Drenajes timpánicos (tubos de ventilación) para la otitis media aguda recurrente en niños
Appendices
Appendix 1. CENTRAL search strategy
| CENTRAL (Cochrane Register of Studies) | MEDLINE (Ovid) | Embase (Ovid) | CINAHL (EBSCO) |
| 1 MESH DESCRIPTOR Otitis AND CENTRAL:TARGET 2 (otitis or inflamm* or infect* or disease):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 3 #1 OR #2 4 MESH DESCRIPTOR Ear, Middle AND CENTRAL:TARGET 5 (middle near ear):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 6 #5 OR #4 7 #3 AND #6 8 MESH DESCRIPTOR Otitis Media AND CENTRAL:TARGET 9 MESH DESCRIPTOR Otitis Media with Effusion EXPLODE ALL AND CENTRAL:TARGET 10 (((otitis near media) or OME)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 11 ((middle near ear near effus*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 12 #7 OR #8 OR #9 OR #10 OR #11 13 MESH DESCRIPTOR Acute Disease EXPLODE ALL AND CENTRAL:TARGET 14 (acute or suppurat* or serous or secretory or secretion* or purulent):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 15 #13 OR #14 16 #12 AND #15 17 MESH DESCRIPTOR Otitis Media, Suppurative EXPLODE ALL AND CENTRAL:TARGET 18 (AOM or TYMPANITIS):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 19 #16 OR #17 OR #18 20 MESH DESCRIPTOR Recurrence EXPLODE ALL AND CENTRAL:TARGET 21 MESH DESCRIPTOR Chronic Disease EXPLODE ALL AND CENTRAL:TARGET 22 MESH DESCRIPTOR Secondary Prevention EXPLODE ALL AND CENTRAL:TARGET 23 (recurrence* or recurrent or chronic or persistent or persistence or prone):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 24 #20 OR #21 OR #22 OR #23 25 #19 AND #24 26 MESH DESCRIPTOR Mastoiditis EXPLODE ALL AND CENTRAL:TARGET 27 (raom or mastoiditis):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 28 #25 OR #26 OR #27 29 MESH DESCRIPTOR Middle Ear Ventilation EXPLODE ALL AND CENTRAL:TARGET 30 ((middle near ear near (tube* or ventilat* or tubulation))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 31 grommet*:AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 32 (((tympanostomy or myringotomy or tympanic) near (tube* or tubulation or ventilat*))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 33 #29 OR #30 OR #31 OR #32 34 #28 AND #33 | 1 Otitis/ 2 (otitis or inflamm* or infect* or disease*).ab,ti. 3 1 or 2 4 exp Ear, Middle/ 5 (middle adj3 ear).ab,ti. 6 4 or 5 7 3 and 6 8 otitis media/ or otitis media with effusion/ 9 (middle adj3 ear adj3 effus*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 10 ((otitis adj3 media) or OME).ab,ti. 11 7 or 8 or 9 or 10 12 exp Acute Disease/ 13 (acute or suppurat* or serous or secretory or secretion* or purulent).ab,ti. 14 12 or 13 15 11 and 14 16 exp Otitis Media, Suppurative/ 17 (AOM or TYMPANITIS).ab,ti. 18 15 or 16 or 17 19 exp Recurrence/ 20 exp Chronic Disease/ 21 exp Secondary Prevention/ 22 (recurrence* or recurrent or chronic or persistent or persistence or prone).ab,ti. 23 19 or 20 or 21 or 22 24 18 and 23 25 Mastoiditis/ 26 (raom or mastoiditis).ab,ti. 27 24 or 25 or 26 28 exp Middle Ear Ventilation/ 29 (middle adj3 ear adj6 (tube* or ventilat* or tubulation)).ab,ti. 30 ((tympanostomy or myringotomy or tympanic) adj6 (tube* or tubulation or ventilat*)).ab,ti. 31 "grommet*".ab,ti. 32 28 or 29 or 30 or 31 33 27 and 32 34 randomized controlled trial.pt. 35 controlled clinical trial.pt. 36 randomized.ab. 37 placebo.ab. 38 drug therapy.fs. 39 randomly.ab. 40 trial.ab. 41 groups.ab. 42 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 43 exp animals/ not humans.sh. 44 42 not 43 45 33 and 44 | 1 otitis/ 2 (otitis or inflamm* or infect* or disease).ti,ab. 3 1 or 2 4 exp middle ear/ 5 (middle adj3 ear).ti,ab. 6 4 or 5 7 3 and 6 8 otitis media/ 9 secretory otitis media/ 10 ((otitis adj3 media) or OME).ti,ab. 11 (middle adj3 ear adj3 effus*).ti,ab. 12 7 or 8 or 9 or 10 or 11 13 exp acute disease/ 14 (acute or suppurat* or serous or secretory or secretion* or purulent).ti,ab. 15 13 or 14 16 12 and 15 17 exp suppurative otitis media/ 18 (AOM or TYMPANITIS).ti,ab. 19 16 or 17 or 18 20 exp recurrent disease/ 21 exp chronic disease/ 22 exp secondary prevention/ 23 (recurrence* or recurrent or chronic or persistent or persistence or prone).ti,ab. 24 20 or 21 or 22 or 23 25 19 and 24 26 mastoiditis/ 27 (raom or mastoiditis).ti,ab. 28 25 or 26 or 27 29 exp middle ear ventilation/ 30 (middle adj3 ear adj6 (tube* or ventilat* or tubulation)).ti,ab. 31 ((tympanostomy or myringotomy or tympanic) adj6 (tube* or tubulation or ventilat*)).ti,ab. 32 "grommet*".ti,ab. 33 29 or 30 or 31 or 32 34 28 and 33 35 (random* or factorial* or placebo* or assign* or allocat* or crossover*).tw. 36 (control* adj group*).tw. 37 (trial* and (control* or comparative)).tw. 38 ((blind* or mask*) and (single or double or triple or treble)).tw. 39 (treatment adj arm*).tw. 40 (control* adj group*).tw. 41 (phase adj (III or three)).tw. 42 (versus or vs).tw. 43 rct.tw. 44 crossover procedure/ 45 double blind procedure/ 46 single blind procedure/ 47 randomization/ 48 placebo/ 49 exp clinical trial/ 50 parallel design/ 51 Latin square design/ 52 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53 exp ANIMAL/ or exp NONHUMAN/ or exp ANIMAL EXPERIMENT/ or exp ANIMAL MODEL/ 54 exp human/ 55 53 not 54 56 52 not 55 57 34 and 56 | S31 S25 AND S30 S30 S26 OR S27 OR S28 OR S29 S29 TX grommet* S28 TX ((tympanostomy or myringotomy or tympanic) n6 (tube* or tubulation or ventilat*)) S27 TX (middle n3 ear n6 (tube* or ventilat* or tubulation)) S26 (MH "Middle Ear Ventilation") S25 S22 OR S23 OR S24 S24 TX (raom or mastoiditis S23 (MH "Mastoiditis") S22 S17 AND S21 S21 S18 OR S19 OR S20 S20 TX recurrence* or recurrent or chronic or persistent or persistence or prone S19 (MH "Chronic Disease") S18 (MH "Recurrence") S17 S15 OR S16 S16 TX (AOM or TYMPANITIS) S15 S11 AND S14 S14 S12 OR S13 S13 TX (acute or suppurat* or serous or secretory or secretion* or purulent) S12 (MH "Acute Disease") S11 S7 OR S8 OR S9 OR S10 S10 TX ((otitis n3 media) or OME) S9 TX middle n3 ear n3 effus* S8 (MH "Otitis Media with Effusion") OR (MH "Otitis Media") S7 S3 AND S6 S6 S4 OR S5 S5 TX middle n3 ear S4 (MH "Ear, Middle") S3 S1 OR S2 S2 TX otitis or inflamm* or infect* or disease S1 (MH "Otitis") |
| Cochrane ENT Register | LILACS | ClinicalTrials.gov | ICTRP |
| 1 otitis or inflamm* or infect* or disease 2 middle near ear 3 #1 AND #2 4 (otitis near media) or OME 5 middle near ear near effus* 6 #3 or #4 or #5 7 acute or suppurat* or serous or secretory or secretion* or purulent 8 #6 AND #7 9 AOM or TYMPANITIS 10 #8 or #9 11 recurrence* or recurrent or chronic or persistent or persistence or prone 12 #10 and #11 13 raom or mastoiditis 14 #12 or #13 15 middle near ear near (tube* or ventilat* or tubulation) 16 grommet* 17 (tympanostomy or myringotomy or tympanic) near (tube* or tubulation or ventilat*) 18 #15 or #16 or #17 19 #14 and #18 | ((TW:"middle ear" OR TW:"Oído Medio" OR TW:"Orelha Média" OR TW:tympanostomy OR TW:myringotomy OR TW:tympanic) AND (TW:Ventila$ OR TW:tube$ OR TW:tubulation)) OR TW:grommet$ AND Controlled Clinical Trial | Via the Cochrane Register of Studies 1 grommet OR grommets OR "tympanostomy tube" OR "tympanostomy tubes" OR "myringotomy tube" OR "myringotomy tubes" OR "middle ear tubulation" OR "tympanic membrane ventilation" OR (middle AND ear AND ventilation) AND INSEGMENT 2 (nct*):AU AND INSEGMENT 3 #1 AND #2 Via ClinicalTrials.gov grommet OR grommets OR "tympanostomy tube" OR "tympanostomy tubes" OR "myringotomy tube" OR "myringotomy tubes" OR "middle ear tubulation" OR "tympanic membrane ventilation" OR (middle AND ear AND ventilation) Study type: Interventional | rAOM OR recurren* AND AOM OR recurren* AND otitis OR recurren* AND tympanitis OR otitis AND prone |
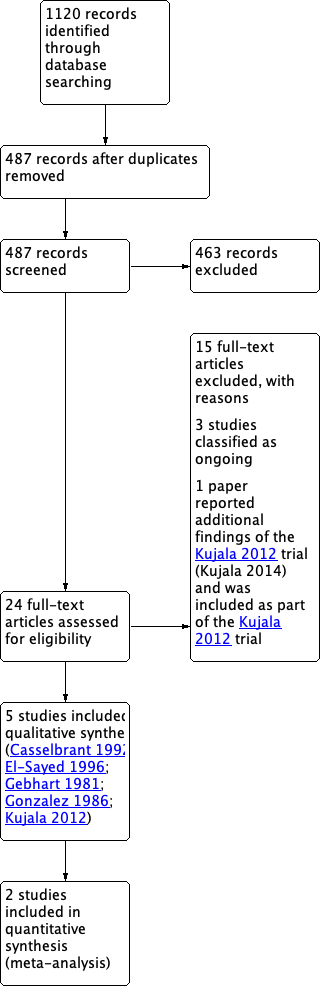
PRISMA flow diagram of search history.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
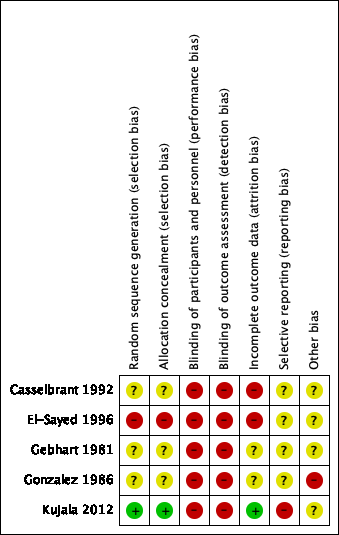
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
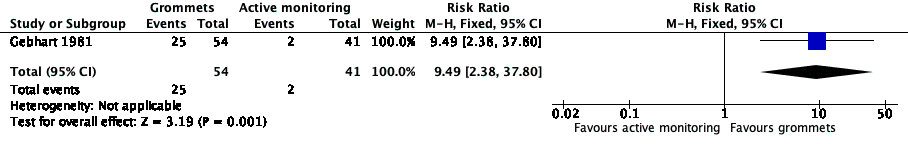
Forest plot of comparison: 1 Grommets versus active monitoring, outcome: 1.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Forest plot of comparison: 2 Grommets versus antibiotic prophylaxis, outcome: 2.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Forest plot of comparison: 3 Grommets versus placebo medication, outcome: 3.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
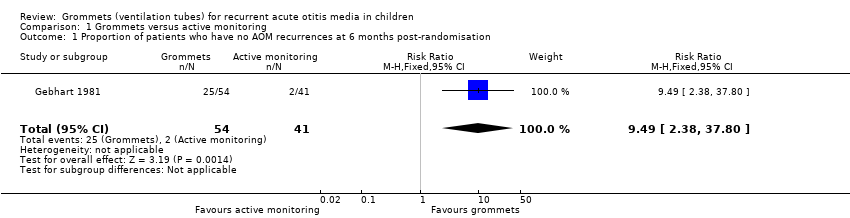
Comparison 1 Grommets versus active monitoring, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
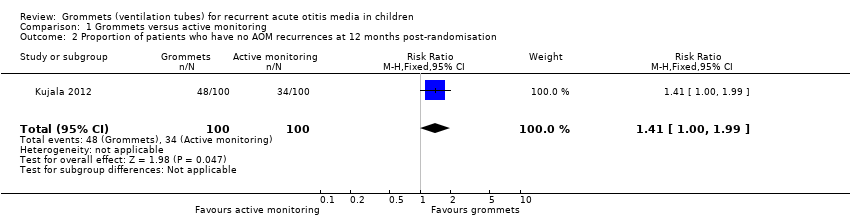
Comparison 1 Grommets versus active monitoring, Outcome 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation.

Comparison 1 Grommets versus active monitoring, Outcome 3 Total number of AOM recurrences at six months post‐randomisation.
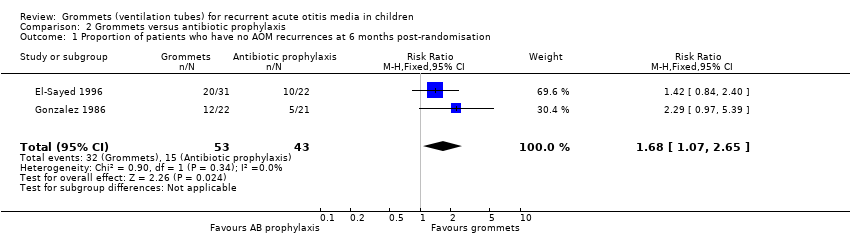
Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
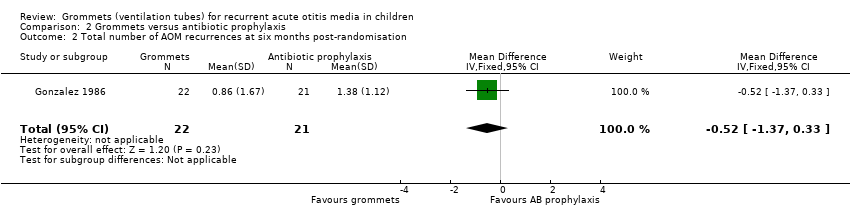
Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.
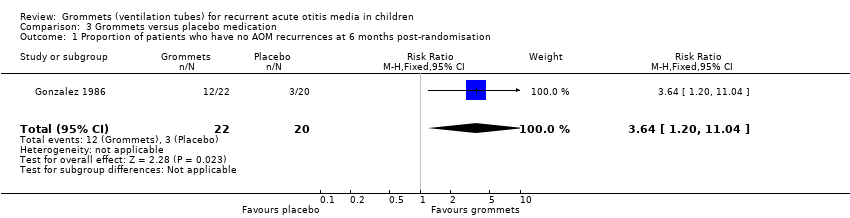
Comparison 3 Grommets versus placebo medication, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
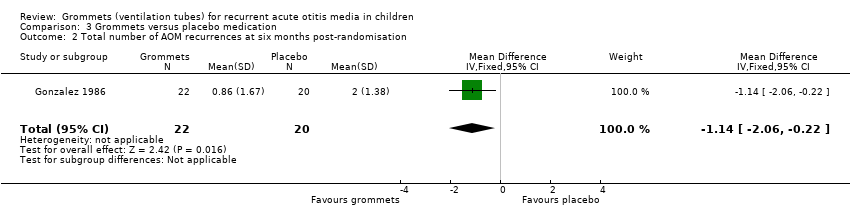
Comparison 3 Grommets versus placebo medication, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.
| Grommets versus active monitoring for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active monitoring | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 9.49 | 95 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (463‐49)* 1000 = 2.41 | |
| 49 per 1000 | 463 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 0 (0/54) | n/a | 54 (1 RCT) | ⊕⊕⊝⊝ | — |
| Proportion of patients who have no AOM recurrences at 12 months post‐randomisation | Study population | RR 1.41 | 200 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (479‐340)* 1000 = 7.19 | |
| 340 per 1000 | 479 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 89 AOM recurrences in 41 children; mean number of AOM recurrences per child: 2.17 | 36 AOM recurrences in 54 children; mean number of AOM recurrences per child: 0.67 | MD ‐1.50, 95% CI ‐1.99 to ‐1.01 | 95 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 12 months post‐randomisation | 119 AOM recurrences in 100 children; incidence rate 1.70 | 92 AOM recurrences in 100 children; incidence rate 1.15 | Incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93 | 200 | ⊕⊕⊝⊝ | — |
| Disease‐specific health‐related quality of life of the child at 4 and 12 months post‐randomisation using the OM‐6 questionnaire | "no statistically significant differences between treatment groups were reported at 4 and 12 months for any of the six subdomains of the OM‐6 questionnaire" | 85 and 81, respectively (1 RCT) | ⊕⊕⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Grommets versus antibiotic prophylaxis for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antibiotic prophylaxis | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 1.68 | 96 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (586‐349)* 1000 = 4.22 | |
| 349 per 1000 | 586 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 29 AOM recurrences in 21 children; mean number of AOM recurrences per child: 1.38 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐0.52, 95% CI ‐1.37 to 0.33 | 43 (1 RCT) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations (when we excluded the trial with high risk of bias from the analysis, no statistically significant difference was observed between groups) and imprecise effect estimates (only two studies with small sample sizes). 2We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). | ||||||
| Grommets versus placebo medication for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo medication | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 3.64 | 42 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (546‐150)* 1000 = 2.53 | |
| 150 per 1000 | 546 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 4% (3/76) | n/a | 76 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 6 months post‐randomisation | 40 AOM recurrences in 20 children; mean number of AOM recurrences per child: 2.0 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐1.14, 95% CI ‐2.06 to ‐0.22 | 42 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). 2We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Study ID | Grommets | Grommets plus adenoidectomy | Active monitoring | Placebo medication | Antibiotic prophylaxis | Adenoidectomy |
| x | x | x | ||||
| x | x | |||||
| x | x | |||||
| x | x | x | ||||
| x | x | x | ||||
| Comparison pairs for this review | ||||||
| # | Intervention | Comparator | Number of trials | Study ID | ||
| 1 | Grommets | Active monitoring | 2 | |||
| 2 | Grommets | Antibiotic prophylaxis | 3 | |||
| 3 | Grommets | Placebo medication | 2 | |||
| Outcomes | |||||
| Primary outcomes | |||||
| Proportion of children who have no AOM recurrences at 3 to 6 months post‐randomisation | x | x | x | ||
| Significant adverse effect: tympanic membrane perforation persisting for 3 months or longer | x | x | |||
| Secondary outcomes | |||||
| Proportion of children who have no AOM recurrences at 6 to 12 months post‐randomisation | x | ||||
| Total number of AOM recurrences | |||||
| < 3 months | |||||
| 3 to 6 months | x | x | |||
| 6 to 12 months | x | ||||
| Disease‐specific health‐related quality of life | |||||
| < 3 months | |||||
| 3 to 6 months | x | ||||
| 6 to 12 months | x | ||||
| Generic health‐related quality of life of the child and parent | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Presence of middle ear effusion | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Other adverse effects: ventilation tube misplaced in middle ear, otorrhoea within 1 week of ventilation tube placement, myringosclerosis | x | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [2.38, 37.80] |
| 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.00, 1.99] |
| 3 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.99, ‐1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.07, 2.65] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.37, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.20, 11.04] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐2.06, ‐0.22] |

