no statistically significant differences between treatment groups were reported at 4 and 12 months for any of the six subdomains of the OM‐6 questionnaire" | 85 and 81, respectively (1 RCT) | ⊕⊕⊝⊝
low1 | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
AOM: acute otitis media; CI: confidence interval;MD: mean difference; n/a: not applicable; NNTB: number needed to treat to benefit; OM‐6: Otitis Media‐6; RCT: randomised controlled trial; RR: risk ratio |
| GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |