鼓膜置管(通气管)治疗儿童复发性急性中耳炎
摘要
研究背景
急性中耳炎(Acute otitis media,AOM)是儿童最常见的疾病之一。虽然许多儿童的个别会经历急性中耳炎的发作,但有一个重要的群体患有复发性急性中耳炎 (recurrent Acute otitis media,rAOM),其定义为六个月内发作三次或三次以上,或一年内发作四次或四次以上。在这部分儿童中,急性中耳炎给他们造成了真正的负担,因为频繁出现的耳痛、全身不适、夜不能寐以及失去在托儿所或学校的时间。鼓膜置管,也被称为通气管或鼓膜通气管,可以用来治疗rAOM。
研究目的
评估在患有rAOM的儿童中,在同时进行/不进行腺样体切除术的情况下,双侧插入鼓膜置管的受益和风险。
检索策略
Cochrane耳鼻喉科文献检索信息专员检索了Cochrane耳鼻喉疾病试验注册库(Cochrane ENT Trials Register);Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL);MEDLINE;EMBASE;CINAHL;Web of Science;ClinicalTrials.gov;ICTRP和其他数据库寻找发表及未发表的试验。检索日期为2017年12月4日。
纳入排除标准
随机对照试验(RCTs)比较了16岁以下rAOM儿童双耳接受鼓膜置管术同时进行/不进行腺样体切除术和不进行耳部手术。我们拟定了两个主要方案:将鼓膜置管作为单一手术干预和鼓膜置管与腺样体切除术同时进行治疗(即干预组和对照组的儿童都进行了腺样体切除术)。比较者包括主动监测、抗生素预防和安慰剂药物治疗。
资料收集与分析
我们使用了Cochrane推荐的标准方法程序。主要结局指标是:随访3至6个月(中期)没有AOM复发的儿童比例和持续鼓膜穿孔(重要不良事件)。次要结局指标是:随访6至12个月(长期)AOM没有复发的儿童比例;短期、中期和长期随访中AOM复发的总数、特定疾病性和一般健康相关的生活质量、中耳积液和其他不良事件的发生。我们使用GRADE评估每个结局的证据质量;见斜体字部分。
主要结果
纳入了5项偏倚风险不明确或高偏移风险的RCTs(805名儿童)。所有研究都是在各国的国家免疫项目中肺炎球菌疫苗接种之前进行的。在所有试验中,没有一项试验是两组同时进行腺样体切除术的。
鼓膜置管对比主动监测
在以下方面,鼓膜置管比主观监测更有效。
6个月没有AOM复发的儿童比例(1项研究,95名儿童,46%比5%:风险比(RR)=9.49,95%置信区间(CI)[2.38, 37.80],获益需治疗人数(NNTB)是3;低质量证据);
12个月没有AOM复发的儿童比例(1项研究,200名儿童,48%对比34%;RR=1.41,95% CI [1.00, 1.99],每增加1例获益的病人所需治疗的病人数(NNTB)= 8;低质量证据);
6个月AOM复发数(1项研究,95名儿童,每名儿童AOM复发的均数:0.67比2.17,均差(MD)=‐1.50,95%CI [‐1.99, ‐1.01]; 低质量证据 );
12个月AOM复发数(1项研究,200名儿童,一年AOM发生率:1.15比1.70,发病率差异是‐0.55,95% CI [‐0.17, ‐0.93];低质量证据 )。
在4个月(1项研究,85名儿童)或12个月(1项研究,81名儿童)中,接受鼓膜置管的儿童并没有获得更好的疾病特异性健康相关的生活质量(中耳炎‐6调查问卷),与接受主动监测管理的儿童相比(低质量证据)。
一项研究报告了54名接受鼓膜置管的儿童中没有出现持续性的鼓膜穿孔( 低质量证据 )。
鼓膜置管对比抗生素预防
根据以下方面,鼓膜置管是否比抗生素预防更有效,尚不确定。
6个月没有AOM复发的儿童比例(2项研究,96名儿童,60%比35%;RR=1.68,95%CI [1.07, 2.65],I2 = 0%,固定效应模型,NNTB=5;极低质量证据)。
6个月AOM复发数(1项研究,43名儿童,每名儿童AOM复发的均数:0.86比1.38,MD=‐0.52,95%CI [‐1.37, 0.33];极低质量证据 )。
鼓膜置管对比安慰剂
在以下方面,鼓膜置管比安慰剂药物更有效。
六个月无AOM复发的儿童比例(1项研究,42名儿童,55%对比15%; RR=3.64,95%CI [1.20, 11.04],NNTB=3; 极低质量证据 );
6个月AOM复发数(1项研究,42名儿童,每名儿童AOM复发平均数:0.86比2.0,MD=‐1.14,95%CI [ ‐2.06, ‐0.22];极低质量证据 )。
一项研究报告了在接受鼓膜置管的76名儿童中,有3名(4%)出现持续性鼓膜穿孔( 低质量证据 )。
亚组分析
没有足够的数据来确定中耳积液的随机存在,鼓膜置管的类型和置管的时间是否会影响鼓膜置管的效果。
作者结论
目前关于鼓膜置管在rAOM患儿中的有效性的证据仅限于5项不明确或高偏倚风险的RCT,这些研究都是在肺炎球菌疫苗接种之前进行的。从低质量到极低质量证据表明,与接受主动监测和安慰剂治疗的儿童相比,接受鼓膜置管的儿童AOM复发的可能性较小,但这种效果是适中的,6个月大约少发作一次,12个月时效果不太明显。低至极低质量证据意味着需要谨慎解释这些数据,因为实际效果可能有很大的不同。鼓膜置管比抗生素预防是否更有效尚不确定。在接受鼓膜置管后,发生持续性鼓膜穿孔的风险很低。
肺炎球菌疫苗接种的广泛使用改变了AOM的细菌学和流行病学,这可能会对之前的试验结果产生怎样的影响尚不清楚。因此,在rAOM患儿中进行鼓膜置管术还需要新的高质量的RCT。这些试验不应仅关注AOM的复发频率,还要收集AOM发作的严重程度、抗生素的消耗以及手术和抗生素的不良反应等数据。这一点尤为重要,因为鼓膜置管可以减少AOM复发的严重程度,并允许进行局部而非口服的抗生素治疗。
PICO
简语概要
鼓膜置管治疗复发性急性中耳感染的患儿
系统综述问题
急性中耳炎反复发作的患儿,在双耳放鼓膜置管(同时手术切除腺样体或不手术)是否有益?
研究背景
急性中耳炎是儿童最常见的疾病之一。虽然大多数孩子都是偶尔发作,但也有一些孩子耳部感染会反复发作(半年内感染三次或三次以上,或一年内感染四次或以上)。这种反复的感染会造成相当大的困扰,因为孩子经常耳痛、发烧、全身不适、夜不能寐,使其失去了在托儿所或学校的时间,也让家长们因此无法去工作。鼓膜置管术,也被称为通气管或鼓膜通气管,可以作为一种治疗手段。它们是由耳鼻喉科医生在一次短暂的手术中放入耳膜的微小塑料管。
研究特征
本综述纳入截至2017年12月4日的证据。我们纳入了5项随机对照试验,共有805名患有复发性急性中耳炎的儿童。所有的研究都是在接种肺炎球菌疫苗前进行的,肺炎球菌是一种常见的引起耳部感染的细菌。在所有试验中,两组都没有进行腺样体的手术切除。
主要结果
我们主要观察随访3到6个月(中期)没有再发生急性中耳炎患儿与耳鼓膜持续穿孔患儿比例差异。我们也研究了一些其他的结局,包括没有再发作急性中耳炎的儿童比例。
鼓膜置管对比主动监测
我们找到的低质量证据表明,接受了鼓膜置管术治疗后6至12个月的随访中,耳部感染进一步发作的儿童少于接受主动监测的儿童;3名和8名儿童分别接受鼓膜置管治疗才能受益于一个。在6个月和12个月的随访中,耳部感染的数量在鼓膜置管组也较少;然而,这种差异不大,6个月时发作减少了一次,12个月时效果不明显(低至极低质量证据)。接受鼓膜置管治疗的儿童在4个月或12个月的随访中并没有获得更好的生活质量( 低质量证据 )。
鼓膜置管对比抗生素预防
我们发现极低质量证据的表明,接受鼓膜置管治疗的儿童在6个月时发生进一步耳部感染的少于接受抗生素预防(预防性抗生素)的儿童;5名儿童需要接受鼓膜置管治疗才能使1名儿童受益。然而,接受鼓膜置管治疗的儿童和接受抗生素预防的儿童在6个月时的耳部感染数量没有显著差异(极低质量证据)。
鼓膜置管对比安慰剂药物
我们发现极低质量证据表明,与接受安慰剂药物治疗的儿童相比,接受鼓膜置管治疗的儿童在6个月时进一步发生耳部感染的人数较少;有3名儿童需要接受鼓膜置管治疗才能使1名儿童受益。6个月时鼓膜置管组耳部感染的数量也较少;然而,这种差异不大,只是少了一次发作(极低质量证据)。
研究中没有系统地报告鼓膜置管的负面影响。2项研究报告了鼓膜持续穿孔的儿童数量;分别发生在0%(0/54)和4%(3/76)接受鼓膜置管治疗的儿童中( 低质量证据 )。
证据质量
由于研究的局限性(偏倚风险)和纳入的研究样本量从小到非常小(导致效果估计不精确),我们将关于复发性急性中耳炎患儿双耳放置鼓膜置管的受益和风险的证据质量评为低到极低。这意味着,对本综述的结果应谨慎解释,因为鼓膜置管组儿童中的实际效果可能与呈现的数据不同。
Authors' conclusions
Summary of findings
| Grommets versus active monitoring for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active monitoring | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 9.49 | 95 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (463‐49)* 1000 = 2.41 | |
| 49 per 1000 | 463 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 0 (0/54) | n/a | 54 (1 RCT) | ⊕⊕⊝⊝ | — |
| Proportion of patients who have no AOM recurrences at 12 months post‐randomisation | Study population | RR 1.41 | 200 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (479‐340)* 1000 = 7.19 | |
| 340 per 1000 | 479 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 89 AOM recurrences in 41 children; mean number of AOM recurrences per child: 2.17 | 36 AOM recurrences in 54 children; mean number of AOM recurrences per child: 0.67 | MD ‐1.50, 95% CI ‐1.99 to ‐1.01 | 95 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 12 months post‐randomisation | 119 AOM recurrences in 100 children; incidence rate 1.70 | 92 AOM recurrences in 100 children; incidence rate 1.15 | Incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93 | 200 | ⊕⊕⊝⊝ | — |
| Disease‐specific health‐related quality of life of the child at 4 and 12 months post‐randomisation using the OM‐6 questionnaire | "no statistically significant differences between treatment groups were reported at 4 and 12 months for any of the six subdomains of the OM‐6 questionnaire" | 85 and 81, respectively (1 RCT) | ⊕⊕⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Grommets versus antibiotic prophylaxis for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antibiotic prophylaxis | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 1.68 | 96 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (586‐349)* 1000 = 4.22 | |
| 349 per 1000 | 586 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 29 AOM recurrences in 21 children; mean number of AOM recurrences per child: 1.38 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐0.52, 95% CI ‐1.37 to 0.33 | 43 (1 RCT) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations (when we excluded the trial with high risk of bias from the analysis, no statistically significant difference was observed between groups) and imprecise effect estimates (only two studies with small sample sizes). 2We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). | ||||||
| Grommets versus placebo medication for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo medication | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 3.64 | 42 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (546‐150)* 1000 = 2.53 | |
| 150 per 1000 | 546 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 4% (3/76) | n/a | 76 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 6 months post‐randomisation | 40 AOM recurrences in 20 children; mean number of AOM recurrences per child: 2.0 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐1.14, 95% CI ‐2.06 to ‐0.22 | 42 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). 2We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
Background
Description of the condition
Acute otitis media (AOM) is one of the most common childhood illnesses; it is defined as the presence of middle ear fluid together with an acute onset of signs and symptoms of middle ear inflammation (Lieberthal 2013). Bulging of the ear drum or new onset of ear discharge not caused by acute otitis externa are the cardinal signs of AOM, while ear pain, fever, irritability, and problems feeding and sleeping are among the typical AOM symptoms (Lieberthal 2013). AOM is one of the most frequent reasons for primary care visits (Ashworth 1995), and the prime indication for antibiotic prescription in children in more economically developed countries (Finkelstein 2000; Grijalva 2009; Williamson 2006). In addition to high direct healthcare costs (Ahmed 2014; Bondy 2000), AOM causes substantial non‐healthcare costs, due to lost days from education or work for parents and the use of over‐the‐counter medications (Alsarraf 1999; Niemelä 1999).
While many children experience sporadic AOM episodes, an important group suffer from recurrent AOM (rAOM), defined as three or more episodes in six months, or four in one year with one episode in the last six months (Goycoolea 1991; Lieberthal 2013). In this subset of children AOM poses a true burden through frequent episodes of ear pain, general illness, sleepless nights and time lost from nursery or school. The impact of rAOM on quality of life is known to equal that of childhood asthma (Brouwer 2005). This causes families with rAOM to repeatedly seek medical attention to relieve the child's symptoms and prevent future episodes. Importantly, AOM is closely related to otitis media with effusion (OME, 'glue ear'); children with OME are at risk of AOM recurrences (Alho 1995), and following an episode of AOM all children have OME for some time (Tapiainen 2014). This extends the burden of rAOM to OME and hearing loss‐related developmental outcomes (Bennett 2001).
The first two years of life represent the period of greatest risk for the first as well as recurrent episodes of AOM (Schilder 2016; Teele 1989). Age‐specific incidence of AOM is highest during the second six months of life, which coincides with the lowest level of serum immunoglobulin (antibody) concentrations. Children prone to AOM may have lower age‐specific immunoglobulin levels, which may reflect a generalised poorer antibody response (Veenhoven 2004). Breastfeeding protects against AOM, whereas craniofacial malformations like cleft palate and early onset of AOM, a family history of recurrent ear disease, day care attendance, low socio‐economic status and passive smoking are associated with increased risk of AOM (Schilder 2016).
Description of the intervention
The surgical procedures under consideration in children with rAOM are insertion of grommets in both ears (also called ventilation or tympanostomy tubes), adenoidectomy, or a combination of the two.
Grommets are tiny plastic tubes that are inserted in the tympanic membrane (eardrum) by an ENT surgeon; in children this usually happens under general anaesthesia as a day‐case procedure. An operating microscope or other magnification is used to visualise the tympanic membrane where a small incision is made (myringotomy), middle ear fluid is aspirated (subject to need and surgical preference) and the grommet is placed in the incision. Grommets facilitate middle ear ventilation and provide a route for drainage of middle ear fluid; they reverse and prevent the formation of middle ear effusions by providing a surrogate to the under‐functioning Eustachian tube and so create a less favourable environment for viruses and bacteria to cause recurrent middle ear infections (Rosenfeld 2013; Schilder 2016).
Grommets may also reduce the severity of AOM recurrences, since they allow for drainage of middle ear fluid that builds up during an acute infection; as such they may prevent ear pain caused by pressure against the tympanic membrane. Finally, grommets allow for topical (local) treatment of AOM recurrences with antibiotic eardrops (van Dongen 2014), thereby avoiding the side effects of systemic antibiotics and potentially reducing the risk of antimicrobial resistance (Weber 2004).
It has been suggested that children suffering from rAOM who have unilateral or bilateral middle ear effusion at the time of evaluation for surgery may benefit more from grommets than children who have an aerated middle ear at this time (Rosenfeld 2013).
Grommets are a temporary treatment. After months or years, depending on the type of grommet, they are extruded into the external ear canal and the tympanic membrane closes. There are different types of grommets, which are made out of various materials. Some are so‐called short‐term grommets that typical stay in place for six to 18 months; others are intermediate/long‐term tubes that usually stay in place for a longer period of time.
Complications of grommet insertion include a persisting perforation of the tympanic membrane causing a conductive hearing loss and the risk of infection, misplacement of the grommet in the middle ear, otorrhoea (drainage of middle ear fluid through the tube) and myringosclerosis (calcification or scarring of the tympanic membrane) that may cause (mild) hearing loss.
Why it is important to do this review
Recommendations regarding the use of grommets in children suffering from rAOM vary within and across countries (CBO Richtlijn 2012; Lieberthal 2013; Rosenfeld 2013). Recent US guidelines on the management of AOM (Lieberthal 2013) and on the use of grommets (tympanostomy tubes; Rosenfeld 2013) recommend grommets as an optional treatment in children with rAOM. The latter suggests that grommets should not be offered to children with rAOM who have no middle ear effusion at the time of evaluation for surgery. In the UK there is guidance on the use of grommets in children with OME (NICE 2008), but national guidance for those with rAOM is lacking.
The role of adenoidectomy in reducing rAOM is not fully established but adenoidectomy as a standalone operation or as an adjunct to grommets may be most beneficial in children below two years of age (Boonacker 2014; van den Aardweg 2010). Furthermore, it has been suggested that adenoidectomy as an adjunct to primary grommet insertion might reduce the rate of further AOM episodes and the risk of re‐insertion of grommets compared to grommet insertion alone (Mikals 2014).
The absence of uniform guidance or consensus on the use of grommets in rAOM contributes to practice variation both within and across countries. For example, a pilot study using UK National Health Service (NHS) Primary Care Trust data showed that in 2012 there was a 40‐ to 60‐fold variation in the rate of grommet insertion for rAOM compared to an eight‐ to nine‐fold variation in grommets for OME (Bohm 2013, personal communication). Moreover, across Western countries the surgical rates for grommets vary from 2 per 1000 children per year in the UK to 20 per 1000 in The Netherlands (Schilder 2004).
An up‐to‐date, comprehensive systematic review is therefore urgently needed, summarising the available evidence on the effects of grommets with or without concurrent adenoidectomy in children with rAOM.
Objectives
To assess the benefits and harms of bilateral grommet insertion with or without concurrent adenoidectomy in children with rAOM.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) irrespective of the randomisation method and blinding procedure used. We excluded the second phase of cross‐over studies and trials where the patient was not the unit of randomisation, i.e. cluster‐randomised trials or trials where 'ears' (right versus left) were randomised.
Types of participants
Children up to age 16 years with rAOM, defined as three or more episodes in the previous six months, or four or more in one year (Goycoolea 1991; Lieberthal 2013).
Types of interventions
Intervention
-
Bilateral grommet insertion (of any type).
Comparisons
The overall comparator was no (ear) surgery. This included the following comparators:
-
active monitoring (grommets versus active monitoring);
-
antibiotic prophylaxis for a minimum period of three months (grommets versus antibiotic prophylaxis);
-
placebo medication (grommets versus placebo medication).
We anticipated that both in the intervention and comparator groups AOM recurrences would be managed with analgesics and antibiotics (topical or systemic) either routinely or in selected cases.
We planned to apply two main scenarios depending on whether adenoidectomy was performed concurrently:
-
grommets as a single surgical intervention: this included studies where children in comparator groups received no other surgical intervention;
-
grommets as concurrent treatment with adenoidectomy: this included studies where children in both the intervention and comparator groups underwent adenoidectomy.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
-
Treatment success, defined as the proportion of children who have no AOM recurrences at three to six months post‐randomisation (intermediate‐term follow‐up).
-
Significant adverse event: tympanic membrane perforation persisting for three months or longer. This has been listed as an important adverse event outcome because a further surgical procedure may ultimately be required to close the perforation if this persists after extrusion of the grommet.
Secondary outcomes
-
Treatment success, defined as the proportion of children who have no AOM recurrences at six to 12 months post‐randomisation (long‐term follow‐up).
In the short‐ (up to three months), intermediate‐ (three to six months) and long‐term (six to 12 months) post‐randomisation:
-
Total number of AOM recurrences.
-
Disease‐specific health‐related quality of life of the child and their parents or carers (using any validated instrument; see Brouwer 2007).
-
Generic health‐related quality of life of the child and parents (using any validated instrument).
-
Presence of middle ear effusion.
-
Other adverse events: grommet misplaced in middle ear, postoperative otorrhoea (in the first week after grommet insertion), myringosclerosis.
We discussed and included within our outcomes other adverse effects and complications recorded in RCTs but not listed above.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 4 December 2017.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies to 4 December 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies to 4 December 2017);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 4 December 2017);
-
Ovid EMBASE (1974 to 4 December 2017);
-
Ovid CAB Abstracts (1910 to 4 December 2017);
-
EBSCO CINAHL (1982 to 4 December 2017);
-
LILACS, lilacs.bvsalud.org (searched to 4 December 2017);
-
KoreaMed (searched via Google Scholar to 4 December 2017);
-
IndMed, www.indmed.nic.in (searched to 4 December 2017);
-
PakMediNet, www.pakmedinet.com (searched to 4 December 2017);
-
Web of Knowledge, Web of Science (1945 to 4 December 2017);
-
ClinicalTrials.gov (via the Cochrane Register of Studies and https://clinicaltrials.gov/ to 4 December 2017);
-
ICTRP, www.who.int/ictrp (searched to 4 December 2017).
In searches prior to December 2017, we also searched PubMed as a top‐up to Ovid MEDLINE (1946 to November 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials, and ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
Three review authors (LL, PTM and RPV) independently screened titles and abstracts obtained from the database searches and the reference lists of relevant systematic reviews to assess their potential relevance for reviewing the full text. The same review authors independently reviewed the full text of potentially relevant articles against the inclusion and exclusion criteria. We resolved any disagreements by discussion.
Data extraction and management
Three review authors (LL, PTM and RPV) independently extracted data from the included studies using standardised data extraction forms. We extracted the following data from each study:
-
Trial characteristics: setting, design, method of data analysis.
-
Participants: study population, number of children in each group, participant characteristics such as age and gender.
-
Interventions: type of surgery including pre‐operative, intra‐operative and postoperative treatment.
-
Outcomes: primary and secondary outcomes recorded, time points, adverse effects and complications related to the intervention and comparators.
-
Aspects of methodology relating to risk of bias (see below).
We also extracted the following summary statistics for each trial and each outcome:
-
For continuous data: mean values, standard deviations and number of patients for each treatment group.
-
For binary data: numbers of participants experiencing an event and number of patients assessed at the particular time point.
-
For ordinal scale data: if the data appeared to be normally distributed or if the analysis suggested that parametric tests were appropriate, we treated those outcome measures as continuous data. Alternatively, if data were available, we converted them into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies reported data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'intermediate‐term' follow‐up periods, our time point was defined as 'three to six months' post‐randomisation. If a study reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Where a study had more than one publication, we retrieved all relevant publications to ensure complete data extraction.
Assessment of risk of bias in included studies
Three review authors (AGMS, RPV and DAN) independently assessed the risk of bias of the included studies and resolved any disagreements by majority opinion. Guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we took the following items into consideration:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective reporting (reporting); and
-
other sources of bias.
We presented the results of the 'Risk of bias' assessment in a 'Risk of bias' graph and summary figure.
Measures of treatment effect
We expressed pooled measures of treatment effect for dichotomous outcomes as risk ratio (RR) with accompanying 95% confidence intervals (CI). For the key outcomes presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We aimed to calculate the number needed to treat to benefit (NNTB) using the pooled results.
We expressed continuous outcome variables either as a mean difference (MD) with 95% CIs, if reported on the same scale, or as a standardised mean difference (SMD) with 95% CIs, if different continuous scales were used.
Unit of analysis issues
This review did not use data from phase two of cross‐over studies or from studies where the patient is not the unit of randomisation, i.e. cluster‐randomised trials or studies where 'ears' (right versus left) were randomised.
Dealing with missing data
For continuous outcomes, we aimed to calculate missing statistics, such as standard deviations (SDs), from other available statistics (e.g. P values) according to the methods described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Apart from imputations for missing SDs, we did not conduct other imputations. We extracted and analysed all data using the available case analysis method.
Assessment of heterogeneity
First, we assessed the level of clinical diversity between trials by reviewing them for potential differences in the types of participants recruited, interventions used and outcomes measured. We did not pool studies where clinical heterogeneity made it unreasonable to do so. Second, we assessed statistical heterogeneity for each outcome by visually inspecting the forest plots and by using the Chi2 test, with a significance level set at P value < 0.10, and the I2 statistic, with I2 values over 50% suggesting substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
We assessed reporting bias as within‐study (outcome reporting) and between‐study reporting (publication) bias.
Outcome reporting bias
We searched the internet, ClinicalTrials.gov (http://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/trialsearch) for available study protocols to determine whether outcomes reported were pre‐defined and whether all outcomes listed in the study protocol were reported in the trial publications. Where there was insufficient information to judge the risk of bias, we classified the risk of bias as unclear (Handbook 2011).
Publication bias
We proposed a more formal method of assessing reporting bias, i.e. by creating funnel plots, if sufficient trials (10 or more) were available for an outcome.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). We analysed the available data according to the intention‐to‐treat principle, i.e. by analysing all participants in the groups to which they were originally randomised. As such, we anticipated that some children allocated to the comparator groups received surgery before the end of the trial (i.e. crossed over into the surgery group).
We calculated treatment differences with the Mantel‐Haenszel method using a fixed‐effect model where no substantial statistical heterogeneity was present (I2 < 50%). If substantial statistical heterogeneity was detected but unresolved by sensitivity analysis and pre‐specified subgroup analyses, we calculated treatment differences using a random‐effects (DerSimonian and Laird) model to provide a more conservative effect estimate. For dichotomous outcomes, we calculated the number needed to treat to benefit (NNTB) using the results of the meta‐analysis (which itself uses risk ratio) based on the average risk of the control groups in the included studies ('study population') (Handbook 2011).
Subgroup analysis and investigation of heterogeneity
We planned to subgroup studies where most participants (80% or more) met the criteria stated below to determine whether the effect of the intervention was different compared to other patients, regardless of whether we observed statistical heterogeneity. We planned to present the main analyses of this review in the form of forest plots based upon our prime subgroup analysis:
-
presence of middle ear effusion at randomisation or at the time of grommet insertion ‐ yes versus no.
For this review, effect modifiers included:
-
type of grommet (short‐term versus intermediate/long‐term length of stay);
-
age (below two years of age versus two years and older).
We therefore planned to consider these subgroup analyses in the presence of statistical heterogeneity.
Sensitivity analysis
We planned to carry out sensitivity analyses for the following factors to assess the robustness of the review findings:
-
risk of bias of included studies: we excluded from analysis studies with high risk of bias defined as high risk of allocation concealment bias and attrition bias (overall loss to follow‐up of more than 20% or differential follow‐up observed, or both).
-
surgical interventions in comparator groups during follow‐up as part of protocol: we excluded from analysis studies in which children in the comparator groups underwent surgical interventions if clinical conditions were met (e.g. paracentesis in case of AOM recurrences).
-
occurrence of AOM recurrences between the date of randomisation and surgery: we excluded from analysis studies that specifically included AOM recurrences occurring between the date of randomisation and surgery.
If any of these investigations found a difference in the effect size or heterogeneity, we reported this in the Effects of interventions section.
GRADE and 'Summary of findings'
We used the GRADE approach to rate the overall quality of evidence for each outcome. We judged the quality of evidence as high, moderate, low or very low. We judged evidence from RCTs that did not have serious limitations as high quality. However, we downgraded the quality of evidence to moderate, low or very low based on the following factors:
-
study limitations (risk of bias);
-
indirectness of evidence (directness of evidence);
-
imprecision (precision of results);
-
inconsistency (consistency of results);
-
publication bias (existence of publication bias).
We presented only the top priority outcomes in the 'Summary of findings' tables:
-
treatment success, defined as the proportion of children who have no AOM recurrences at three to six months post‐randomisation;
-
significant adverse effects: a tympanic membrane perforation persisting for three months or longer;
-
treatment success, defined as the proportion of children who have no AOM recurrences at six to 12 months post‐randomisation;
-
total number of AOM recurrences at three to six months post‐randomisation;
-
total number of AOM recurrences at six to 12 months post‐randomisation;
-
disease‐specific health‐related quality of life;
-
generic health‐related quality of life.
Results
Description of studies
Results of the search
The searches retrieved a total of 1120 records. Removing duplicates left 487 unique articles. After screening titles and abstracts
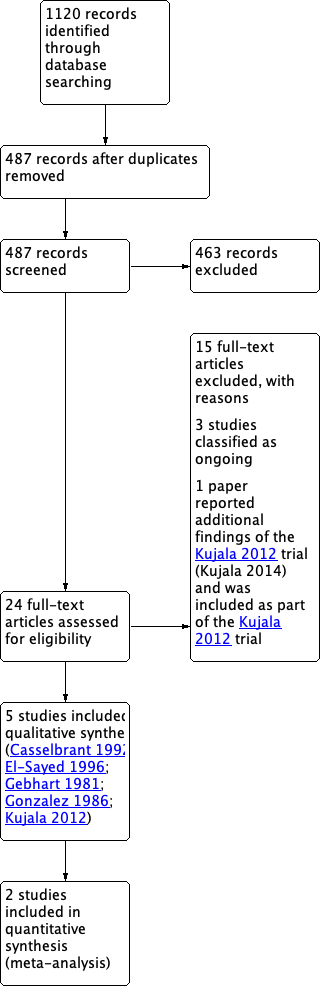
we identified 24 potentially eligible records. We excluded 15 records with reasons (see Characteristics of excluded studies), three studies were classified as ongoing (Aabel 2011; Hoberman 2015; SIUTIT Trial 2015: for details see Characteristics of ongoing studies) and one paper reported additional findings of the Kujala 2012 trial (Kujala 2014) and was therefore included as part of Kujala 2012. This left five studies eligible for inclusion (Casselbrant 1992; El‐Sayed 1996; Gebhart 1981; Gonzalez 1986; Kujala 2012). Figure 1 shows the flow chart of study retrieval and selection.

PRISMA flow diagram of search history.
Included studies
For details of the included studies see the Characteristics of included studies table.
Design
All five studies were RCTs. Two were two‐armed trials, whereas three trials had a three‐armed parallel design. Due to the nature of the intervention and comparators, all studies were open‐label (for the grommets versus no (ear) surgery comparisons).
Setting
Studies were conducted in a secondary and/or tertiary care setting in the USA (three studies), Saudi Arabia (one study) and Finland (one study).
Participants
The number of participants in the included studies ranged from 65 to 300. Participants' ages ranged from 0 to 10 years and 55% to 63% were boys. Children with middle ear effusion at baseline were excluded in two studies. The proportion of children with OME at baseline was not reported in two studies and was 29% (18/63) in one study.
Interventions
In the five included studies insertion of grommets in both ears was compared to active monitoring, antibiotic prophylaxis or placebo medication. None of the studies performed adenoidectomy as background therapy and the effectiveness of grommets as add‐on therapy to adenoidectomy could therefore not assessed in this review. Table 1 provides an overview of interventions and comparison pairs included in this review. Further details of the specific interventions can be found in the Characteristics of included studies table.
| Study ID | Grommets | Grommets plus adenoidectomy | Active monitoring | Placebo medication | Antibiotic prophylaxis | Adenoidectomy |
| x | x | x | ||||
| x | x | |||||
| x | x | |||||
| x | x | x | ||||
| x | x | x | ||||
| Comparison pairs for this review | ||||||
| # | Intervention | Comparator | Number of trials | Study ID | ||
| 1 | Grommets | Active monitoring | 2 | |||
| 2 | Grommets | Antibiotic prophylaxis | 3 | |||
| 3 | Grommets | Placebo medication | 2 | |||
Outcome measures
Table 2 summarises whether the included studies did (or did not) report on our pre‐specified outcomes. All outcomes were reported in at least one study, but adverse events were not systematically assessed in any of the studies.
| Outcomes | |||||
| Primary outcomes | |||||
| Proportion of children who have no AOM recurrences at 3 to 6 months post‐randomisation | x | x | x | ||
| Significant adverse effect: tympanic membrane perforation persisting for 3 months or longer | x | x | |||
| Secondary outcomes | |||||
| Proportion of children who have no AOM recurrences at 6 to 12 months post‐randomisation | x | ||||
| Total number of AOM recurrences | |||||
| < 3 months | |||||
| 3 to 6 months | x | x | |||
| 6 to 12 months | x | ||||
| Disease‐specific health‐related quality of life | |||||
| < 3 months | |||||
| 3 to 6 months | x | ||||
| 6 to 12 months | x | ||||
| Generic health‐related quality of life of the child and parent | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Presence of middle ear effusion | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Other adverse effects: ventilation tube misplaced in middle ear, otorrhoea within 1 week of ventilation tube placement, myringosclerosis | x | ||||
Funding and conflicts of interest
Two studies received non‐commercial (governmental) funding. One study was performed without funding, whereas no details were provided in one study. Pharmaceutical companies provided the study medications in two studies.
Excluded studies
We excluded 15 articles after reviewing the full text. Reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
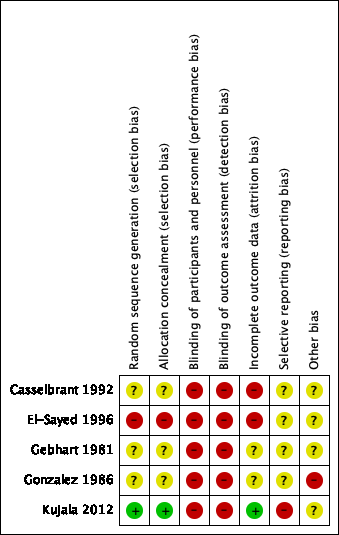
Summaries of the 'Risk of bias' assessments of the included studies are presented in Figure 2 and Figure 3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged the risk of selection bias due to sequence generation and concealment of allocation to be low in one study (20%), high in one study (20%) and unclear in three studies (60%).
Blinding
Due to the nature of the studies (comparing surgical and non‐surgical interventions), blinding of participants and personnel (performance bias) is not possible. Blinding of outcome assessment (detection bias) was not performed in the included studies. As such, we judged both the risk of performance bias and detection bias to be high in all studies.
Incomplete outcome data
We judged the risk of bias for incomplete outcome data to be low in one study (20%), high in two studies (40%) and unclear in two studies (40%).
Selective reporting
We judged the risk of outcome reporting bias to be high for Kujala 2012. We could not retrieve trial protocols for the remaining four studies (80%) and therefore we could not determine the risk of selective outcome reporting bias for these studies.
Other potential sources of bias
We judged the risk of other potential sources of bias to be unclear in four studies (80%) and high in one study (20%).
Effects of interventions
See: Summary of findings for the main comparison Grommets versus active monitoring for recurrent acute otitis media in children; Summary of findings 2 Grommets versus antibiotic prophylaxis for recurrent acute otitis media in children; Summary of findings 3 Grommets versus placebo medication for recurrent acute otitis media in children
See: summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3. We have reported all available outcome data for all comparison pairs (those not listed were not available).
1. Grommets versus active monitoring
Primary outcomes
Treatment success, defined as the proportion of children who have no acute otitis media (AOM) recurrences at six months post‐randomisation (intermediate‐term follow‐up)
For this outcome, we could use data from only one study (108 randomised children; 95 (88%) included in analysis) (Gebhart 1981). Children receiving grommets were more likely to have no AOM recurrences at six months post‐randomisation than those managed by active monitoring (46% versus 5%; risk ratio (RR) 9.49, 95% confidence interval (CI) 2.38 to 37.80, number needed to treat to benefit (NNTB) 3) (Analysis 1.1; Figure 4).
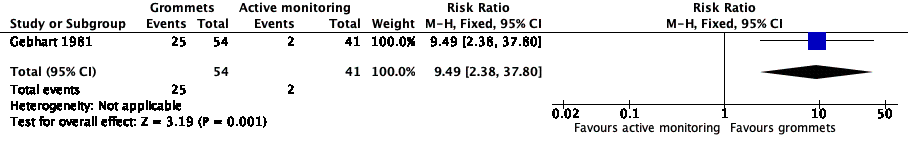
Forest plot of comparison: 1 Grommets versus active monitoring, outcome: 1.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Significant adverse event: tympanic membrane perforation persisting for three months or longer
For this outcome, we could use data from only one study (Gebhart 1981). In this study, no persistent tympanic membrane perforations were reported among 54 children receiving grommets.
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Secondary outcomes
Treatment success, defined as the proportion of children who have no AOM recurrences at 12 months post‐randomisation (long‐term follow‐up)
For this outcome, we could use data from only one study (200 randomised children; 200 (100%) included in analysis) (Kujala 2012). Children receiving grommets were more likely to have no AOM recurrences at 12 months post‐randomisation than those managed by active monitoring (48% versus 34%; RR 1.41, 95% CI 1.00 to 1.99, NNTB 8) (Analysis 1.2).
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a relatively small sample size).
Total number of AOM recurrences at six months post‐randomisation
One study reported on this outcome (108 randomised children; 95 (88%) included in analysis) (Gebhart 1981). At six months post‐randomisation, a total of 36 AOM recurrences were observed in the grommets group (54 children) and 89 in the active monitoring group (41 children); the mean number of AOM recurrences per child was 0.67 versus 2.17, respectively (MD ‐1.50, 95% CI ‐1.99 to ‐1.01) (Analysis 1.3).
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Total number of AOM recurrences at 12 months post‐randomisation
One study reported on this outcome (200 randomised children; 200 (100%) included in analysis) (Kujala 2012). At 12 months post‐randomisation, a total of 92 AOM recurrences were observed in the grommets group (100 children) and 119 in the active monitoring group (100 children). The one‐year AOM incidence rate was estimated at 1.15 versus 1.70, respectively (incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93).
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a relatively small sample size).
Disease‐specific health‐related quality of life of the child at four months post‐randomisation
One study reported on this outcome for a subset of participating children using the OM‐6 questionnaire (105 randomised children; 85 (81%) included in analysis) (Kujala 2012). At four months post‐randomisation, "no statistically significant differences" were reported between groups for any of the six sub‐domains.
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Disease‐specific health‐related quality of life of the child at 12 months post‐randomisation
One study reported on this outcome for a subset of participating children using the OM‐6 questionnaire (105 randomised children; 81 (77%) included in analysis) (Kujala 2012). At 12 months post‐randomisation, "no statistically significant differences" between groups were reported for any of the six sub‐domains.
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Other secondary outcomes
None of the studies reported on generic health‐related quality of life, presence of middle ear effusion or other adverse events.
2. Grommets versus antibiotic prophylaxis
Primary outcomes
Treatment success, defined as the proportion of children who have no AOM recurrences at six months post‐randomisation
For this outcome, we could combine data from two studies (96 children) (Gonzalez 1986; El‐Sayed 1996). Children receiving grommets were more likely to have no AOM recurrences at six months post‐randomisation than those receiving antibiotic prophylaxis (60% versus 35%; RR 1.68, 95% CI 1.07 to 2.65, I2 = 0%, fixed‐effect model, NNTB 5) (Analysis 2.1; Figure 5).

Forest plot of comparison: 2 Grommets versus antibiotic prophylaxis, outcome: 2.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
When we excluded the study with high risk of bias (El‐Sayed 1996), we observed no statistically significant difference between groups (RR 2.29, 95% CI 0.97 to 5.39). The data did not allow us to perform any of the planned subgroup analyses and remaining sensitivity analyses.
Quality of the evidence
The evidence for this outcome was of very low quality; we downgraded it from high to very low quality due to study limitations (no statistically significant difference between groups was observed after exclusion of the trial with high risk of bias) and imprecise effect estimates (only two studies with small sample sizes).
Significant adverse event: tympanic membrane perforation persisting for three months or longer
For this outcome, we could use data from only one study (Casselbrant 1992). In this study, a persistent tympanic membrane perforation was reported in 3 of 76 children (4%) who were randomised to grommet insertion.
Secondary outcomes
Total number of AOM recurrences at six months post‐randomisation
One study reported on this outcome (number of randomised children unknown; 43 included in analysis) (Gonzalez 1986). At six months post‐randomisation, a total of 19 AOM recurrences were observed in the grommets group (22 children) and 29 in the antibiotic prophylaxis group (21 children); the mean number of AOM recurrences per child was 0.86 versus 1.38, respectively (MD ‐0.52, 95% CI ‐1.37 to 0.33) (Analysis 2.2).
Quality of the evidence
The evidence for this outcome was of very low quality; we downgraded it from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size).
Other secondary outcomes
None of the studies reported on disease‐specific or generic health‐related quality of life, presence of middle ear effusion or other adverse events.
3. Grommets versus placebo medication
Primary outcomes
Treatment success, defined as the proportion of children who have no AOM recurrences at six months post‐randomisation
For this outcome, we could use data from only one study (number of randomised children unknown; 42 included in analysis) (Gonzalez 1986). Children receiving grommets were more likely to have no AOM recurrences at six months post‐randomisation than those receiving placebo medication (55% versus 15%; RR 3.64, 95% CI 1.20 to 11.04, NNTB 3) (Analysis 3.1; Figure 6).
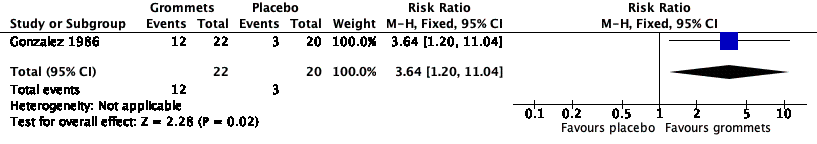
Forest plot of comparison: 3 Grommets versus placebo medication, outcome: 3.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
Quality of the evidence
The evidence for this outcome was of very low quality; we downgraded it from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size).
Significant adverse event: tympanic membrane perforation persisting for three months or longer
Only one study reported on the occurrence of persistent tympanic membrane perforation in the grommets group (Casselbrant 1992). The findings are illustrated above (in the grommets versus antibiotic prophylaxis comparison).
Quality of the evidence
The evidence for this outcome was of low quality; we downgraded it from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size).
Secondary outcomes
Total number of AOM recurrences at six months post‐randomisation
One study reported on this outcome (number of randomised children unknown; 42 included in analysis) (Gonzalez 1986). At six months post‐randomisation, a total of 19 AOM recurrences were observed in the grommets group (22 children) and 40 in the placebo medication group (20 children); the mean number of AOM recurrences per child was 0.86 versus 2.0, respectively (MD ‐1.14, 95% CI ‐2.06 to ‐0.22) (Analysis 3.2).
Quality of the evidence
The evidence for this outcome was of very low quality; we downgraded it from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size).
Other secondary outcomes
None of the studies reported on disease‐specific or generic health‐related quality of life, presence of middle ear effusion or other adverse events.
Subgroup analyses
There were insufficient data to determine whether presence of middle ear effusion at randomisation, type of grommet or age modified the effectiveness of grommets.
Discussion
Summary of main results
Current evidence on the effectiveness of bilateral grommet insertion in children with recurrent acute otitis media (rAOM) is limited to five RCTs with unclear or high risk of bias, which were conducted prior to the introduction of pneumococcal vaccination. In none of the studies was adenoidectomy performed concurrently in both groups.
Low to very low‐quality evidence suggests that children receiving grommets are less likely to have AOM recurrences at six and 12 months' follow‐up compared to those managed by active monitoring and placebo medication, but the magnitude of the effect is modest with around one fewer episode at six months and a less noticeable effect by 12 months.
Low‐quality evidence suggests that disease‐specific quality of life is similar at four and 12 months in children receiving grommets and those managed by active monitoring.
It is uncertain whether or not grommets are more effective than antibiotic prophylaxis.
The risk of persistent tympanic membrane perforation after grommet insertion is low (0/54 children in one study and 3/76 in another (low‐quality evidence)).
Overall completeness and applicability of evidence
The children participating in the five RCTs included in this review represent those most commonly encountered in clinical practice, that is children below six years of age suffering from rAOM. However, we judged the overall completeness and applicability of the evidence to be low.
All trials were conducted at a time when pneumococcal conjugate vaccination had not yet been introduced to national immunisation programmes. Since then pneumococcal vaccination has been introduced in most countries; this may have changed the pathogens causing AOM, its clinical features and the recurrence rate (Coker 2010; Fortanier 2014). How this might impact the results of prior trials is unknown.
None of the studies reported the effect of grommets on the severity of AOM recurrences or antibiotic consumption. This is particularly important since grommets may reduce the severity of AOM recurrences because they allow for drainage of middle ear fluid that builds up during an acute infection; as such they may prevent ear pain caused by pressure against the tympanic membrane. They also allow for topical (local) treatment of AOM recurrences with antibiotic eardrops (van Dongen 2014), and thereby avoid the side effects of systemic antibiotics and potentially reduce the risk of antimicrobial resistance (Weber 2004).
Finally, the included studies did not record adverse events systematically; nor did they compare effects with costs. A thorough evaluation of benefits, harms and cost‐effectiveness of grommets versus active monitoring in children with rAOM in the post‐pneumococcal conjugate vaccine era is therefore urgently needed.
Quality of the evidence
The quality of the evidence for the outcomes included in the studies comparing grommets versus active monitoring, antibiotic prophylaxis and placebo medication in children with rAOM was very low to low. Our confidence in the effect estimates is therefore (very) limited and the findings of this review should be interpreted with caution since the true effects of grommets in this group of children may be quite different than the effect estimates presented.
Potential biases in the review process
We closely adhered to the methods and analyses presented in our protocol, which was developed and published prior to the conduct of this review (Lau 2015). We used an extensive search strategy without language or publication restrictions and reviewed citation lists of all potentially relevant records; it is therefore unlikely that we have missed relevant studies. The decision, however, to downgrade the quality of evidence according to sample size, i.e. the determination of 'imprecise effect estimate', was not prespecified, but based on a post hoc subjective interpretation by the review authors.
Agreements and disagreements with other studies or reviews
Several systematic reviews of the effects of grommets in children with rAOM have been published in recent years (Cheong 2012; Damoiseaux 2011; Hellstrom 2011; Lous 2011; Steele 2017). Hellstrom concluded in 2011 that "there was insufficient evidence to support an effect of grommet insertion for rAOM" (Hellstrom 2011). Others came to a similar conclusion, i.e. that the "evidence on the effects of grommet insertion for children with rAOM is (severely) limited" (Damoiseaux 2011; Steele 2017).
Despite this limitation, Damoiseaux, Lous and Steele concluded that grommets seem "to have only a short‐term benefit" (Damoiseaux 2011), "seems to prevent one attack of AOM or keep one child out of three free from AOM in six months" (Lous 2011) and "may be associated with fewer AOM recurrences" (Steele 2017).
Two important clinical practice guidelines were launched in the USA in 2013: one published by the American Academy of Pediatrics on the management of AOM (Lieberthal 2013), and one by the American Academy of Otolaryngology ‐ Head and Neck Surgery on tympanostomy tubes (Rosenfeld 2013). Both guidelines recommend grommets as an optional treatment in children with rAOM. The latter suggests that grommets should not be offered to children with rAOM who have no middle ear effusion at the time of evaluation for surgery (Rosenfeld 2013).
In our review, we planned to present the main analyses of the review in the form of forest plots based on whether middle ear effusion was present at randomisation or at the time of surgery. The data, however, did not allow us to perform such analysis. Children with middle ear effusion at baseline were excluded in two trials and presence of OME was only 29% in one study. In this latter study, results were stratified according to the presence or absence of middle ear effusion at the initial visit (Gonzalez 1986), indicating that the effect of grommets may be larger in children with rAOM and concomitant middle ear effusion (no AOM recurrence at six months in 8/9 of the grommets group, 1/6 of the antibiotic prophylaxis group and 1/3 of the placebo medication group) than in those with no middle ear effusion (no AOM recurrence at six months in 4/12 of the grommets group, 4/15 of the antibiotic prophylaxis group and 2/17 of the placebo medication group). However, we performed this analysis post hoc and it was based upon a very small number of children.
We found that the risk of persistent tympanic membrane perforation after grommet insertion is low. This is in line with a previous meta‐analysis of tympanostomy tube sequelae, which indicated that persistent perforation occurred in 2.2% of children receiving short‐term grommets (Kay 2001).
PRISMA flow diagram of search history.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
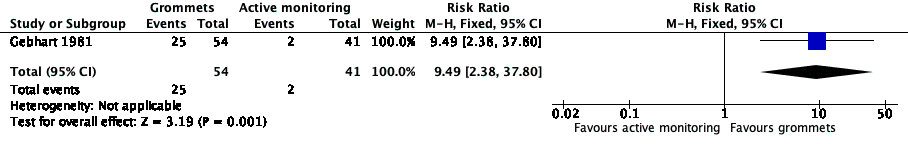
Forest plot of comparison: 1 Grommets versus active monitoring, outcome: 1.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Forest plot of comparison: 2 Grommets versus antibiotic prophylaxis, outcome: 2.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Forest plot of comparison: 3 Grommets versus placebo medication, outcome: 3.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
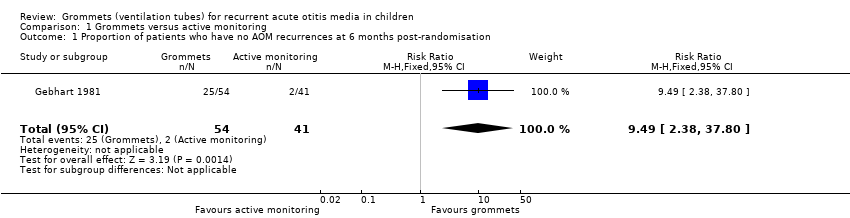
Comparison 1 Grommets versus active monitoring, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
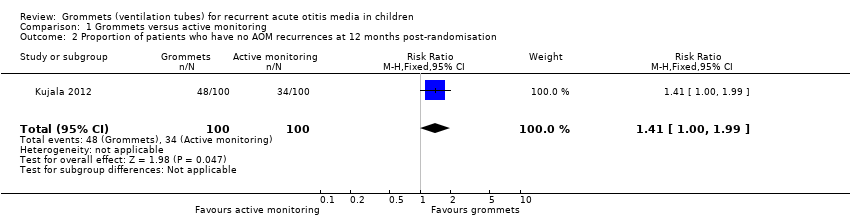
Comparison 1 Grommets versus active monitoring, Outcome 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation.

Comparison 1 Grommets versus active monitoring, Outcome 3 Total number of AOM recurrences at six months post‐randomisation.
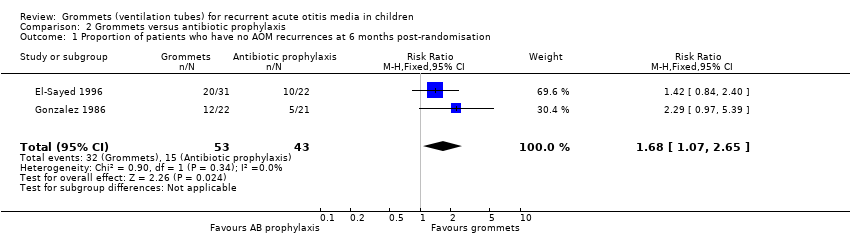
Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
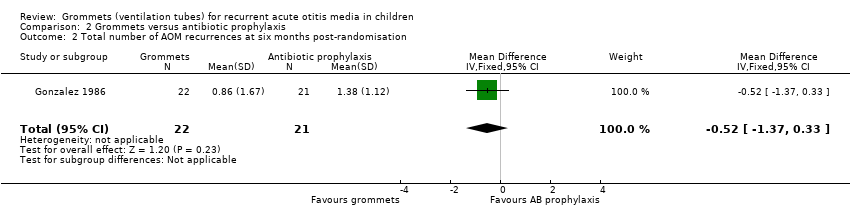
Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.
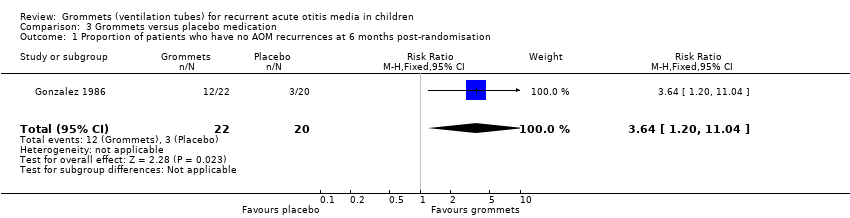
Comparison 3 Grommets versus placebo medication, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
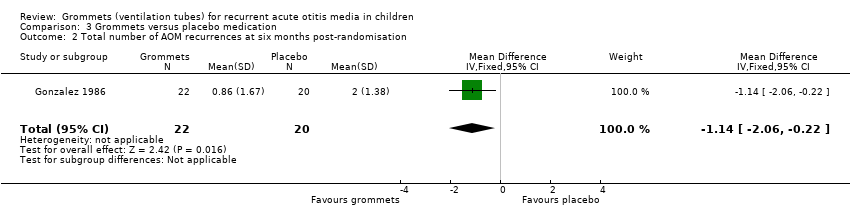
Comparison 3 Grommets versus placebo medication, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.
| Grommets versus active monitoring for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active monitoring | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 9.49 | 95 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (463‐49)* 1000 = 2.41 | |
| 49 per 1000 | 463 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 0 (0/54) | n/a | 54 (1 RCT) | ⊕⊕⊝⊝ | — |
| Proportion of patients who have no AOM recurrences at 12 months post‐randomisation | Study population | RR 1.41 | 200 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (479‐340)* 1000 = 7.19 | |
| 340 per 1000 | 479 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 89 AOM recurrences in 41 children; mean number of AOM recurrences per child: 2.17 | 36 AOM recurrences in 54 children; mean number of AOM recurrences per child: 0.67 | MD ‐1.50, 95% CI ‐1.99 to ‐1.01 | 95 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 12 months post‐randomisation | 119 AOM recurrences in 100 children; incidence rate 1.70 | 92 AOM recurrences in 100 children; incidence rate 1.15 | Incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93 | 200 | ⊕⊕⊝⊝ | — |
| Disease‐specific health‐related quality of life of the child at 4 and 12 months post‐randomisation using the OM‐6 questionnaire | "no statistically significant differences between treatment groups were reported at 4 and 12 months for any of the six subdomains of the OM‐6 questionnaire" | 85 and 81, respectively (1 RCT) | ⊕⊕⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Grommets versus antibiotic prophylaxis for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antibiotic prophylaxis | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 1.68 | 96 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (586‐349)* 1000 = 4.22 | |
| 349 per 1000 | 586 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 29 AOM recurrences in 21 children; mean number of AOM recurrences per child: 1.38 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐0.52, 95% CI ‐1.37 to 0.33 | 43 (1 RCT) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations (when we excluded the trial with high risk of bias from the analysis, no statistically significant difference was observed between groups) and imprecise effect estimates (only two studies with small sample sizes). 2We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). | ||||||
| Grommets versus placebo medication for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo medication | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 3.64 | 42 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (546‐150)* 1000 = 2.53 | |
| 150 per 1000 | 546 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 4% (3/76) | n/a | 76 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 6 months post‐randomisation | 40 AOM recurrences in 20 children; mean number of AOM recurrences per child: 2.0 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐1.14, 95% CI ‐2.06 to ‐0.22 | 42 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). 2We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Study ID | Grommets | Grommets plus adenoidectomy | Active monitoring | Placebo medication | Antibiotic prophylaxis | Adenoidectomy |
| x | x | x | ||||
| x | x | |||||
| x | x | |||||
| x | x | x | ||||
| x | x | x | ||||
| Comparison pairs for this review | ||||||
| # | Intervention | Comparator | Number of trials | Study ID | ||
| 1 | Grommets | Active monitoring | 2 | |||
| 2 | Grommets | Antibiotic prophylaxis | 3 | |||
| 3 | Grommets | Placebo medication | 2 | |||
| Outcomes | |||||
| Primary outcomes | |||||
| Proportion of children who have no AOM recurrences at 3 to 6 months post‐randomisation | x | x | x | ||
| Significant adverse effect: tympanic membrane perforation persisting for 3 months or longer | x | x | |||
| Secondary outcomes | |||||
| Proportion of children who have no AOM recurrences at 6 to 12 months post‐randomisation | x | ||||
| Total number of AOM recurrences | |||||
| < 3 months | |||||
| 3 to 6 months | x | x | |||
| 6 to 12 months | x | ||||
| Disease‐specific health‐related quality of life | |||||
| < 3 months | |||||
| 3 to 6 months | x | ||||
| 6 to 12 months | x | ||||
| Generic health‐related quality of life of the child and parent | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Presence of middle ear effusion | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Other adverse effects: ventilation tube misplaced in middle ear, otorrhoea within 1 week of ventilation tube placement, myringosclerosis | x | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [2.38, 37.80] |
| 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.00, 1.99] |
| 3 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.99, ‐1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.07, 2.65] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.37, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.20, 11.04] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐2.06, ‐0.22] |

