Contraste hidrosoluble oral para la obstrucción intestinal maligna
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012014.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 07 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
William Syrmis: formulated question, drafted and wrote final protocol, searched for studies, reviewed titles and abstracts, retrieved articles, assessed article quality, drafted and wrote review.
Russell Richard: provided review and general advice on the protocol, reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Sue Jenkins‐Marsh: drafted protocol, reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Siew Chin Chia: provided review and general advice on the protocol, reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Phillip Good: formulated question, drafted protocol, reviewed titles and abstracts, retrieved articles, assessed article quality, performed critical revision of review.
All the authors agreed on the final version.
Declarations of interest
-
William Syrmis: none known; WS is a Palliative Care specialist and manages patients with inoperable malignant bowel obstructions. He will be an investigator in an open label pilot study of oral water soluble contrast (Gastrografin) in addition to conservative medical management for the resolution of malignant bowel obstruction in adult patients to take place in late 2017.
-
Russell Richard: none known; RR is a Palliative Care specialist and manages patients with inoperable malignant bowel obstructions. He will be an investigator in an open label pilot study of oral water soluble contrast (Gastrografin) in addition to conservative medical management for the resolution of malignant bowel obstruction in adult patients to take place in late 2017.
-
Sue Jenkins‐Marsh: none known.
-
Siew Chin Chia: none known; SC is a Palliative Care physician and manages patients with inoperable malignant bowel obstructions.
-
Phillip Good: none known; PG is a Palliative Care physician and manages patients with inoperable malignant bowel obstructions. He will be an investigator in an open label pilot study of oral water soluble contrast (Gastrografin) in addition to conservative medical management for the resolution of malignant bowel obstruction in adult patients to take place in late 2017.
Acknowledgements
We thank Joanne Abbott, the Information Specialist for the Cochrane Pain, Palliative and Supportive Care Review Group for assisting to develop the search strategy.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 07 | Oral water soluble contrast for malignant bowel obstruction | Review | William Syrmis, Russell Richard, Sue Jenkins-Marsh, Siew C Chia, Phillip Good | |
| 2015 Dec 22 | Oral water soluble contrast for malignant bowel obstruction | Protocol | William Syrmis, Russell Richard, Sue Jenkins‐Marsh, Siew C Chia, Phillip Good | |
Notes
A restricted search in March 2020 did not identify any potentially relevant eligible studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abdominal Neoplasms [*complications];
- Administration, Oral;
- *Conservative Treatment;
- Contrast Media [*administration & dosage, adverse effects];
- Diatrizoate Meglumine [*administration & dosage, adverse effects];
- Feasibility Studies;
- Intestinal Obstruction [*diagnostic imaging, etiology, *therapy];
- Length of Stay;
- Pilot Projects;
Medical Subject Headings Check Words
Adult; Humans;
PICO
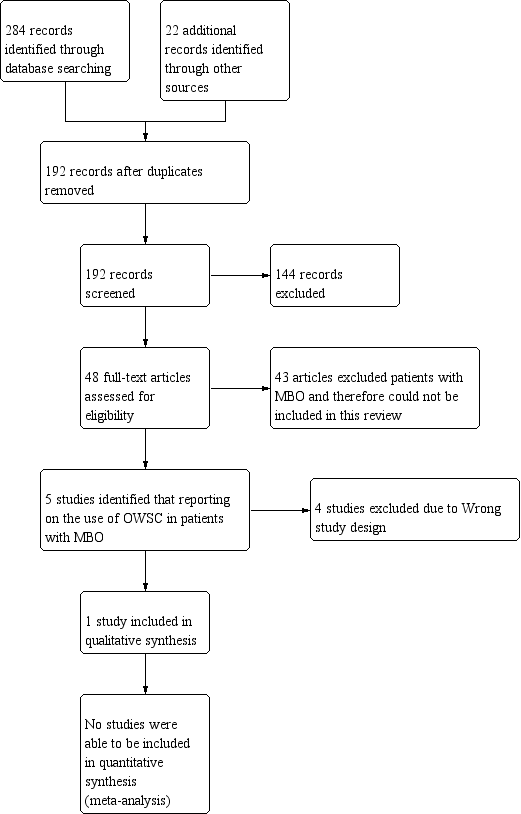
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Oral Water Soluble Contrast compared with Placebo for Malignant Bowel Obstruction | |||
| Patient or population: Adults 18 yrs and over with malignant bowel obstruction (MBO) as defined by the International Conference on MBO, with no indication for other treatments e.g. surgery, endoscopy, etc Settings: Hospital inpatients Intervention: 100 mL of gastrografin administered orally Comparison: Placebo (100m L of distilled water flavoured with aniseed oil in order to mimic the taste and smell of gastrografin) | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
|---|---|---|---|
| 1. The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic). | no data | no data | no data |
| 2. The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic). | no data | no data | no data |
| 3. Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 4. Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 5. Length of hospital stay. | no data | no data | no data |
| 6. Time from administration of OWSC to resolution of MBO. | no data | no data | no data |
| 7. Survival time from onset of inoperable MBO until death. | no data | no data | no data |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgraded once for imprecision (sparse data). OWSC = oral water soluble contrast | |||

