Oral water soluble contrast for malignant bowel obstruction
Abstract
Background
Malignant bowel obstruction (MBO) is a common problem in patients with intra‐abdominal cancer. Oral water soluble contrast (OWSC) has been shown to be useful in the management of adhesive small bowel obstruction in identifying patients who will recover with conservative management alone and also in reducing the length of hospital stay. It is not clear whether the benefits of OWSC in adhesive small bowel obstruction are also seen in patients with MBO.
Objectives
To determine the reliability of OWSC media and follow‐up abdominal radiographs in predicting the success of conservative treatment in resolving inoperable MBO with conservative management.
To determine the efficacy and safety of OWSC media in reducing the duration of obstruction and reducing hospital stay in people with MBO.
Search methods
We identified studies from searching Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and MEDLINE in Process, Embase, CINAHL, Science Citation Index (Web of Science) and Conference Proceedings Citation Index ‐ Science (Web of Science). We also searched registries of clinical trials and the CareSearch Grey Literature database. The date of the search was the 6 June 2017.
Selection criteria
Randomised controlled trials (RCTs), or prospective controlled studies, that evaluated the diagnostic potential of OWSC in predicting which malignant bowel obstructions will resolve with conservative treatment.
RCTs, or prospective controlled studies, that assessed the therapeutic potential of OWSC in managing MBO at any level compared with placebo, no intervention or usual treatment or supportive care.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We assessed risk of bias and assessed the evidence using GRADE and created a 'Summary of findings' table.
Main results
We found only one RCT meeting the selection criteria for the second objective (therapeutic potential) of this review. This study recruited nine participants. It compared the use of gastrografin versus placebo in adult patients with MBO with no indication for further intervention (surgery, endoscopy) apart from standardised conservative management.
The overall risk of bias for the study was high due to issues with low numbers of participants, selective reporting of outcomes and a high attrition rate for the intervention arm.
Primary outcomes
The included trial was a pilot study whose primary outcome was to test the feasibility for a large study. The authors reported specifically on the number of patients screened, the number recruited and reasons for exclusion; this was not the focus of our review.
Due to the low number of participants, the authors of the study decided not to report on our primary outcome of assessing the ability of OWSC to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic effect). It also did not report on our primary outcome of rate of resolution of MBO in patients receiving OWSC compared with those not receiving it (therapeutic effect).
The study reported that no issues regarding safety or tolerability of either gastrografin or placebo were identified. The overall quality of the evidence for the incidence of adverse events with OWSC was very low, downgraded twice for serious limitations to study quality (high risk of selective reporting and attrition bias) and downgraded once for imprecision (sparse data).
Secondary outcomes
The study planned to report on this review’s secondary outcome measures of length of hospital stay and time from administration of OWSC to resolution of MBO. However the authors of the study decided not to do so due to the low numbers of patients recruited. The study did not report on our secondary outcome measure of survival times from onset of inoperable MBO until death.
Authors' conclusions
There is insufficient evidence from RCTs to determine the place of OWSC in predicting which patients with inoperable MBO will respond with conservative treatment alone. There is also insufficient evidence from RCTs to determine the therapeutic effects and safety of OWSC in patients with malignant small bowel obstruction.
PICO
Plain language summary
Oral water soluble contrast for cancer‐related bowel obstruction
Background
Intra‐abdominal cancer is where the cancer has either started elsewhere in the body and spread into the stomach area or started in the bowel itself. It can commonly grow and block the movement of food or faeces through the bowel. A blockage of the bowel caused by cancer is known as malignant bowel obstruction (MBO). Some patients with MBO benefit from surgery to reverse the blockage. In others it is not possible to reverse the MBO and it either resolves or becomes permanent (limiting prognosis to days or weeks).
Scar tissue around the bowel (called adhesions) can also cause a blockage, this is known as adhesive small bowel obstruction. Adhesive obstructions often resolve with conservative management in hospital (allowing the bowel to "rest" and recover). However in some, the obstruction requires surgery to remove the scar tissue and fix the blockage.
Oral water soluble contrast (OWSC) is often prepared in liquid form and when swallowed before an x‐ray or computed tomography (CT) scan will show up clearly in the bowel. It can predict which people with adhesive small bowel obstruction will require surgery. In some patients it may also speed recovery leading to reduced hospital stay.
It is not clear whether the benefits of OWSC in adhesive small bowel obstruction are also seen in MBO.
Study characteristics
We searched for evidence that OWSC could be used to identify which patients with inoperable MBO would recover with conservative management. We also wanted to know if OWSC increased the likelihood of recovery from MBO, reduced hospital stay or improved prognosis. Finally, we wished to know what side effects OWSC might cause for patients with MBO. We conducted the search in June 2017 and found one study. It only recruited nine participants and did not fully report on the outcomes of using OWSC in MBO.
Key Findings
We found insufficient evidence that OWSC can identify which patients with MBO will recover with conservative management. We found insufficient evidence that patients with MBO benefit from OWSC in terms of length of hospital stay, recovery time or survival. No conclusions could be made about side effects from OWSC.
Quality of Evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results. We rated the quality of the evidence as very low due to the lack of studies reporting on the benefits of using OWSC in MBO and the only study we found reporting on side effects recruited very few participants (nine). The low quality of the evidence means that we are very uncertain about the use of OWSC in the management of MBO and cannot confirm its benefits or harms in patients with this condition.
Authors' conclusions
Summary of findings
| Oral Water Soluble Contrast compared with Placebo for Malignant Bowel Obstruction | |||
| Patient or population: Adults 18 yrs and over with malignant bowel obstruction (MBO) as defined by the International Conference on MBO, with no indication for other treatments e.g. surgery, endoscopy, etc Settings: Hospital inpatients Intervention: 100 mL of gastrografin administered orally Comparison: Placebo (100m L of distilled water flavoured with aniseed oil in order to mimic the taste and smell of gastrografin) | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
|---|---|---|---|
| 1. The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic). | no data | no data | no data |
| 2. The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic). | no data | no data | no data |
| 3. Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 4. Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 5. Length of hospital stay. | no data | no data | no data |
| 6. Time from administration of OWSC to resolution of MBO. | no data | no data | no data |
| 7. Survival time from onset of inoperable MBO until death. | no data | no data | no data |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgraded once for imprecision (sparse data). OWSC = oral water soluble contrast | |||
Background
Description of the condition
Bowel obstruction refers to any mechanical or functional obstruction of the intestine that prevents physiological transit and digestion (Tuca 2012). For the purposes of research, malignant bowel obstruction (MBO) has been defined using the following criteria (Anthony 2007):
-
that the patient has clear clinical evidence of a bowel obstruction (based on history, examination and radiological criteria), and
-
that the bowel obstruction be located distal to the Ligament of Treitz (at the junction of the duodenum and jejunum), and either
-
that the patient has an intra‐abdominal primary cancer with incurable disease, or
-
that the patient has a non intra‐abdominal primary cancer with clear intra‐peritoneal disease.
MBO is reported to have a prevalence of 3% to 15% in cancer patients (Tuca 2012). Its symptoms, such as nausea, vomiting, abdominal pain and distension, can cause significant physical distress. MBO is common in patients with ovarian cancer (20% to 50% of ovarian cancer patients present with symptoms of bowel obstruction) and colorectal cancer (10% to 28% developing an obstruction through the course of their disease) (Ripamonti 2008). Breast cancer and melanoma are the most common extra‐abdominal cancers associated with MBO (Ripamonti 2008).
MBO may be directly related to intra‐abdominal tumour growth causing extrinsic compression of the bowel, intraluminal obstruction or by intramural infiltration (Tuca 2012). In patients with cancer, bowel obstructions may also be caused by post‐surgical adhesions or radiation fibrosis. Intestinal motility disorders leading to obstruction may be caused by direct infiltration by cancer of the coeliac plexus, mesentery, bowel wall or nerves. Intestinal dysmotility may also be caused by paraneoplastic syndromes or opioid‐related constipation. Obstruction may be partial or complete, may occur in the small or large bowel, and may occur singularly or at multiple levels.
Bowel obstruction may be diagnosed with plain abdominal x‐rays showing distension of the intestinal loops, the presence of air‐fluid levels in the zone proximal to the occlusion as well as a reduction in gas and stools in the segments distal to the obstruction. Computed tomography (CT) scans or magnetic resonance imaging (MRI) provide better sensitivity and specificity in diagnosing the level of obstruction, in addition to providing information on other sites of metastatic disease that may influence management decisions. Where curative surgery is not possible, palliative surgery or endoscopic stenting may be appropriate management for patients with suitable sites of disease, performance status and prognosis. In other patients whose disease is considered inoperable, palliation of symptoms is performed until either the obstruction resolves or the patient dies.
Spontaneous resolution of an inoperable bowel obstruction may occur in approximately one third of cases (Tuca 2012). In a prospective cohort study, the mean survival was “12 days (95% CI = 9.0 – 14.1) for participants with no spontaneous resolution of their malignant bowel obstruction, and 57 days for participants with complete resolution, (P < 0.001)” (Tuca 2012). There is also a suggestion that early and aggressive medical management of an MBO may induce resolution and prevent the obstruction from becoming irreversible (Mercadante 2004). There appears to be little evidence to determine which inoperable MBOs are likely to resolve with conservative management (i.e. spontaneously or with medical management).
Description of the intervention
Oral water soluble contrast (OWSC) is an iodinated contrast medium that shows up opaque on plain x‐ray. The most common form used is gastrografin ‐ a hyperosmolar solution that is a combination of sodium diatrizoate and meglumine diatrizoate.
A previous Cochrane review assessed the benefit of OWSC for adhesive small bowel obstruction (Abbas 2007). OWSC was administered either orally or via a nasogastric tube with an abdominal x‐ray taken at a set time afterwards. It showed that OWSC seen in the colon on abdominal x‐ray within 24 hours from administration was highly predictive of resolution of adhesive small bowel obstruction. However the use of OWSC did not decrease the need for surgical intervention, but did decrease hospital stay. Two more recent systematic reviews and meta‐analyses of the diagnostic and therapeutic role of OWSC in adhesive small bowel obstruction have been performed (Branco 2010; Ceresoli 2016). Both concluded that OWSC was effective in predicting the need for surgery in patients with adhesive small bowel obstruction. But both also concluded that the intervention reduced the need for surgery and reduced length of hospital stay. In this review, we searched for studies using the same or a similar intervention in an attempt to predict the likelihood of malignant small bowel obstruction resolving with conservative management. That is, does OWSC reaching the colon in a patient with malignant small bowel obstruction indicate with an acceptable sensitivity and specificity that the obstruction will resolve with conservative or medical treatment alone? This was the diagnostic arm of the review.
Administration of water soluble contrast (in most studies, 100 mL of gastrografin) was tested as a therapeutic agent in adhesive bowel obstruction (Abbas 2007). This review also searched for studies with similar interventions to treat MBO (small bowel obstruction or inoperable large bowel obstruction). This was the therapeutic arm of the review.
Although the potential adverse effects of using OWSC include vomiting, diarrhoea, hypersensitivity reactions and rarely bowel perforation or aspiration pneumonia, the reviews into its use in adhesive small bowel obstruction have concluded that it was safe in that setting, as there was no increase in morbidity or mortality through its use in the reported studies (Abbas 2007; Ceresoli 2016).
How the intervention might work
OWSC agents are generally felt to be more useful than barium for imaging in small bowel obstructions, as they promote peristalsis and are less irritating to the peritoneum in the event of a perforation (Joyce 1992; Mercadante 2004; Riccabona 2014). The hyperosmolar nature of OWSC is thought to attract fluid from the bowel wall into the lumen, potentially assisting to resolve the obstruction by decreasing oedema in the bowel wall (Khasawneh 2013). By shifting fluid into the bowel lumen, it also increases the pressure gradient across an obstructive site and, as the bowel content is also diluted, this may assist its movement through a narrower or partially‐obstructed lumen (Mercadante 2004; Vather 2015).
Why it is important to do this review
Published literature suggests that gastrografin may be of benefit in the management of reversible MBOs (Khasawneh 2013; Mercadante 2004; Ripamonti 2001; Ripamonti 2002; Ripamonti 2008). However it is not clear what level of evidence supports this recommendation. Also, while there is evidence that gastrografin assists in predicting which adhesive bowel obstructions may resolve with conservative management (Abbas 2007), it is not clear whether this also applies to MBO, given the large number of other possible causes (extrinsic compression, intraluminal narrowing, etc). In this review we attempted to identify whether the use of OWSC is able to identify patients with inoperable malignant small bowel obstruction in whom the obstruction is likely to resolve with conservative management. This may be important in judging the patients' prognoses and influence their planning for end‐of‐life care. We will also seek evidence as to whether water soluble contrast has a therapeutic role in the reversal of MBO at any level, which may reduce morbidity and the duration of hospital stay for patients undergoing palliative care. If the administration of OWSC is able to prevent the obstruction from becoming irreversible, then this may also improve survival time from the onset of the obstruction until death.
Objectives
To determine the reliability of oral water soluble contrast (OWSC) media and follow up abdominal radiographs in predicting the success of conservative treatment in resolving inoperable malignant bowel obstruction with conservative management.
To determine the efficacy and safety of OWSC media in reducing the duration of obstruction and reducing hospital stay in people with malignant bowel obstruction.
Methods
Criteria for considering studies for this review
Types of studies
We planned to review prospective studies to evaluate the diagnostic potential of oral water soluble contrast (OWSC) in predicting which malignant small bowel obstructions will resolve with conservative treatment.
We planned to review randomised controlled trials (RCTs) that assessed the therapeutic potential of OWSC in managing malignant bowel obstruction (MBO) at any level. If no RCTs were found, relevant prospective controlled studies would be discussed but not included in a meta‐analysis.
Types of participants
Adult patients (aged 18 and over) who meet the criteria for MBO as listed above where the obstruction is limited to the small bowel only (diagnostic).
Adult patients (aged 18 and over) who meet the criteria for MBO listed above and where surgery or endoscopic stenting are not appropriate, or with malignant small bowel obstruction who are having a trial of conservative management before a decision on surgery/stenting is made (therapeutic).
Types of interventions
-
The administration of OWSC in patients with the diagnosis of malignant small bowel obstruction followed by interval abdominal radiographs to identify contrast in the colon (diagnostic).
-
The administration of OWSC to patients with MBO (at any level), to assess its ability to resolve the obstruction.
Comparison
-
Placebo.
-
No intervention.
-
Usual treatment or supportive care.
Types of outcome measures
Primary outcomes
-
The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic).
-
The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic).
-
Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting).
-
Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions).
Secondary outcomes
-
Length of hospital stay.
-
Time from administration of OWSC to resolution of MBO.
-
Survival time from onset of inoperable MBO until death.
Search methods for identification of studies
The search strategy was not limited by language, publication type or status, or by date.
Electronic searches
The following electronic databases were searched on the 6 June 2017 by the Information Specialist of the Cochrane Pain, Palliative and Supportive Care Review Group.
-
The Cochrane Central Register of Controlled Trials (CENTRAL, via CRSO).
-
MEDLINE (Ovid)1946 to Jun 5 2017.
-
MEDLINE in Process to Jun 05 2017.
-
Embase (Ovid) 1974 to 2017 week 23.
-
CINAHL1982 to Jun 5 2017.
-
Science Citation Index (Web of Science) to Jun 05 2017.
-
Conference Proceedings Citation Index ‐ Science (Web of Science) to Jan 04 2016.
The search strategies used can be seen in Appendix 1. The Cochrane Highly Sensitive Search Strategy filter for identifying randomised trials in MEDLINE via Ovid (Lefebvre 2011) was also applied. The MEDLINE search was adapted and modified across the other databases.
Searching other resources
We also searched clinicaltrials.gov (www.Clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) to identify any ongoing trials, in June 2017. The metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com/mrct/),was under review and not available in June 2017, so we searched the UKCTG (http://www.ukctg.nihr.ac.uk/default.aspx) instead. For grey literature, we searched the Internet using the Google scholar search engine (http://scholar.google.com.au/) and CareSearch Grey Literature databases (http://www.caresearch.com.au) with selected terms from the strategy in Appendix 1.
Data collection and analysis
Selection of studies
All titles and abstracts of the studies identified by the searches were divided amongst all the review authors, (WS, RR, SJM, SC, PG) so that each was reviewed by at least two people. Each review author then independently selected all potentially‐relevant studies by applying the selection criteria outlined in the Criteria for considering studies for this review. Our selections were then compared, any differences discussed and then the papers were either included or excluded based on a majority decision.
A PRISMA study flow diagram (Liberati 2009) (Figure 1) documents the screening process, as recommended in Part 2, Section 11.2.1 of the Cochrane Handbookfor Systematic Reviews of Interventions (Higgins 2011).

Study flow diagram.
Data extraction and management
Two review authors (WS, PG) independently extracted data using a piloted data extraction form. Data extracted included information about the year of study, study design, number of participants treated, participant demographic details, type of cancer, drug and dosing regimen, study design (placebo or active control) and methods, study duration and follow‐up, outcome measures (reduction in duration of obstruction, length of stay, likelihood of resolution if contrast reaches colon), withdrawals and adverse events. Had we included studies with more than two intervention arms, we planned only to include the intervention and control arms that met the eligibility criteria. Multi‐arm studies included in the review would have had their multiple intervention arms analysed in an appropriate way to avoid arbitrary omission of relevant groups and double‐counting of participants. We planned to resolve potential disagreements by discussion and a majority decision.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for the one included study. This was done using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We prepared a 'Risk of bias' table for the included study using the 'Risk of bias' tool in RevMan (RevMan 2014).
We used the following methods to assess the risk of bias in the included study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) would have been deemed to be at high risk of bias and excluded.
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). Studies that did not conceal allocation (e.g. open list, date of birth or any other explicitly unconcealed procedure) would have been deemed to be at high risk of bias and excluded.
-
Blinding of participants, personnel (checking for possible performance bias). We planned to assess studies as low risk of bias if they adequately described how the blinding of key study participants and personnel has been assured. Studies would have been assessed as high risk of bias if there was no blinding or incomplete blinding which may impact on the assessment of adverse effects from OWSC. An assessment of unclear risk of bias would apply to studies where there was insufficient information to permit judgement of 'high' or 'low' risk.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies where there was no blinding of outcome assessment or where the blinding may have been broken which may affect outcome measurements (particularly adverse effects of OWSC) would have been deemed high risk of bias and excluded.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
-
Selective reporting (checking for reporting bias). We planned to assess studies as being at low risk of bias (all of the relevant pre‐specified outcomes listed in the protocol and reported in the pre‐specified way); high risk of bias (one or more outcomes of interest are reported incompletely so that they could not be entered in a meta‐analysis); or unclear risk of bias (insufficient information to permit judgement of 'high risk' or 'low risk').
-
Size of study (checking for possible biases confounded by small size). We planned to assess studies as being at low risk of bias (≥ 200 participants per intervention arm); unclear risk of bias (50 to 199 participants per intervention arm); or high risk of bias (<50 participants per intervention arm).
Measures of treatment effect
We planned to estimate and compare the risk ratio (RR) using a 95% confidence interval (CI) for dichotomous outcomes between groups. We planned to measure arithmetic means and standard deviation (SD) for continuous outcomes between groups and to report the mean difference (MD) with 95% CI. We planned to use standardised mean difference (SMD) with 95% CI where an outcome was derived with different instruments measuring the same outcomes.
We planned to calculate the number needed to treat for an additional harmful outcome (NNTH) for unwanted effects using dichotomous data to calculate RR with 95% CI. We planned using the following terms to describe adverse outcomes in terms of harm or prevention of harm.
-
We would have used the term 'number needed to treat to prevent one event' (NNTp) when significantly fewer adverse outcomes occurred with OWSC than with control (placebo or active).
-
We would have used the term 'number needed to harm or cause one event' (NNTH) when significantly more adverse outcomes occurred with water soluble contrast compared with control (placebo or active) we would have used the term 'number needed to harm or cause one event' (NNTH).
Unit of analysis issues
We planned to only include studies in which randomisation was by the individual patient; this would have included cross‐over studies or n = 1 study.
Dealing with missing data
We planned to contact the authors to request any missing data if they were relevant in cases where data were missing. We planned to perform an intention‐to‐treat (ITT) analysis. Missing participants or information would be assigned to a zero improvement category where possible. The method of assessing data processed from withdrawals would have been ascertained where possible. We planned on performing sensitivity analyses where there were substantial numbers (> 10%) of participants missing from analyses.
Assessment of heterogeneity
We planned to undertake a meta‐analysis only if participants, interventions, comparisons and outcomes were judged to be sufficiently similar to ensure an answer would be clinically meaningful. There may be an effect due to differences between patients, level of obstruction (small bowel versus large bowel) and outcome measures. We planned on assessing heterogeneity by using the I2 statistic. We would have considered I2 values above 50% to represent substantial heterogeneity in line with Higgins 2011, and would have assessed potential sources of heterogeneity through subgroup analyses.
Assessment of reporting biases
We planned to assess the likelihood of publication bias by examining funnel plot symmetry to interpret the results of statistical analysis if there had been at least 10 studies in the meta‐analysis. We would have considered publication bias as only one of a number of possible explanations (Higgins 2011) if there had been evidence of small‐study effects.
Data synthesis
We planned to enter the data into Review Manager software (RevMan 2014) for data synthesis. Where appropriate, we would have pooled data for each dichotomous outcome and calculated RRs with 95% CIs using the fixed‐effect model, together with numbers needed to treat for an additional beneficial outcome (NNTBs) with 95% CIs and, for adverse events, numbers needed to treat for an additional harmful outcome (NNTHs) with 95% CIs.
Quality of the evidence
Two review authors (WS, PG) independently rated the quality of the outcomes. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (GRADEpro GDT 2015), and the guidelines provided in Chapter 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2011).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence:
-
high: we are very confident that the true effect lies close to that of the estimate of the effect;
-
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
-
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
-
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased grade if we identified:
-
serious (‐1) or very serious (‐ 2) limitation to study quality;
-
important inconsistency (‐ 1);
-
some (‐1) or major (‐ 2) uncertainty about directness;
-
imprecise or sparse data (‐ 1);
-
high probability of reporting bias (‐ 1).
'Summary of findings' table
We included a 'Summary of findings' table(s) to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the following outcomes.
-
The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic).
-
The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic).
-
Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting).
-
Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions).
-
Length of hospital stay.
-
Time from administration of OWSC to resolution of MBO.
-
Survival time from onset of inoperable MBO until death.
Subgroup analysis and investigation of heterogeneity
We planned, if sufficient data were available, to perform the following subgroup analyses.
-
Level of obstruction (small bowel or large bowel).
-
Degree of obstruction (partial or complete).
-
Dose of OWSC used.
-
Timing of follow‐up abdominal x‐ray (diagnostic).
-
Type of cancer.
Sensitivity analysis
We planned, if sufficient data were available, to examine the robustness of the meta‐analyses by conducting sensitivity analyses using different components of the 'Risk of bias' assessment, particularly with those relating to whether allocation concealment and patient/assessor blinding were adequate. We planned to conduct further sensitivity analyses to examine the impact of missing data on the results if a large proportion of the studies were at an ‘unknown’ or ‘high risk’ of attrition bias, and finally, to examine whether publication status and trial size influenced the results.
Results
Description of studies
See: 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
We performed the searches for this review in June 2017. The PRISMA diagram (Figure 1) outlines the number of records identified in the search and the screening process for these papers. In the initial database search 284 records were identified and 22 records were identified from other sources. One hundred and ninety‐two records remained after duplicates were removed. We were able to exclude 144 of those articles based on information provided in the title and abstract. We selected 48 publications for full‐text review but the majority of the full‐text articles reviewed concentrated on the treatment of adhesive bowel obstruction and excluded participants with known malignant obstruction from their studies. Only five studies were found to include participants with malignant bowel obstruction (MBO). We excluded four of those studies and the reasons are described in the 'Characteristics of excluded studies' table. We found only one randomised controlled trial (RCT) meeting the selection criteria for this review.
We did not identify any ongoing RCTs at the time of our search. One of the authors of this review (Dr Phillip Good) had registered an open‐label pilot study of oral water soluble contrast (OWSC) (gastrografin) in addition to conservative medical management for the resolution of MBO in adult patients with the International Clinical Trials Registry Platform, but recruitment has not commenced. It is unlikely to be included for analysis in future updates of this review as it will not be a controlled trial.
Included studies
We identified one RCT that met the inclusion criteria for this review (Lee 2013; a detailed description of the study can be found in the Characteristics of included studies table).
This was a phase II pilot study whose primary outcome was to assess the feasibility of performing a larger phase III RCT investigating the therapeutic use of gastrografin in MBO. To do so, it measured the number of participants screened, enrolled and completing assessment over an eight‐month period at a single hospital. However its other stated outcome measures of efficacy, safety and tolerability of OWSC in MBO were relevant to the therapeutic arm of this review.
Participants in the study were aged 18 years and over, presenting to hospital with MBO as defined by the International Conference on MBO (those with clinical evidence of bowel obstruction on history/physical/radiographic examination, with obstruction distal to the ligament of Treitz, in the setting of known incurable primary intra‐abdominal cancer or non intra‐abdominal primary cancer with clear intraperitoneal disease). To be eligible for inclusion in the study, the participants had to have no other intervention such as surgery or endoscopy indicated as determined by the relevant clinicians. Exclusion criteria included allergy to the study drug gastrografin or iodine, pregnancy, inability to give informed consent, evidence of gross gastric distension on radiologic examination, and the presence of a venting or feeding gastrostomy or jejunostomy.
All participants randomised to the study had their MBO treated following a standardised protocol with parenteral hydration, 16 mg of parenteral dexamethasone each day and anti‐emetics and/or antimuscarinics according to their symptoms. The intervention arm also received 100 mL of gastrografin orally while the control arm received 100 mL of distilled water in identical bottles, flavoured to resemble gastrografin. To measure the safety and tolerability of the intervention the participants had to complete assessments of their symptoms and quality of life prior to the intervention, then at 30 minutes, six hours, 12 hours and 24 hours after the intervention, and then daily for 14 days or discharge, whichever came first. Measures to record the efficacy of gastrografin as a therapeutic measure for MBO included days from administration to resolution of the obstruction, length of hospital stay and 30‐day readmission rate.
This study screened 57 patients over eight months but was only able to enrol nine participants, four in the intervention arm and five in the control arm. Only two participants in the intervention arm completed assessments with one participant lost to follow‐up and the other declining further participation in the study due to ongoing vomiting two hours after administration of the study drug. Four of the five participants in the control arm completed the study. The study concluded that a phase III trial was not feasible using their current protocol. Unfortunately, due to the low numbers of participants in the intervention arm who completed the outcome assessments the authors stated they were unable to comment on the efficacy of gastrografin in MBO and did not publish any recorded data relating to symptom and quality of life assessment scores or measurements of time to resolution of the obstruction following administration of the OWSC agent. They reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified".
The authors declared that there were no conflicts of interest nor financial support for the research, authorship and/or publication of their article.
Excluded studies
We excluded four studies and the reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed the included study using the Cochrane 'Risk of bias' tool. Our judgements about each risk of bias for the included study are shown in the 'Risk of bias' summary (Figure 2).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The included study reported that participants were block randomised in a one‐to‐one ratio to receive either gastrografin or placebo. Central randomisation by the hospital pharmacy was described with the pharmacy having no contact with the patient. A "predetermined" allocation code was provided to the pharmacy but the study does not describe the method used to generate the allocation sequence. Therefore the adequacy of random sequence generation is unclear.
Allocation concealment
The pharmacy was provided with a "predetermined" allocation code prior to the study commencing, therefore the concealment of allocation sequence appeared adequate and the study was assessed as having a low risk of bias in this regard.
Blinding
Blinding of participants and personnel was adequately described in the study therefore it was deemed to be at low risk of possible performance bias. The study confirmed that it was blinded in outcome assessment and adequately described the use of identical 100 mL bottles with the placebo matched in odour and taste to gastrografin using aniseed oil flavouring. Therefore it was deemed to be at low risk of possible detection bias.
Incomplete outcome data
Of the four participants who received gastrografin only two completed the intervention and assessment while assessment was completed for four of the five participants on placebo. With the small numbers enrolled, the 50% attrition rate for the intervention arm represents a high risk of bias.
Selective reporting
The study planned to compare the rate of resolution of MBO in patients receiving gastrografin compared to those receiving placebo. However they decided not to report on this outcome writing that “a comment on efficacy could not be made due to the small number of patients enrolled in the intervention arm”. They also did not report on planned outcome measures of length of hospital stay or time from administration of OWSC to resolution of MBO. This represents a high risk of bias from selective reporting.
Other potential sources of bias
Size of study
There were fewer than 50 participants in the intervention arm, and so we judged the study to be at a high risk of bias for this domain.
No other bias suspected.
Effects of interventions
See: Summary of findings 1 Summary of findings
We planned to assess the following as the primary outcome measures of this review.
-
The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic).
-
The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic).
-
Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting).
-
Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions).
We also planned to assess the following secondary outcome measures.
-
Length of hospital stay.
-
Time from administration of OWSC to resolution of MBO.
-
Survival time from onset of inoperable MBO until death.
We planned to search for studies comparing the use of OWSC in patients with MBO against the following.
-
Placebo;
-
No intervention;
-
Usual treatment or supportive care.
We did not find any randomised controlled trials (RCTs) that assessed the use of OWSC to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone.
The single RCT that met inclusion criteria for this review planned to compare the rate of resolution of MBO between those receiving gastrografin (OWSC) and those receiving placebo with both arms receiving standardised supportive care. The trial authors planned on reporting length of hospital stay and time from administration of OWSC to resolution of MBO. However the trial authors decided not to report the data collected on these outcomes due to the low number of participants recruited (nine participants).
The authors also planned on reporting on the safety of gastrografin covering our two outcome measures examining adverse effects. They reported that they found no issues regarding safety or tolerability of gastrografin. We assessed the quality of that evidence to be very low using GRADE, downgrading twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgrading once for imprecision (sparse data).
Discussion
Summary of main results
Our review did not find any randomised controlled trials (RCTs) examining the use of oral water soluble contrast (OWSC) as a diagnostic agent in malignant bowel obstruction (MBO) to predict which patients will achieve resolution of their inoperable obstruction with conservative management alone.
In assessing the efficacy of OWSC as a therapeutic agent for patients with MBO the following outcome measures may be of use.
-
The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic).
-
Length of hospital stay.
-
Time from administration of OWSC to resolution of MBO.
-
Survival time from onset of inoperable MBO until death.
We did not find any evidence from RCTs reporting on those outcomes that would support the use of OWSC as a therapeutic agent for MBO.
The single RCT we found compared the use of OWSC with placebo in patients with MBO receiving usual treatment. We did not find any studies comparing the use of OWSC alone versus no intervention or versus usual treatment.
Although retrospective studies and case series, (Goussous 2013; Kao 1967; Khasawneh 2013; Mercadante 2004) have suggested that the use of OWSC is safe in patients with MBO, confirmation of its tolerability and safety was not possible as the single RCT identified by this review had only four participants in its intervention arm.
Overall completeness and applicability of evidence
We identified only one RCT studying the use of OWSC as a therapeutic agent, compared with placebo, in patients with MBO meeting the criteria for inclusion in this review. That RCT failed to recruit enough participants and subsequently did not report fully on the outcomes that were the focus of this review. We did not find any studies examining the use of OWSC as a diagnostic agent in patients with MBO. This review therefore has not found enough evidence to determine the place of OWSC in the management of patients with MBO.
Quality of the evidence
We had two objectives for this review.
-
To determine the reliability of OWSC and to follow up abdominal radiographs in predicting the success of conservative treatment in resolving inoperable MBO with conservative management (diagnostic agent).
-
To determine the efficacy and safety of OWSC in reducing the duration of obstruction and reducing hospital stay in people with MBO (therapeutic agent).
We did not find any evidence from RCTs that looked at the use of OWSC as a diagnostic agent in patients with MBO.
We found a single RCT looking at outcomes associated with the therapeutic use of OWSC (gastrografin) in patients with MBO. This RCT failed to recruit more than four participants to the intervention arm and elected not to report or comment on the therapeutic efficacy of gastrografin (as determined by time from administration to resolution of the MBO). Therefore, we found no evidence from RCTs on the therapeutic effects of OWSC in patients with MBO.
The single RCT did report that no issues regarding safety or tolerability of gastrografin were identified. It stated it would do so by serial recording of the patient’s reported symptom and quality of life scores as assessed by the Edmonton Symptom Assessment System throughout the study period. We rated the evidence for the safety and tolerability of gastrografin in patients with MBO as very low using the GRADE approach, downgrading twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgrading once for imprecision (sparse data).
Potential biases in the review process
We searched all available databases, checked reference lists of all relevant trials and searched available grey literature databases for registered or unpublished trials. Although the search strategy for this review was wide, it is possible that the search missed some studies due to the variety of OWSC agents available and that they may be named differently in non‐English speaking countries.
Four of the five review authors have prescribed gastrografin for patients with MBO. None of the review authors have received any support or sponsorship from companies manufacturing or distributing OWSC. None of the authors of this review were involved in any of the excluded or included studies. Dr Phillip Good was involved in a retrospective audit of the use of gastrografin in a Palliative Care unit which commenced after the literature search was performed. Dr Phillip Good and Dr William Syrmis are planning a prospective controlled trial of gastrografin to treat MBO in palliative care patients and this review was performed partly to assist in the design of that trial.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews of the use of OWSC in patients with MBO.
The previous Cochrane review of the use of OWSC in adhesive small bowel obstruction found evidence that gastrografin assists in predicting which obstructions may resolve with conservative management, that OWSC was safe for use in patients with adhesive small bowel obstruction and that it may reduce the length of hospital stay for those patients (Abbas 2007). Our review could not find sufficient evidence to support the same benefits of OWSC in patients with MBO as found in those with adhesive small bowel obstruction.
Also, while retrospective studies and case series, Goussous 2013, Kao 1967, Khasawneh 2013, and Mercadante 2004 have suggested OWSC is safe for use in patients with MBO and may be of benefit in their management, our review could not find evidence from RCTs to support those statements.
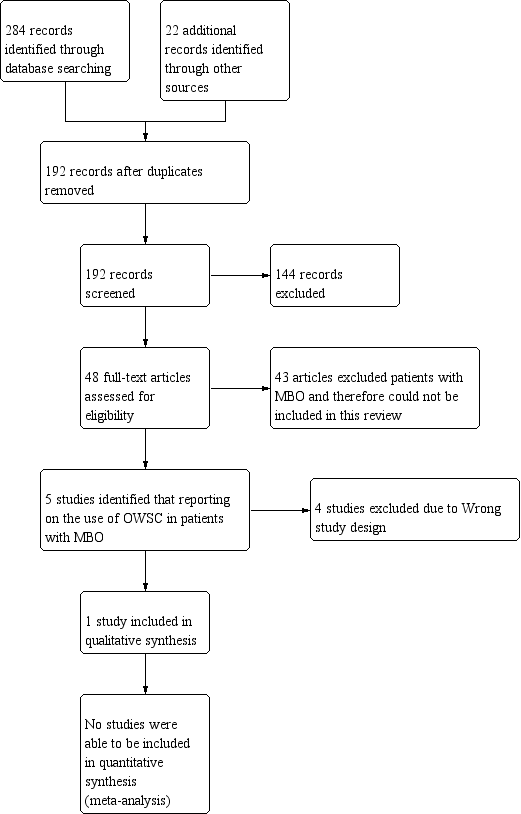
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Oral Water Soluble Contrast compared with Placebo for Malignant Bowel Obstruction | |||
| Patient or population: Adults 18 yrs and over with malignant bowel obstruction (MBO) as defined by the International Conference on MBO, with no indication for other treatments e.g. surgery, endoscopy, etc Settings: Hospital inpatients Intervention: 100 mL of gastrografin administered orally Comparison: Placebo (100m L of distilled water flavoured with aniseed oil in order to mimic the taste and smell of gastrografin) | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
|---|---|---|---|
| 1. The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic). | no data | no data | no data |
| 2. The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic). | no data | no data | no data |
| 3. Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 4. Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 5. Length of hospital stay. | no data | no data | no data |
| 6. Time from administration of OWSC to resolution of MBO. | no data | no data | no data |
| 7. Survival time from onset of inoperable MBO until death. | no data | no data | no data |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgraded once for imprecision (sparse data). OWSC = oral water soluble contrast | |||

