Oral water soluble contrast for malignant bowel obstruction
Appendices
Appendix 1. Search strategies
CENTRAL (CRSO)
("water soluble contrast"):TI,AB,KY
MESH DESCRIPTOR Contrast Media
MESH DESCRIPTOR Diatrizoate Meglumine
("water‐soluble contrast"):TI,AB,KY
("water‐soluble contrast"):TI,AB,KY
((gastrografin or urografin)):TI,AB,KY
((sodium diatrizoate or meglumine
diatrizoate)):TI,AB,KY
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
MESH DESCRIPTOR Intestinal Obstruction
EXPLODE ALL TREES
( ((bowel* or intestin*) adj3 (obstruct*
or block*))):TI,AB,KY
(((bowel* or intestin*) adj3 adhes*)):TI,AB,KY
#9 OR #10 OR #11
#8 AND #12
MEDLINE & MEDLINE in Process (OVID)
(N.B MEDLINE in Process was searched without the RCT filter)
1 "water soluble contrast".tw. (1055)
2 Contrast Media/ (67222)
3 Diatrizoate Meglumine/ (1980)
4 "water‐soluble contrast".tw. (1055)
5 (gastrografin or urografin).tw. (749)
6 (sodium diatrizoate or meglumine diatrizoate).tw. (475)
7 or/1‐6 (69062)
8 exp Intestinal Obstruction/ (38615)
9 ((bowel* or intestin*) adj3 (obstruct* or block*)).tw. (408)
10 ((bowel* or intestin*) adj3 adhes*).tw. (1684)
11 or/8‐10 (40137)
12 7 and 11 (738)
13 randomized controlled trial.pt. (382255)
14 controlled clinical trial.pt. (88491)
15 randomized.ab. (306631)
16 placebo.ab. (157203)
17 drug therapy.fs. (1728855)
18 randomly.ab. (222443)
19 trial.ab. (316184)
20 groups.ab. (1406392)
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (3430383)
22 exp animals/ not humans.sh. (3974624)
23 21 not 22 (2944461)
24 12 and 23 (81)
Embase (OVID)
1 "water soluble contrast".tw. (1335)
2 Contrast Media/ (51233)
3 Diatrizoate Meglumine/ (3975)
4 "water‐soluble contrast".tw. (1335)
5 (gastrografin or urografin or amidotrizoato).tw. (2278)
6 (sodium diatrizoate or meglumine diatrizoate).tw. (560)
7 or/1‐6 (56082)
8 exp Intestinal Obstruction/ (65812)
9 ((bowel* or intestin*) adj3 (obstruct* or block*)).tw. (23938)
10 ((bowel* or intestin*) adj3 adhes*).tw. (2375)
11 or/8‐10 (72748)
12 7 and 11 (1087)
13 random$.tw. (1042367)
14 factorial$.tw. (26683)
15 crossover$.tw. (55638)
16 cross over$.tw. (24913)
17 cross‐over$.tw. (24913)
18 placebo$.tw. (230237)
19 (doubl$ adj blind$).tw. (163757)
20 (singl$ adj blind$).tw. (16959)
21 assign$.tw. (276849)
22 allocat$.tw. (99768)
23 volunteer$.tw. (201215)
24 Crossover Procedure/ (45359)
25 double‐blind procedure.tw. (229)
26 Randomized Controlled Trial/ (390745)
27 Single Blind Procedure/ (21228)
28 or/13‐27 (1638839)
29 (animal/ or nonhuman/) not human/ (4918012)
30 28 not 29 (1453787)
31 12 and 30 (52)
CINAHL
S21 S11 AND S20
S20 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S19 (allocat* random*)
S18 (MH "Quantitative Studies")
S17 (MH "Placebos")
S16 placebo*
S15 (random* allocat*)
S14 (MH "Random Assignment")
S13 (Randomi?ed control* trial*)
S12 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or
(trebl* blind* ) or (trebl* mask* ) or
(tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S11 S6 AND S10
S10 S7 OR S8 OR S9
S9 ((bowel* or intestin*) N3 adhes*)
S8 ((bowel* or intestin*) N3 (obstruct* or block*))
S7 (MH "Intestinal Obstruction+")
S6 S1 OR S2 OR S3 OR S4 OR S5
S5 (sodium diatrizoate or meglumine diatrizoate)
S4 (gastrografin or urografin)
S3 "water‐soluble contrast".
S2 (MH "Contrast Media")
S1 "water soluble contrast".
Web of Science (ISI)
#16 #15 AND #11
# 15 #14 OR #13 OR #12
# 14 TS=((((singl* OR doubl* OR trebl*
OR tripl*) SAME (blind* OR mask*))))
#13 TS= (((controlled clinical trial OR
controlled trial OR clinical trial OR
placebo)))
#12 TS= (((randomised OR randomized OR
randomly OR random order OR random
sequence OR random allocation OR randomly
allocated OR at random OR randomized
controlled trial)))
#11 #10 AND #7
#10 #9 OR #8
#9 TOPIC: (((bowel* or intestin*) near/3
adhes*))
#8 TS=(((bowel* or intestin*) near/3
(obstruct* or block*)))
#7 #6 OR #5 OR #4 OR #3 OR #2 OR #1
#6 TOPIC: ((sodium diatrizoate or
meglumine diatrizoate))
#5 TOPIC: ((gastrografin or urografin))
#4 TOPIC: ("water‐soluble contrast")
#3 TOPIC: (Diatrizoate Meglumine)
#2 TOPIC: ("Contrast Media")
#1 TOPIC: ("water soluble contrast")
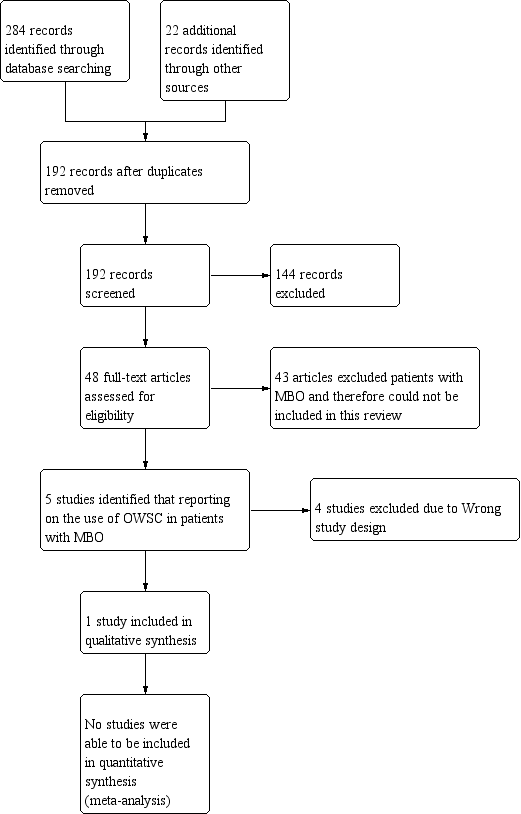
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Oral Water Soluble Contrast compared with Placebo for Malignant Bowel Obstruction | |||
| Patient or population: Adults 18 yrs and over with malignant bowel obstruction (MBO) as defined by the International Conference on MBO, with no indication for other treatments e.g. surgery, endoscopy, etc Settings: Hospital inpatients Intervention: 100 mL of gastrografin administered orally Comparison: Placebo (100m L of distilled water flavoured with aniseed oil in order to mimic the taste and smell of gastrografin) | |||
| Outcomes | No of Participants | Quality of the evidence | Comments |
|---|---|---|---|
| 1. The ability of OWSC, when seen to reach the colon on follow‐up imaging, to predict the likelihood of malignant small bowel obstruction resolving with conservative treatment alone (diagnostic). | no data | no data | no data |
| 2. The rate of resolution of MBO with medical management only in patients receiving OWSC compared with those not receiving it (therapeutic). | no data | no data | no data |
| 3. Gastrointestinal adverse effects (increased abdominal pain, nausea, vomiting). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 4. Extra‐abdominal complications (aspiration pneumonia, hypersensitivity reactions). | 9 (1) | ⊕⊝⊝⊝ | Although they reported that "no issues regarding safety or tolerability of either gastrografin or placebo were identified", no data were actually reported. |
| 5. Length of hospital stay. | no data | no data | no data |
| 6. Time from administration of OWSC to resolution of MBO. | no data | no data | no data |
| 7. Survival time from onset of inoperable MBO until death. | no data | no data | no data |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded twice for serious limitations to study quality (high risk of selective reporting and of attrition bias) and downgraded once for imprecision (sparse data). OWSC = oral water soluble contrast | |||

