引流液中的淀粉酶含量用于诊断胰腺切除术后胰瘘
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. | ||
| Patient characteristics and setting | Sample size: 182. | ||
| Index tests | Index test: postoperative day 3 drain fluid amylase. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. | ||
| Patient characteristics and setting | Sample size: 471. | ||
| Index tests | Index test: postoperative day 5 drain fluid amylase. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. | ||
| Patient characteristics and setting | Sample size: 65. | ||
| Index tests | Index test: post operative day 3 to 5 drain fluid amylase. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: prospective study.. | ||
| Patient characteristics and setting | Sample size: 50. | ||
| Index tests | Index test: postoperative day 5 drain fluid amylase. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. | ||
| Patient characteristics and setting | Sample size: 100. | ||
| Index tests | Index test: postoperative day 4 drain fluid amylase | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
ISGPF = International Study Group on Pancreatic Fistula
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition (target condition not defined adequately) | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Not a diagnostic test accuracy study. | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| Inappropriate population (patients without pancreatic fistula were excluded from the study) | |
| Inappropriate index test (drain fluid amylase was measured on post‐operative day 1 only) | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate population (not in patients undergoing pancreatic resection) | |
| Inappropriate index test (not on drain fluid amylase) | |
| Inappropriate index test | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| Inappropriate target condition | |
| Inappropriate population (people with amylase > 5000 IU were initially excluded; in addition, people with low unvalidated fistula risk score were excluded from the analysis) | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Not a diagnostic test accuracy study | |
| Inappropriate target condition | |
| Inappropriate index test | |
| Inappropriate target condition | |
| Not a diagnostic test accuracy study | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| Inappropriate target condition | |
| Inappropriate target condition | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| Inappropriate target condition | |
| Inappropriate target condition. | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Inappropriate target condition | |
| Inappropriate target condition | |
| Not a diagnostic test accuracy study | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Not a diagnostic test accuracy study | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| No diagnostic test accuracy data using appropriate reference standard | |
| Not a diagnostic test accuracy study (systematic review) | |
| Inappropriate target condition |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 POD:3 DFA > 600 IU/L Show forest plot | 1 | 182 |
| Test 1 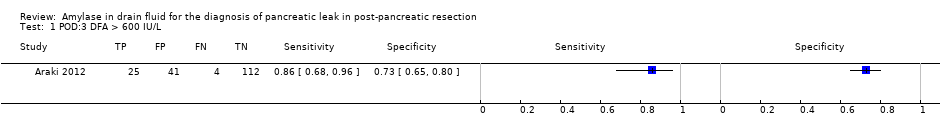 POD:3 DFA > 600 IU/L. | ||
| 2 POD:3 to 5 DFA > 3 times serum amylase Show forest plot | 1 | 65 |
| Test 2  POD:3 to 5 DFA > 3 times serum amylase. | ||
| 3 POD:4 DFA > 647 U/L Show forest plot | 1 | 100 |
| Test 3  POD:4 DFA > 647 U/L. | ||
| 4 POD:5 DFA > 3 times serum amylase Show forest plot | 1 | 50 |
| Test 4 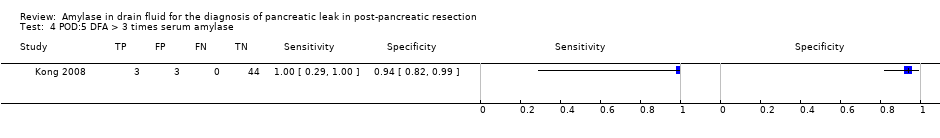 POD:5 DFA > 3 times serum amylase. | ||
| 5 POD:5 DFA > 4000 U/L Show forest plot | 1 | 471 |
| Test 5  POD:5 DFA > 4000 U/L. | ||
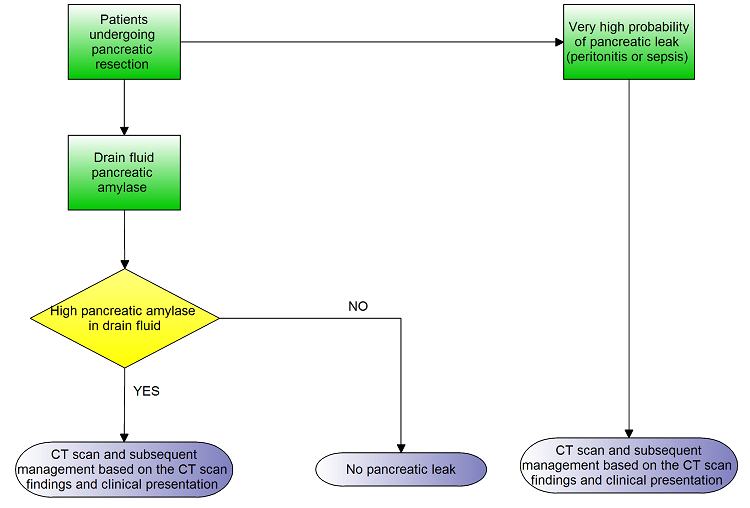
Clinical pathway

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Forest plot of tests: The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold. The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings.

Plot of sensitivity and specificity in the ROC (receiver operating characteristics) space: The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold. The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings.

POD:3 DFA > 600 IU/L.

POD:3 to 5 DFA > 3 times serum amylase.

POD:4 DFA > 647 U/L.

POD:5 DFA > 3 times serum amylase.

POD:5 DFA > 4000 U/L.
| Population | People undergoing pancreatic resection | ||||||||
| Setting | Secondary care in various countries | ||||||||
| Target condition | Clinically significant pancreatic leak | ||||||||
| Reference standard | International Study Group on Pancreatic Fistula (ISGPF) grade B or C | ||||||||
| Median prevalence of pancreatic leak | 15.9% | ||||||||
| Index test1 | Sensitivity | Specificity | Post‐test probability of a positive test2 | Post‐test probability of a negative test2 | Number of studies | Number of participants | Risk of bias | Applicability concerns | Plain language interpretation |
| POD:3 DFA > 600 IU/L | 0.86 (95% CI 0.68 to 0.96) | 0.73 (95% CI 0.65 to 0.80) | 37.9% (95% CI 31.1% to 45.1%) | 3.4% (95% CI 1.4% to 8.2%) | 1 | 182 | Unclear | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 38 people (95% CI 31 to 45) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 3 people (95% CI 1 to 8) have clinically significant pancreatic leak. |
| POD:3 to 5 DFA > 3 times serum amylase | 0.79 (95% CI 0.49 to 0.95) | 0.78 (95% CI 0.65 to 0.89) | 40.8% (95% CI 27.7% to 55.5%) | 4.9% (1.8% to 12.5%) | 1 | 65 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 41 people (95% CI 28 to 56) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 5 people (95% CI 2 to 13) have clinically significant pancreatic leak. |
| POD:4 DFA > 647 U/L | 0.72 (95% CI 0.53 to 0.86) | 0.91 (95% CI 0.82 to 0.97) | 60.7% (95% CI 41.1% to 77.4%) | 5.5% (95% CI 3.2% to 9.3%) | 1 | 100 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 61 people (95% CI 41 to 77) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 6 people (95% CI 3 to 9) have clinically significant pancreatic leak. |
| POD:5 DFA > 3 times serum amylase | 1.00 (95% CI 0.29 to 1.00) | 0.94 (95% CI 0.82 to 0.99) | 74.8% (95% CI 49.8% to 89.9%) | 0% (95% CI not estimable) | 1 | 50 | Unclear | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 75 people (95% CI 50 to 90) have clinically significant pancreatic leak. It was not possible to estimate the number of people with clinically significant pancreatic leak when the test was negative. |
| POD:5 DFA > 4000 U/L | 0.75 (95% CI 0.58 to 0.88) | 0.99 (95% CI 0.98 to 1.00) | 95.4% (95% CI 86.8% to 98.5%) | 4.6% (95% CI 2.6% to 7.8%) | 1 | 471 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 95 people (95% CI 87 to 99) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 5 people (95% CI 3 to 8) have clinically significant pancreatic leak. |
| Interpretation | The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. A negative test more or less rules out pancreatic leak. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings. | ||||||||
| 1The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold. 2All post‐test probabilities were calculated at the median prevalence (pre‐test probability) of pancreatic leak in the studies. At the lower quartile of the prevalence of 7.6%, the post‐test probabilities of pancreatic leak of positive POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 21.0% (95% CI 16.5% to 26.4%), 23.2% (95% CI 14.3% to 35.2%), 40.3% (95% CI 23.4% to 59.9%), 56.5% (95% CI 30.3% to 79.5%), and 90.0% (95% CI 74.2% to 96.6%) respectively. At the same pre‐test probability, the post‐test probabilities of pancreatic leak of negative POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 1.5% (95% CI 0.6% to 3.7%), 2.2% (95% CI 0.8% to 5.9%), 2.5% (95% CI 1.4% to 4.3%), 0% (95% CI not estimable), and 2.0% (95% CI 1.2% to 3.5%) respectively. At the upper quartile of the prevalence of 21.5%, the post‐test probabilities of pancreatic leak of positive POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 46.9% (95% CI 39.6% to 54.4%), 50.0% (95% CI 35.7% to 64.3%), 69.1% (95% CI 50.3% to 83.2%), 81.1% (95% CI 59.0% to 92.8%), and 96.8% (95% CI 90.5% to 98.9%) respectively. At the same pre‐test probability, the post‐test probabilities of pancreatic leak of negative POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 4.9% (95% CI 2.0% to 11.4%), 7.0% (95% CI 2.7% to 17.1%), 7.8% (95% CI 4.6% to 12.9%), 0% (95% CI not estimable), and 6.5% (95% CI 3.8% to 10.8%) respectively. CI = confidence intervals | |||||||||
| Grade | A | B | C |
| Clinical conditions | Well | Often well | Usually ill |
| Ultrasound/CT (computed tomogram) (if obtained) | Negative | Negative/positive | Positive |
| Persistent drainage (after 3 weeks) | No | Usually yes | Yes |
| Reoperation | No | No | Yes |
| Death related to postoperative pancreatic fistula | No | No | Possibly yes |
| Signs of infections | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Readmission | No | Yes/no | Yes/no |
| Modified from Bassi 2005. | |||
| Domain 1: Patient selection | Patient sampling | Patients who have undergone pancreatic resection with drain fluid at least 48 hours after pancreatic resection irrespective of the volume of the drain fluid. |
| Was a consecutive or random sample of patients enrolled? | Yes: if a consecutive sample or a random sample of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was included in the study. | |
| Was a case‐control design avoided? | Yes: if a cohort of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was studied. | |
| Did the study avoid inappropriate exclusions? | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included. | |
| Could the selection of patients have introduced bias? | Low risk of bias: if 'yes' classification for all of the above 3 questions. High risk of bias: if 'no' classification for any of the above 3 questions. Unclear risk of bias: if 'unclear' classification for any of the above 3 questions but without a 'no' classification for any of the above 3 questions. | |
| Patient characteristics and setting | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included. | |
| Are there concerns that the included patients and setting do not match the review question? | Low concern: if the patient characteristics and setting were classified as 'yes'. Unclear concern: if the patient characteristics and setting were classified as 'unclear'. High concern: if the patient characteristics and setting were classified as 'no'. | |
| Domain 2: Index test | Index test(s) | Amylase in drain fluid. |
| Were the index test results interpreted without knowledge of the results of the reference standard? | The index test would always be conducted though not interpreted before the reference standard. Yes: if the index test was conducted and interpreted without the knowledge of the results of the reference standard. | |
| If a threshold was used, was it pre‐specified? | Yes: if a pre‐specified threshold was used. No: if a pre‐specified threshold was not used. Unclear: if it was not clear whether the threshold used was pre‐specified. | |
| Could the conduct or interpretation of the index test have introduced bias? | Low risk of bias: if 'yes' classification for both of the above questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: if the criteria for positive index test was clearly stated. High concern: if the criteria for positive index test was not stated. | |
| Domain 3: Target condition and reference standard | Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak (requiring clinical intervention). Planned reference standards (see below).
|
| Is the reference standard(s) likely to correctly classify the target condition? | Yes: if pancreatic leak was confirmed at reoperation. Unclear: although we planned to exclude studies if the reference standard was not described adequately or was not one of the above planned reference standards, this would have meant that there would have been no studies included in the review. So, we accepted the ISGPF grades B and C as an appropriate references standard and classified the answer to this signalling question as unclear. | |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes: if the reference standard was interpreted without the knowledge of the results of the index test. | |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk of bias: if 'yes' classification for both of the above 2 questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. | |
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Although we anticipated that all of the included studies would be classified as 'low concern' because of the reference standards we planned to use, we have classified all the studies as 'high concern' because of the reference standards that we accepted. | |
| Domain 4: Flow and timing | Flow and timing | Patients may have progression or resolution of pancreatic leak if there is a long delay between index test and reference standard. An arbitrary 2 weeks was chosen as an acceptable delay between index test and reference standard. |
| Was there an appropriate interval between index test and reference standard? | Yes: if the time interval between index test and reference standard was less than 2 weeks. | |
| Did all patients receive a reference standard? | Yes: if all patients received a reference standard. | |
| Did all patients receive the same reference standard? | Yes: if all the patients received the same reference standard. Unclear: if this information was not clear. Because of the inclusion criteria, all the studies in this review were classified as 'yes' for this signalling question. | |
| Were all patients included in the analysis? | Yes: if all the patients are included in the analysis irrespective of whether the results were interpretable. | |
| Could the patient flow have introduced bias? | Low risk of bias: if 'yes' classification for all the above 4 questions. High risk of bias: if 'no' classification for any of the above 4 questions. Unclear risk of bias: if 'unclear' classification for any of the above 4 questions but without a 'no' classification for any of the above 4 questions. | |
| ISGPF = International Study Group on Pancreatic Fistula | ||
| Test | No. of studies | No. of participants |
| 1 POD:3 DFA > 600 IU/L Show forest plot | 1 | 182 |
| 2 POD:3 to 5 DFA > 3 times serum amylase Show forest plot | 1 | 65 |
| 3 POD:4 DFA > 647 U/L Show forest plot | 1 | 100 |
| 4 POD:5 DFA > 3 times serum amylase Show forest plot | 1 | 50 |
| 5 POD:5 DFA > 4000 U/L Show forest plot | 1 | 471 |

