Auf Eltern beschränkte Interventionen bei übergewichtigen oder adipösen Kindern im Alter von 5 bis 11 Jahren
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Cluster randomised controlled trial Randomisation ratio: initially 1 : 1 : 1, final 5 sites randomised 1 : 2 : 2 owing to higher drop‐out in the practices providing the 2 interventions Superiority design | |
| Participants | Inclusion criteria: aged 2‐8 years with a BMI ≥ 85th and ≤ 97th percentile based on Centers for Disease Control cut‐points (reference provided) Exclusion criteria: type 1 or type 2 diabetes, non‐English‐speaking parent, no working telephone, chronic medical disorders, chromosomal disorders, syndromes and non‐ambulatory conditions, medications known to affect growth, enrolment in a weight loss programme, seen by weight loss specialist in past 12 months Diagnostic criteria: as above | |
| Interventions | Number of study centres: ‐ Treatment before study: ‐ Titration period: ‐ BMI2 (Brief Motivational Interviewing to reduce body mass index): 1. Moderate‐intensity (4 sessions, 3 in year 1) primary care providers (PCP) motivational interviewing (MI) based counselling. PCPs received 2 days of in‐person training in MI and behaviour therapy and an interactive MI DVD training. Provided counselling sessions with a parent of the index child in year 1 and 1 additional "booster" visit in year 2 as well as usual care (described below). MI uses specific techniques such as reflective listening, autonomy support, shared decision‐making, and eliciting change talk. Focused on discrete behaviours, such as snack foods, sweetened beverages, fruits, vegetables, TV/screen time and physical activity/exercise. Provided positive feedback for healthy behaviours and then, collaboratively with the parent, identify behaviours that might be modified. Pre‐existing or new materials written in a style consistent with MI and self determination theory. Content emphasised child choice in making behaviour change. Self monitoring logs could be used, clinicians educational materials and logs specific to the family 2. High‐Intensity, PCP and dietician, intervention. Same intervention components as moderate‐intensity group (4 sessions with PCP) but added 6 MI‐based counselling (4 in year 1) from a trained dietician. Sessions were delivered either in‐person or by telephone. Dieticians given same training in MI as the PCPs 3. Usual care: routine care by the PCP, as well as standard educational materials for parents on healthy eating and exercise. Practitioners attended a half‐day orientation session that included current treatment guidelines PCPs and dieticians were incentivised for the number of sessions and the number of participants recruited additional payments for retaining 50% and 80% of cohort | |
| Outcomes | Outcomes reported in abstract of publication: BMI percentile | |
| Study details | Run‐in period: ‐ Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "to test the efficacy of moderate‐intensity (4 sessions) PCP MI‐based counselling and the effect of adding 6 MI‐based counselling sessions by trained dietitians delivered to parents of overweight youth aged 2 to 8 years recruited through primary care offices" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "randomly assigned" Comment: no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "open label" Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "open label" Comment: investigator‐assessed, low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: reported with reasons per practice |
| Selective reporting (reporting bias) | High risk | Comment: no secondary outcomes reported |
| Other bias | Low risk | Comment: no other bias |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: BMI ≥ the 85th percentile who primarily resided with the participating carer. Participants also had to speak English, be able to understand basic instructions and perform simple exercises Exclusion criteria: carers: non‐ambulatory, pregnant or have a medical diagnosis that may be negatively impacted by exercise. Parents whose children have a medical condition or developmental disorder that precludes weight loss using conventional diet and exercise methods Diagnostic criteria: BMI ≥ 85th percentile | |
| Interventions | Number of study centres: not reported Treatment before study: none Titration period: none NOURISH parenting intervention Based in Social Cognitive Theory (SCT), and emphasises parental role modelling as a primary way children learn health behaviours. Focuses on enhancing parents' self efficacy to make positive changes in eating and exercise behaviours. In addition, cognitive‐behavioural strategies such as self monitoring, contingency management and stimulus control incorporated. All sessions involved participatory activities, including self assessments, group discussions and experiential activities. These participatory experiences aimed to enhance overall intervention efficacy All sessions led by doctoral students in psychology working under the supervision of a licensed, clinical psychologist with specific training in group facilitation. Parents had a 1‐hour booster session 2 months after the intervention to allow parents to share with one another their successes, and to elicit suggestions from group leaders and fellow parents regarding barriers they have encountered Control parenting intervention Parents in the control group attended a group session moderated by an independent interventionist (a doctoral student in psychology). The session addressed the role of diet and exercise in paediatric overweight. Control participants were mailed publicly available brochures on paediatric overweight on 3 occasions during the study: between weeks 4 and 5, between weeks 8 and 9, and 2 months after post‐testing (the latter of which was meant to match the NOURISH booster session) Parents and children in both the intervention and control groups received a pedometer. Intervention parents also received a raffle ticket at each session for a USD 75 gift card, which will take place at the final session. Participants who attend the final session were given Certificates of Completion. All parents (i.e. intervention and control groups) were given USD 20 gift cards for completing the pre‐test, post‐test and the 6‐month follow‐up. The study provided childcare for all programme sessions and assessments | |
| Outcomes | Outcomes reported in abstract of publication: child BMI, parents satisfaction, parent behaviour change | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The primary aim of this study is to implement and evaluate the feasibility, acceptability, and preliminary effectiveness of NOURISH, a culturally sensitive, parent‐only skills‐based group intervention, and a single‐session, education‐only, control group (parent‐only) intervention on overweight children's BMI percentile. The secondary aim of this study is to evaluate the effectiveness of the intervention for improving children's dietary intake, body dissatisfaction, and quality of life. The impact of these two programs on adult participants will also be evaluated, including parental BMI and dietary intake. Parent satisfaction and feedback regarding the NOURISH intervention will also be elicited..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants will be randomly assigned (using a random number generator) to one of two parent‐only interventions..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: carers in the control arm were blinded to the aims and hypotheses of this study otherwise no details masking of treatment assignment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers of drop‐outs and withdraws provided and similar across groups. ITT analysis ‐ analysed all the data according to participants' assigned group, whether or not they actually completed the intervention. Used participants' most recent data as post intervention scores |
| Selective reporting (reporting bias) | High risk | Comment: many outcomes were collected but not reported ‐ though it was reported that these were not significantly different from baseline |
| Other bias | Unclear risk | Comment: although it was made clear why the trial team changed the protocol intervention time from 12 weeks to 6 weeks, this did not seem to help retain participants and meant if there was to be an effect it could not be shown in the health‐related quality of life of participants' children |
| Methods | Cluster randomised controlled trial Randomisation ratio: 1 : 1 Superiority design: | |
| Participants | Inclusion criteria: child classified as being overweight (not obese) according to international age and gender specific cut‐off points for BMI at the well‐child visit (country wide health visit in the year a child turns 5 years), attended between September 2007 and October 2008. Parents with at least basic Dutch language skills Exclusion criteria: obese children with chronic health problems that many influence outcomes Diagnostic criteria: not reported | |
| Interventions | Number of study centres: 9 (44 teams) Treatment before study: none Titration period: none Parent‐only intervention: motivational interviewing if needed, with information about overweight prevention and healthy lifestyle choices. Initiated at the well‐child visit and up to 3 structured counselling sessions could be offered at approximately 3, 6 and 12 months later. The session content depended on the stage of behavioural change of the parents (individually tailored). Motivation was assessed by Youth Health Care professionals by creating awareness of the child's weight status and associated consequences. 4 lifestyle‐related behaviours could be promoted: playing outdoors, eating breakfast, reducing sweet drinks and sedentary behaviour. Parents choose 1 or 2 behaviours to target during the sessions. Advice was by international guidelines. Information materials provided, diet and activity diaries discussed and family‐oriented action plans to change health‐related behaviour documented Youth Health care professionals were provided with a half‐day training in motivational interviewing techniques, were provided with a workbook with information and practical examples and an information sheet with step‐by‐step guide to how the information could be applied Control group: parents informed of overweight of their child but usual care (general information about a healthy lifestyle) given | |
| Outcomes | Outcomes reported in abstract of publication: BMI, minutes of outside play or TV viewing, having breakfast, number drinks of sweet beverages | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐review publication | |
| Stated aim for study | Quote from publication: "Children who are overweight (not obese) visiting YHC teams…would have a less increase in BMI and waist circumference at follow‐up compared to overweight children visiting YHC teams allocated to the control condition, performing usual care" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "within each centre, YHC teams were randomised... by means of a computer‐generated random number list… random permuted blocks… lengths were 4 or 6 depending on the number of teams per Municipal Health Service" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of allocation concealment provided by study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from publication: "parents were not aware of the research condition they were allocated to" Comment: personnel were aware of allocation. Investigator‐assessed outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "... research assistants [measuring weight and height] were blinded to the research condition." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2 clusters (1 per arm) not analysed. Also states used ITT, numbers analysed for BMI and waist circumference outcomes differ, assume because of ICC for clustering |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes stated are reported and response from study author concurs |
| Other bias | Unclear risk | Comment: 211 parent‐child dyads in the intervention group did not receive any of the counselling sessions |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: parents of overweight or obese 4‐ to 8‐year‐old Exclusion criteria: children who had uncontrolled medical problems (e.g. asthma) that might preclude them from fully participating in the intervention Diagnostic criteria: described only as overweight or obese | |
| Interventions | Number of study centres: unclear Treatment before study: none Titration period: none Before each of the sessions for either group, parents were provided with age‐appropriate, audio‐taped, educational information on a range of topics. Each group had 4 face‐to‐face sessions held for 30‐60 minutes and 3 telephone calls between sessions (duration unclear) Parent intervention: Theoretically based intervention. Parents were offered educational information about the establishment of healthy habits in young children, nutritional information, information regarding increasing physical activity and decreasing sedentary time, and age‐specific information regarding the child's behaviour in response to change 4 face‐to‐face sessions during which trained research assistants used principles of brief motivational interviewing (i.e. elicit information from the participant, provide non‐judgemental information, and elicit the participant's understanding; to collaborate with parents on identifying specific realistic healthy lifestyle goals, developing clear steps to reach those goals, routinely having the parents re‐evaluate progress, and identifying new goals as needed) Parents were provided with specific feedback about their child (i.e. physical activity and dietary intake) before establishing goals for their child and family. All face‐to‐face parent intervention sessions were separated by 4‐6 weeks to provide each family with time to enact planned changes, encounter child responses to those changes and review new educational information before the next face‐to‐face session Telephone calls were made to each parent to:
At each of the 4 measurement time points, parents were offered USD 35 as remuneration for their time in completing the various measurements. Each child was given a group specific (e.g. treatment group and control group) bag of toys to facilitate activities that parents would be encouraged to complete with their child Sessions taken by trained research assistants who were also supervised Control: Parents were provided with educational age‐appropriate, evidence‐based health and safety information (e.g. care for thermal injuries, first‐aid care, and care for insect bites and stings) that is specific to parenting in the southwest US. Parents met with a control interventionist and in a similar way were encouraged to make health and safety goals for their family (e.g. development of first‐aid materials and identification of a fire escape plan) | |
| Outcomes | Outcomes reported in abstract of publication: waist circumference, waist‐by‐height ratio, BMI and BMI percentile | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The purpose of this randomised control pilot study was to determine the feasibility and preliminary effects of a theoretically based, primary care intervention on the physical outcomes of 60 overweight/obese preschool/early school‐aged 4‐ to 8‐year‐old children..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "each parent‐child dyad was randomly assigned to the treatment or control condition..." Comment: no description provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | Low risk | Quote from publication: "Seven parent‐child dyads returned incomplete T1 data, and thus we removed data from those dyads from all other analyses. As a result, experimental and control group sample sizes were 33 and 27, respectively" Comment: multiple imputation techniques to account for the missing data in subsequent analyses for the remaining 60 parent‐child dyads |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1 : 1 Superiority design | |
| Participants | Inclusion criteria: aged 7 years, ≥ 95th percentile of BMI for age Exclusion criteria: mental retardation, psychiatric symptoms, current obesity treatment, chronic disease and use of medication Diagnostic criteria: obesity defined as BMI 95th percentile for age by 2000 reference standards | |
| Interventions | Number of study centres: 1 Treatment before study: none Titration period: none Parent‐only: 12 session training programme over 6 months 8 weekly sessions for the first 2 months, then 4 monthly sessions. Each session lasts 4 hours, including a review of parent progress in implementing strategies developed for changing child's eating or exercise habits, and the specific topic of the day, such as learning about the reasons of the childhood obesity, receiving nutritional information (e.g. food pyramid, food choices, food labels, food preparation and cooking, eating habits, regular meals, controlling environments that stimulate overeating, special dietary consideration during holidays and at the restaurants) and guidelines for physical activity and reducing sedentary behaviours (e.g. reduce watching TV and playing computer games, use stairs instead of lifts and play outside instead of inside) Control: 2 sessions of training programme (occurred after intervention group's 6‐month training programme), no details provided | |
| Outcomes | Outcomes reported in abstract of publication: weight, waist and hip circumference, cholesterol, serum triglycerides, food group consumption, TV and computer time, walking time | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Peer review publication | |
| Stated aim for study | Quote from publication: "to assess the effect of lifestyle modification family‐based intervention in young Iranian children" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "children got a code number and were randomly divided into two groups" Comment: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to judge |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: adjudicated/investigator‐assessed, no other details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | High risk | Comment: numbers only reported, numbers in flow chart and results do not match, differential drop‐out rates |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: child aged 6‐12 years, 20‐85% overweight, medical clearance form a physician Exclusion criteria: secondary overweight caused by endocrinological, chromosomal or hypothalamic disease or mental retardation Diagnostic criteria: as above | |
| Interventions | Number of study centres: 1 Treatment before study: none Titration period: none Parent‐only intervention: 6 group meetings of 2 hours each over a 5‐month period. Provided information with a focus on weight control not weight loss to re‐establish a sense of healthy balance between energy intake and energy expenditure. Parent workbook. Education on different food groups, detailed product information and child‐friendly recipes. Used the Food Pyramid. Parenting skills focused on understanding of eating habits and lifestyles, cognitive and behavioural barriers to change, general parenting skills of positive involvement, monitoring, problem‐solving skills and maintaining positive changes already effected; self control and healthier lifestyle sessions (full details of content provided) Sessions conducted by dietician and a psychologist under supervision of a behavioural therapist and a manual for each session was available Control: Waiting list control | |
| Outcomes | Outcomes reported in abstract of publication: BMI, parental report of child's eating behaviour, familial health principles | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Commercial funding Peer review publication | |
| Stated aim for study | Quote from publication "To evaluate a parent‐led intervention for overweight children between 6 and 12 years old" | |
| Notes | Also report a follow‐up study comparing all families post intervention with a sample of families who did not respond to the original invitation. Not relevant here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from publication: "randomly assigned on the basis of the date on which they contacted the research group" Comment: high risk |
| Allocation concealment (selection bias) | High risk | Comment: assignment by a co‐worker, no other details |
| Blinding of participants and personnel (performance bias) | High risk | Quote from study author: "Participants knew whether they would start immediately (intervention group); or had to wait (waitlist condition)" Comment: no details blinding of study personnel |
| Blinding of participants and personnel (performance bias) | High risk | Quote from study author: "Participants knew whether they would start immediately (intervention group); or had to wait (waitlist condition)" Comment: no details blinding of study personnel |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no details, as height and weight considered by reviewers as a subjective outcome as were obtained by parental report |
| Blinding of outcome assessment (detection bias) | High risk | Quote from study author: "height and weight were obtained by parental report" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: questionnaire completion rates provided and some withdrawn by study authors as missing items |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: reports numbers but not reasons for drop‐out. All drop‐outs from waiting list control group so some imbalance. No description of how analysed |
| Selective reporting (reporting bias) | High risk | Comment: subjective outcomes not reported at baseline or follow‐up for the 2 groups separately but are reported for both combined |
| Other bias | Unclear risk | Comment: is reported to be a pilot study so unclear if sample size is appropriate to detect a difference |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1 : 1 : 1 Superiority design | |
| Participants | Inclusion criteria: ≥ 85th percentile for BMI as determined by the Centers for Disease Control growth charts, having no dietary or physical activity restrictions Exclusion criteria: participating parent could not read English, had a psychological disorder that would impair ability to participate or if the family was planning to move out of the area during the programme Diagnostic criteria: BMI ≥ 85th percentile | |
| Interventions | Number of study centres: not reported Treatment before study: none Titration period: none Behavioural parent‐only intervention: 8 sessions, 45 minutes each Focused on increasing child growth monitoring and providing feedback to families 2 interventions that combined the parent‐only intervention with a 6‐month, behavioural, parent‐only intervention that focused on 2 energy‐balance (diet) behaviours: Decreasing sugar sweetened beverage and sweet and salty snack food intake. Children and parents reduced intake of sweet and salty snack foods (i.e. candy, cookies, ice cream, chips, nuts) to ≤ 3 servings/week, and sugar sweetened beverages (i.e. soda, Kool‐aid, sweetened tea, non‐100% fruit juice, sports drinks) to ≤ 3 servings/week Increasing fruit, vegetable and low‐fat dairy intake. Children and parents were encouraged to consume 2 servings/day of whole fruit, 3 servings/day of vegetables and 2 servings/day of low‐fat dairy products Families received USD 20 for completing each of the 6‐ and 12‐month assessments Based on behavioural economics theory (changing a substitute behaviour of a target behaviour enhances the feeling of choice for engaging in and liking the targeted behaviour, which could increase long‐term adherence). Meetings were led by an experienced research‐staff therapist (either master or doctoral level) with expertise in nutrition or exercise science, and behaviour modification Following the 6‐month intervention, all families received feedback on growth at 9 months, and final assessments were conducted at 12 months | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, energy intake | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "to examine the efficacy of U.S. primary care paediatric obesity treatment recommendations, within two randomised trials" | |
| Notes | Authors provided change data from baseline to immediate and longest follow‐up for BMI z score, following contact to request further data. There are 2 comparisons of relevance to this review, the parent‐only vs. the decrease group and the parent‐only vs. the increase group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: masking of carers and participants to allocation but unclear if blinded to intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "Dependent measures were collected by trained research‐staff blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis. Missing data filled using a multiple imputation strategy. Specifically, for each participant with a missing BMI z score value, 5 random variables from a normal distribution that has a mean equal to the baseline BMI z score and variance equal to the estimated variance for BMI z score of other participants at the time where BMI z score is missing |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1 : 1 : 1 Superiority design | |
| Participants | Inclusion criteria: ≥ 85th percentile for BMI as determined by the Centers for Disease Control growth charts, having no dietary or physical activity restrictions Exclusion criteria: participating parent could not read English, had a psychological disorder that would impair ability to participate or if the family was planning to move out of the area during the programme Diagnostic criteria: BMI ≥ 85th percentile | |
| Interventions | Number of study centres: not reported Treatment before study: none Titration period: none Description of interventions Behavioural parent‐only intervention: as above. 2 additional interventions were: Decreasing sugar‐sweetened beverage intake and increasing physical activity. Encouraged children to reach 60 minutes/day (parents 30 minutes/day) of moderate‐intensity physical activity most days of the week and for children and parents to consume ≤ 3 servings of sugar‐sweetened beverages/week. Increasing low‐fat milk intake and decreasing TV watching, encouraged children and parents to watch ≤ 2 hours of TV/day and to consume 2 servings of low‐fat milk/day | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, energy intake | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "to examine the efficacy of U.S. primary care paediatric obesity treatment recommendations, within two randomised trials" | |
| Notes | There are 2 comparisons of relevance to this review: the parent‐only vs. the substitute group and the parent‐only vs. the increase group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments..." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: masking of carers and participants to allocation but unclear if blinded to intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "Dependent measures were collected by trained research‐staff blinded to treatment assignment.." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis. Missing data filled using a multiple imputation strategy. Specifically, for each participant with a missing BMI z score value, 5 random variables from a normal distribution that has a mean equal to the baseline BMI z score and variance equal to the estimated variance for BMI z score of other participants at the time where BMI z score is missing |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: aged 5.0‐9.9 years, overweight (International Obesity Task Force definition) and prepubertal (Tanner stage 1), having a carer willing to attend sessions and able to speak English Exclusion criteria: BMI z score of 4.0, having a syndromal cause of obesity, using medications that influence weight, having a physical or developmental disability, having a chronic illness or having a sibling enrolled in the study Diagnostic criteria: overweight (International Obesity Task Force definition) | |
| Interventions | Number of study centres: 3 Treatment before study: none Titration period: none 24‐week intervention delivered by a dietician (accredited training for the parenting skills component) 2 groups: parent healthy lifestyle group and healthy lifestyle group The interventions included 12 (parent group) or 8 (healthy lifestyle) 90‐ to 120‐minute group sessions (open to both parents but mostly attended by mothers) and 4 telephone sessions, delivered over 6 months with tapered frequency (weekly, bimonthly, then monthly). Details of the standardised parenting skills programme and the healthy lifestyle education sessions reported (references given). The 4 telephone sessions alternated with the last 4 group sessions for both arms, using a standard protocol Parenting healthy lifestyle: The Positive Parenting Program (Triple P) was delivered in 4 sessions before the healthy lifestyle component. It is a standardised and evaluated generic parenting programme widely used in Australia and provides comprehensive facilitator training The 8 sessions included recommendations on specific core food servings; practical skills for healthy eating, reduced sedentary behaviours and increased activity; and monitoring of lifestyle behaviours and roles and responsibilities around eating, managing appetite, self esteem and teasing. Children and siblings participated in fun, non‐competitive activity sessions run by physical activity educators. These sessions provided optional active child care for participants in both groups and were not part of the intervention | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist z score | |
| Study details | Run‐in period: no clear run‐in period Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "... to evaluate (1) the effectiveness of a healthy lifestyle intervention for overweight children aged 5 to 9 years that targets parents as the agents of change and (2) whether additional specific parenting skills training would improve parenting skills and enhance the intervention effect. The long‐term effect (2 years from baseline) and the immediate postintervention effect (at completion of the intervention, 6 months from baseline) were assessed. We also aimed to confirm gender differences reported in our previous study..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: ".. After baseline measurements, participants were allocated to intervention groups using computer‐generated randomisation schedules stratified according to gender and recruitment site and prepared by staff not otherwise involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Allocation was concealed in opaque, sequentially numbered, sealed envelopes and opened by parents after completion of baseline measurements..." |
| Blinding of participants and personnel (performance bias) | High risk | Comment: a single‐blinded randomised controlled trial blind at point of allocation, but not blinded to which intervention they received |
| Blinding of participants and personnel (performance bias) | High risk | Comment: a single‐blinded randomised controlled trial blind at point of allocation, but not blinded to which intervention they received |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: those who took measurements were blinded to type of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: those who took measurements were blinded to type of intervention |
| Incomplete outcome data (attrition bias) | High risk | Comment: stated ITT was conducted using all available data according to allocation, regardless of attendance (details provided). However, a second per‐protocol analysis was performed that included only those who attended ≥ 75% of the programme sessions. The potential effect of missing data were explored using T tests to compare the baseline and 6‐month BMI z scores of those who remained and those who were lost to follow‐up. No reasons for drop‐outs were given. It is unclear whether data presented were ITT or not |
| Incomplete outcome data (attrition bias) | High risk | Comment: stated ITT was conducted using all available data according to allocation, regardless of attendance (details provided). However, a second per‐protocol analysis was performed that included only those who attended ≥ 75% of the programme sessions. The potential effect of missing data were explored using T tests to compare the baseline and 6‐month BMI z scores of those who remained and those who were lost to follow‐up. No reasons for drop‐outs were given. It is unclear whether data presented were ITT or not |
| Selective reporting (reporting bias) | High risk | Comment: states health‐related quality of life measured but no data reported |
| Other bias | Unclear risk | Comment: no true control group. Parenting outcomes only given for the whole sample, not split into intervention and control groups ‐ therefore success of intervention in each group cannot be compared. Retention rates moderate for the long follow‐up period. Unclear if ITT analysis was performed. Likely the participants who dropped out the study were more overweight |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: parents participated in the treatment voluntarily. Children's percentage of overweight had to be at least 130% Exclusion criteria: none stated Diagnostic criteria: overweight (as above) | |
| Interventions | Number of study centres: 3 Treatment before study: none Titration period: none Description of interventions: Parental CBT: 'Finger in the pie' was a cognitive behavioural treatment and each session addressed a different theme associated with childhood overweight. The purpose was not purely to present information, but to teach parents to think of alternatives and possible solutions themselves. This way, future coping abilities were addressed. The following themes were included: creating realistic expectations concerning the development of their children's weight status, modifying eating and exercising habits, knowledge on how parents can influence the behaviour of their children (e.g. by modelling and by the use of control and rewards), information on the development of overweight, handling feelings of guilt, and recognising and handling a child with low self esteem. So, instead of purely focusing on nutrition and physical activity, a substantial part of the treatment was devoted to enhancing parenting tactics (e.g. teaching parents to ignore undesirable behaviours and to reward desirable behaviours). This aspect of the treatment combined with extensively discussing parental control makes the current intervention distinguishing Behavioural and nutritional components Intervention carried out according to a protocol, written by the first and the second authors and carried out by trained cognitive behavioural therapists Waiting list control: Offered the treatment after 6 months | |
| Outcomes | Outcomes reported in abstract of publication: BMI percentile, relapse, psychopathology, self esteem and negative thoughts | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Funding not stated Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The aim of the current study is to investigate whether a treatment that aims at parents exclusively (by targeting eating and exercise behaviours, and supporting self‐esteem of the child in a cognitive‐behavioural manner) would be successful in reducing their children's overweight. Such a treatment might lead to better results than treatments focusing on children, as parents play an important role in their children's eating and exercising behaviours, and in promoting their self‐esteem..." | |
| Notes | Randomisation was broken because 9 participant families from the waiting list control were included in the intervention arm, the study did not report the numbers randomised to each group, just the total numbers randomised, the group sizes were unbalanced (59 vs. 39), and the paper states that 9 were 'included in the treatment group' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Parents were randomly assigned to either the treatment group or the waiting‐list control group" Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis. The missing values at the follow‐up meeting were replaced by the last‐observation‐carried‐forward. The missing in‐between values (post‐treatment) were replaced by the mean of the values before treatment and at 3‐month follow‐up. Numbers of drop‐outs reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | High risk | Comment: as only 9 of the 48 families in the waiting‐list control group eventually decided to participate in the treatment after the waiting period, it was decided to include these 9 families in the treatment group, and to disregard their data from the waiting period. These 9 families did not differ from the original treatment group or the control group |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: overweight or obese (defined according to age‐ and sex‐specific international BMI cut‐off points (reference provided); aged 5‐9 years; pre‐pubertal (no pubic hair ‐ Tanner stage 1); generally healthy Exclusion criteria: extreme obesity (BMI z score > 4); known syndromal cause of obesity; long‐term steroid use; medications associated with weight gain; chronic illness; significant dietary restrictions Diagnostic criteria: overweight or obese (defined according to age‐ and sex‐specific international BMI cut‐off points | |
| Interventions | Number of study centres: not reported Treatment before study: not reported Titration period: not reported Parent DIET Intervention: Aimed at parents, not children. Informs parents of how to improve their child's diet. Based on the "Health Belief Model" (reference provided). Delivered by a dietician Parent and child physical activity (child was main focus): Parent encouraged to set realistic short‐ to medium‐term SMART goals for increasing physical activity and reducing sedentary behaviours. Asked to identify barriers in their family lives that may prevent their child from participating in sufficient physical activity or that leads to their child spending excessive amounts of time in small screen recreation Children attended 10 x 2‐hour face‐to‐face weekly sessions. Each week children participate in a variety of activities aimed at improving their mastery of 12 fundamental movement skills (run, jump, leap, hop, slide, gallop, strike, roll, kick, throw, catch, bounce). Each session covered 3 fundamental movement skills, such that over the course of the 10 weeks each skill is re‐visited, although the focus is on more complex components of the skill, in subsequent sessions. Skill mastery is aided by adherence to lesson plans for each skill incorporating several learning stages:
1 Refresher session (2 hours) attended at week 18; 3 monthly telephone calls at weeks 14, 18 and 22 of the invention To maximise the children's competence and confidence, they were strongly encouraged to practice the fundamental movement skills at home with their parents or siblings (or both), between each group session. Each participant given a 'Home‐challenge folder', which included fun, relevant and developmentally appropriate activities enabling practice of skills at home. The home challenges took approximately 30 minutes and children were encouraged to complete 3 sessions each week Based on the "Competence Motivation Theory" (reference provided). Delivered by physical education teachers Same components as the DIET and physical activity interventions. 25 sessions. Delivered by physical education teachers and dieticians. | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist measurements, metabolic outcomes | |
| Study details | Run‐in period: not reported Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "Outcomes of childhood obesity interventions are rarely reported beyond 1 year. We hypothesized that the impact on the BMI z score from a child‐centred physical‐activity program in combination with a parent‐entered dietary‐modification program would be greater than either program conducted alone at 24 months' after baseline" | |
| Notes | There were 2 comparisons of relevance to this review: the parent‐only vs. parent‐child physical activity group and the parent‐only vs. the parent‐child physical activity and diet group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "To randomly allocate participants to one of the three intervention groups the bias coin method of allocation, using a computer‐based random number‐producing algorithm, is used. This method ensures an equal chance of allocation to each group. Stratification by gender and site is done to ensure an equal representation in groups at each site. Only one study member at each site has access to the allocation codes and these are stored on a password‐protected computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "Outcome measures were assessed at baseline and 6, 12, and 24 months by trained assessors blinded to group assignment..." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: "Outcome measures were assessed at baseline and 6, 12, and 24 months by trained assessors blinded to group assignment..." |
| Incomplete outcome data (attrition bias) | High risk | Comment: ITT performed, however, high numbers not completing (35% to 51% completers after 2 years) |
| Incomplete outcome data (attrition bias) | High risk | Comment: ITT performed, however, high numbers not completing (35% to 51% completers after 2 years) |
| Selective reporting (reporting bias) | High risk | Comment: only the 24‐month results are shown |
| Other bias | Unclear risk | Comment: not enough information to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Non‐inferiority design: 1‐sided confidence interval | |
| Participants | Inclusion criteria: parents and their overweight or obese (> 85th BMI percentile) children, aged 8‐12 years. At least 1 parent or guardian participated with the child. If 2 children in the family met criteria for the study, both were invited to attend the treatment groups but a coin‐flip was used to determine which child's data would be part of the study Exclusion criteria: either the child or parent currently involved in psychological or weight loss treatment, using medications that affected weight or appetite, or had a psychiatric or physical condition (e.g. eating disorder, psychosis) that would interfere with participation Diagnostic criteria: as above (> 85th BMI percentile) | |
| Interventions | Number of study centres: 2 Treatment before study: none Titration period: none Parent‐only: Behavioural change skills; included self monitoring of targeted behaviours, positive reinforcement, stimulus control, pre‐planning and modelling. Parents in the parent‐only group were coached on how to assist their children in weight monitoring at home and reflect on the behaviours that influenced weight. Goal setting for the parent‐only group occurred during the treatment groups. Completed quizzes each week to assure knowledge of the treatment protocol. The intervention was theoretically based: using a current state‐of‐the‐art behavioural treatment for childhood obesity described by Epstein and colleagues (references provided) All interventionists attended a 3‐day training regarding the behavioural intervention for the study, and were supervised by the first author on a weekly basis during treatment. The intervention was provided by a psychologist Parent‐child intervention: Components of the interventions in common between the groups included:
| |
| Outcomes | Outcomes reported in abstract of publication: inferiority of treatment group on child weight loss, parent weight loss and child physical activity, caloric intake | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "to evaluate whether a standardized behavioral parent only treatment program would not be inferior to a standardized behavioral parent‐child program on child weight loss and other relevant markers of change" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 80 parent‐child dyads were randomly assigned to either parent‐child or parent‐only groups. Random assignment was conducted after completing the initial assessment using a computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | High risk | Comment: ITT analysis but data not presented. A multiple imputation approach was used as a form of sensitivity analysis, which yielded non‐substantive differences with full‐likelihood approach to analysing the data that are reported. Parent‐only: 24/40 pairs completed follow‐up. Parent‐child: 28/40 pairs completed follow‐up. No further details |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: parent described the child's body size as overweight, the child was 4‐11 years of age, and the parent agreed to attend a 12‐week intervention Exclusion criteria: child taking medication that affected growth or weight control, or had a severe developmental delay or disability Diagnostic criteria: parental description of child as overweight | |
| Interventions | Number of study centres: 6 Treatment before study: none Titration period: none Parent‐only: 12‐week intervention, 1 session per week. Group Lifestyle Triple P is a modification of Level 4 Group Triple P tailored to the concerns of parents of overweight and obese children. The intervention is a 12‐week intervention that consists of 9 x 90‐minute group sessions and 3 x 20‐minute telephone sessions. To help parents acquire new knowledge and skills, all sessions used an active skills training process (e.g. demonstrating and rehearsing skills) within a self regulation framework (e.g. self selecting goals and self evaluating progress). Each parent received a workbook summarising the session content, and suggested between‐session tasks Triple P programme was standardised. All sessions were facilitated by a clinical psychologist and accredited provider of Group Triple P (who co‐authored the intervention materials), with assistance from graduate students in nutrition and dietetics, physical education and psychology Control: Waiting list control for 12 weeks. Included a physical activity and nutritional advice components | |
| Outcomes | Outcomes reported in abstract of publication: child BMI z score and weight‐related problem behaviour, confidence in managing children's weight‐related behaviour | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "..to evaluate the effects of the intervention on parenting and child weight‐related behaviour, relative to a waiting list control condition. This study describes the evaluation of a lifestyle‐specific parenting program (Group Lifestyle Triple P) on multiple child and parent outcomes..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Families were randomly allocated to either the intervention...or the waiting list control...Groups were allocated to conditions according to a list of computer generated random numbers..." |
| Allocation concealment (selection bias) | High risk | Quote from publication: "Allocation concealment and blinded outcome assessment were not possible due to limited staff and resources" Comment: no masking of allocation to intervention or control |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no masking |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no masking |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no masking |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no masking |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers missing reported and reasons explained; ITT analysis with the last point‐carried‐forward |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers missing reported and reasons explained; ITT analysis with the last point‐carried‐forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: eligible parents must have had a child: (a) with a BMI ≥ 85th percentile, (b) enrolled at 1 of the 2 study schools, (c) between Grades K and 5, (d) parents also had to agree to be randomised to either 1 of the 2 study conditions. In instances when families had multiple children enrolled at a study school with BMIs ≥ 85th percentile, the oldest child was considered the index child Exclusion criteria: none stated Diagnostic criteria: 85th percentile BMI | |
| Interventions | Number of study centres: 2 Treatment before study: none Titration period: none Focus groups with 9 parents helped to determine study content, messages, and potential use of the 2 delivery methods, 1‐to‐1 counselling and mailed materials Educational mailed materials: Posted approximately every 5 weeks, 6 mailings over 30 weeks The educational material included: tips to increase walking, talk with children about TV viewing. Received a physical activity book and a pedometer. Read nutrition labels, shop more healthfully at grocery stores, talk with children about eat out healthfully. Received a cookbook, had a hands‐on activity about portion sizes Educational material plus personal encounters: The type of encounter (home visit, telephone call, etc.) was based on participating parents' preferences and schedules. Parents selected the topics discussed during each visit from choices provided by community health workers (CHWs) (i.e. epidemiology of childhood overweight, biological, social and environmental influences on childhood overweight; basic nutrition; label reading; grocery shopping strategies, including a tour of a local grocery store; physical activity guidelines) Mean 3.4 personal encounters over the 30 weeks. On average, parents received encounters for 18 weeks Both interventions were delivered by CHWs who attended a 36‐hour training programme over the course of 6 days. The purpose of the training was to prepare CHWs to make evidence‐based recommendations to families such as changing to reduced fat milk, reducing the intake of snack foods, replacing sugar‐sweetened beverages with water, increasing fruit and vegetable intake, decreasing TV viewing and increasing physical activity. The topics covered during the training included guidelines for home visits; the epidemiology of childhood overweight, biological, social and environmental influences on childhood overweight; basic nutrition; label reading; grocery shopping strategies, including a tour of a local grocery store; physical activity guidelines and counselling strategies. CHWs practiced counselling skills during their training by engaging in role‐plays with each other. After training and throughout the study, the study staff and CHWs met monthly to discuss specific concerns or difficulties with study participants | |
| Outcomes | Outcomes reported in abstract of publication: BMI | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The goal was to create an easy‐to‐use parent outreach model that could ultimately be used by school nurses, paediatricians, community health agencies, and CHWs" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Parents from two schools were randomly selected to participate in one of two study groups and were randomised to either one of the two study conditions..." Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | High risk | Comment: no ITT analysis. In all, 42/46 (91%) parents completed post‐intervention surveys |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: BMI ≥ 85th percentile for their age Exclusion criteria: plans to move out of the state during the course of the study, a request by the child's paediatrician that the family not be contacted Diagnostic criteria: BMI ≥ 85th percentile for their age | |
| Interventions | Number of study centres: not reported Treatment before study: not reported Titration period: not reported Based on social‐ecological theory (reference provided). 3 intervention groups: workbook, group sessions, group sessions + interactive voice response (IVR) counselling Parent group + IVR counselling: Group sessions as above. Then 10 follow‐up sessions by IVR, 1 per week. Included behavioural, physical activity and nutritional components (12‐week intervention) Physical activity get at least 2.5 hours of moderate physical activity this week; make a list of your family's barriers to physical activity around the house, and come up with a way to overcome them; do something active with your child for 15 minutes at least 3 times this week; review the family goal sheet with your family and set measurable, specific and objective goals this week; decrease your sitting time by 0.5 hours each day this week; increase your physical activity by 1 day per week; set a goal to take your family to a new park or trail that you have not visited before; survey your family regarding physical activity that they would like to do as a family, and try to do that activity at least 3 times per week Nutrition parent healthy habits: set a good example for your child by eating 5‐9 servings of fruits and vegetables every day this week; drink low‐fat milk at 1 meal each day; clear the kitchen cupboards of unhealthy snacks; prepare at least 1 healthy meal together with your child; post signs at least twice this week about your family's positive changes with healthy eating; decrease your soda and sugared‐drink consumption by 1 serving per day; increase your servings of fruits and vegetables by 3 per day; check your library for cookbooks, recipes or videos that help your family to prepare nutritious meals; change 1 food item that is high‐fat to a healthy snack of fruit or vegetable Behavioural consistency and contingency; communication; praise; parenting skills; support; plan; commitment Group sessions were led by a dietician (no further details) Parent group sessions: 2 group sessions. Utilised the workbook used in the control condition. Addressed parents' behavioural health skills and knowledge of weight, nutrition and physical activity. It also identified key parenting skills: limit setting, effective communication and role modelling. This session concluded with role playing, problem solving and the development of an action plan. 24‐week intervention Parent workbook group: 61‐page workbook to promote physical activity and the consumption of fruits and vegetables and decrease sugared‐drink consumption and TV viewing/recreational computer time. Activity to explore parental beliefs about eating and physical activity, healthy habits for creating a healthy family, defining the division of responsibility for eating and activity. Physical activity: using FITT (frequency, intensity, time, type) principles. Nutrition: helping children to avoid fad diets, reading labels, selecting healthy food options, sample menus, tips for preparing healthy snacks and meals. Assessing and calculating BMI in children and adults, causes of overweight in children (biological, cognitive, environmental), 5 reasons children gain weight, impact of being overweight, parenting skills to support weight reduction, survey of the family home environment, ways to promote a healthy home environment, goal setting: creating a family action plan, process of goal setting and keeping objectives clear, parent's personal action plan, barriers and strategies to maintaining family action plan. 24‐week intervention | |
| Outcomes | Outcomes reported in abstract of publication: child BMI z scores, symptoms of eating disorders and body image | |
| Study details | Run‐in period: not reported Study terminated before regular end (for benefit/because of adverse events): not reported | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "A practical RCT [randomised controlled trial] evaluated the relative effectiveness of three interventions to support parents of overweight or at‐risk children to change the home environment to foster more healthful child eating and activity behaviours, thereby reducing child BMI and BMI z scores. A secondary purpose was to determine the patterns of use and potential dose effect for the highest‐intensity intervention" | |
| Notes | There were 3 comparisons of relevance to this review: the parent‐only group and IVR vs. the control (workbook); the parent‐only group vs. the control; the parent‐only group and IVR vs. the parent group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Through a random‐numbers table, participants were assigned randomly (families/staff unblinded) to the FC‐workbook, the FC‐group, or the FC‐IVR intervention..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) | High risk | Comment: families/staff were both unblinded |
| Blinding of participants and personnel (performance bias) | High risk | Comment: families/staff were both unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Comment: families/staff were both unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: families/staff were both unblinded, but probably no substantial impact on outcome measures |
| Incomplete outcome data (attrition bias) | Low risk | Comment: study says they carried out ITT analysis; however, results were only given for completers. 72% in workbook intervention, 66% in group intervention and 74% in group + IVR intervention completed 12‐month follow‐up ‐ drop‐outs rates average for this length of follow‐up |
| Incomplete outcome data (attrition bias) | High risk | Comment: study says they carried out ITT analysis; however, results were only given for completers. 72% in workbook intervention, 66% in group intervention and 74% in group + IVR intervention completed 12‐month follow‐up ‐ drop‐out rates average for this length of follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: states health‐related quality of life reported but no data reported |
| Other bias | Unclear risk | Comment: unsure if all the participants stayed in their randomised groups |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Equivalence design | |
| Participants | Inclusion criteria: BMI > 85th percentile adjusted for gender and age. Participating family members were able to speak and write in German. All participants were free from diabetes, heart disease and endocrine disorders Exclusion criteria: parents and children meeting the criteria of the DSMIV‐TR for mental disorders warranting immediate treatment (assessed in a clinical interview), such as suicidal tendency, psychosis, mania, organic dementia or substance abuse disorder. Parents' or children's participation in a diet programme or other psychotherapy treatment with weight loss medication. There were only 4 fathers eligible for treatment, therefore, excluded from the analyses Diagnostic criteria: BMI > 85th percentile adjusted for gender and age | |
| Interventions | Number of study centres: 2 Treatment before study: none Titration period: no Mother's CBT: 10 sessions in 5 phases over 10 weeks (120‐minute sessions) Phase 1 (nutrition and eating behaviour, covered across 8 sessions including psychoeducation about childhood obesity, risks, nutritional counselling, goal setting, stimulus control, family rules); phase 2 (physical activity, covered within 2 sessions including psychoeducation about physical activity, motivation, suitable sports); phase 3 (social competences, covered in 1 session, including social skills training, parental modelling and support of children dealing with difficulty situations); phase 4 (body concept, covered in 1 session, included supporting child's developments of a positive body concept); phase 5 (relapse prevention, covered in 1 session, included training of maintenance skills, appraisal of goal attainment, developing coping strategies). Children attended a relaxation training (progressive muscle relaxation training) of equal frequency and duration to the disorder‐specific CBT of children in the mother‐child group 10 weekly treatment sessions of 120 minutes. Mothers received CBT including the same components as the Mothers‐only group. Children received sessions on nutrition and eating behaviour, basic nutritional education, reinforcement and tokens, lessons in physical activity, social competencies (self assertiveness, social skills, saying 'no' to food offers, role modelling, anti‐bullying plans), developing a positive body concept and relapse prevention Sessions were undertaken by psychologists and trained co‐therapists. All therapists were trained and supervised weekly by 1 of the authors | |
| Outcomes | Outcomes reported in abstract of publication: per cent overweight, general behaviour problems (externalising and internalising behaviour problems), global and social anxiety, and depression | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal and Word document | |
| Stated aim for study | Quote from publication: "we investigated whether the treatment of parents only would be as efficacious as a parent‐child treatment in a randomised controlled clinical trial. Our group treatment approach, TAKE [Training adipöser Kinder und deren Eltern ('training of obese children and their parents')], targeted weight stabilization and reduction of behavioral problems of obese children aged 8‐12 years" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "families were randomly assigned according to a permuted block design to either the mother‐child (condition A) or the mother‐only (condition B) cognitive behavioral therapy (CBT) treatment..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) | High risk | Comment: high rates of drop‐out (mother‐child 11/31 dropped out, in mother‐only 18/25 dropped out), no ITT analysis |
| Incomplete outcome data (attrition bias) | High risk | Comment: high rates of drop‐out (mother‐child 11/31 dropped out, in mother‐only 18/25 dropped out), no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: families were randomly assigned according to a permuted block design to either the mother‐child (condition A) or the mother‐only (condition B) CBT treatment. Selection bias: there were only 4 fathers eligible for treatment and they were excluded from the analyses. Also underpowered |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: BMI ≥ 85th percentile for age and gender, required to live within the same dwelling in a rural county that is designated in whole or in part as a "Health Professional Shortage Areas" by the U.S. Department of Health and Human Services, to obtain physician approval to participate in the study, with documentation provided via letter signed by a physician medically clearing individuals to participate in a weight management programme Exclusion criteria: child or participating parent has a dietary or exercise restriction, or a medical condition that contraindicates mild energy restriction or moderate physical activity (including a history of musculoskeletal condition that limits walking; heart condition; chronic lung diseases limiting physical activity; uncontrolled diabetes; uncontrolled hypertension; thyroid disease; or uncontrolled exercise‐induced asthma as determined by a physician). Children or participating parents on antipsychotic agents, systemic corticosteroids or who were currently using prescription weight‐loss drugs, insulin or other diabetic medications. Not engaged in another weight control programme, exhibit conditions or behaviours that were likely to affect their participation in the trial, such as being unwilling or unable to give informed consent, parent(s) or legal guardian(s) unable to read English at approximately the 5th grade level, unwilling to accept random assignment, unable to travel to extension office for intervention sessions, or likely to move out of the county within the next 12 months Diagnostic criteria: BMI ≥ 85th percentile for age and gender | |
| Interventions | Number of study centres: not reported Treatment before study: none Titration period: none Detailed description of interventions Behavioural parent‐based intervention: 12 sessions of 90 minutes Changes in dietary habits were addressed via a modified version of the Stoplight Diet Parents participated in role‐play activities to practice negotiation of goals with their child. As children were not attending group sessions, an emphasis was placed on teaching parents how to work with their children to set goals together. Parents encouraged to utilise praise, incentives and modelling to encourage participation and goal achievement. Parents provided handouts to guide them in discussing programme material and setting weekly goals with their children. Parents weighed every other group session to monitor their weight status Delivered as described below Behavioural family‐based intervention: 12 sessions of 90 minutes. Parent‐child dyads participated in simultaneous but separate groups. The primary objectives were to build healthier dietary habits, increase moderate intensity physical activity, establish a healthier weight status and build positive self worth Increased physical activity encouraged through a pedometer‐based step programme. Children and parents encouraged to monitor their physical activity and gradually increase their steps per day. Programme goals based on their baseline level of steps and targeting an increase of at least 3000 steps per day by the end of the programme. Goals set for gradually decreasing sedentary activities (children spend no more than 2 hours per day watching TV or playing video games). If excessive TV viewing was not a concern for a given family, group leaders targeted non‐homework‐based computer time Incentivised by providing payment for transportation costs (USD 5 per session) and USD 50 for completing post‐treatment and 6‐month assessment visits Delivered by Family and Consumer Sciences Agents, in collaboration with a post‐doctoral clinical psychologist and graduate students in clinical health psychology who had extensive training and certification in the treatment protocols. The principal investigator of the study conducted periodic direct observation of group sessions to monitor interventionist's performance and assess treatment fidelity. The interventionists also participated in weekly supervision meetings to review each family's progress, discuss group interactions and prepare for the next group session Waiting list control: | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, self esteem, cost | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The goals of the study are to (a) assess the feasibility of recruitment in rural settings, (b) develop and evaluate training protocol for group leaders, (c) determine strategies to increase adherence to monitoring and goal setting protocol, (d) evaluate strategies for participant retention, (e) assess the relative cost‐effectiveness of the interventions, (f) assess the acceptability of the intervention to families and Cooperative Extension administrators and personnel, and (g) if successful, estimate the sample size needed for a full scale trial" | |
| Notes | There were 2 comparisons of relevance to this review: the parent‐only vs. parent‐child group and the parent‐only vs. the waiting list control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: during the initial telephone screen, families were informed as to which 2 evenings the group intervention sessions would be held in their county. Families then indicated which of these evenings they were available to attend. Before the baseline assessment, all families were randomised via computer assignment, based on availability, to 1 of the 2 specific week nights or the waiting list control condition. After randomisation of all families, the interventions (parent‐only or family based) were assigned randomly to the specific week nights |
| Allocation concealment (selection bias) | Unclear risk | Quote from study author: "Randomization was conducted by a research team member who did not participate in assessments. Assignments were written down and put in an envelope by the person making the assignments for each dyad. Envelope was opened with family at end of baseline visit" Comment: unclear whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) | High risk | Quote from study author: "Participants and treatment personnel were not blind" Comment: families were notified of their group assignment at pre‐treatment assessment |
| Blinding of participants and personnel (performance bias) | High risk | Quote from study author: "Participants and treatment personnel were not blind" Comment: families were notified of their group assignment at pre‐treatment assessment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from study author: "Outcome assessors were blinded in that we used personnel to complete outcome assessments that did not serve as treatment personnel in each respective county" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from study author: "Outcome assessors were blinded in that we used personnel to complete outcome assessments that did not serve as treatment personnel in each respective county" |
| Incomplete outcome data (attrition bias) | High risk | Comment: no ITT analysis. Numbers completing assessment provided only |
| Incomplete outcome data (attrition bias) | High risk | Comment: no ITT analysis. Numbers completing assessment provided only |
| Selective reporting (reporting bias) | High risk | Comment: collected other measures that were not reported |
| Other bias | Unclear risk | Comment: not enough information to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: overweight (according to the International Obesity Task Force definition), and Tanner stage with a carer willing to attend sessions and able to read and understand English Exclusion criteria: BMI z score > 3.5, diagnosed with a syndromal cause of obesity, using medications that influence weight gain or loss, a diagnosis of physical or developmental disability or chronic illness, and a sibling enrolled in the study Diagnostic criteria: overweight (defined as above) | |
| Interventions | Number of study centres: 2 Treatment before study: none Titration period: none Parenting‐skills training: 11 sessions over 24 weeks, 4 weekly 2‐hour group sessions followed by 4 weekly, then 3 monthly 15‐ to 20‐minute individual telephone sessions. Parenting‐skills training was used to facilitate and support parents to undertake family lifestyle change. Positive, Parenting Program (Triple P) based on child development theory and social learning principles and aimed to promote parental competence to manage their child's behaviour. Standard Triple P resource materials were used with programme examples adapted to reflect dietary and activity behaviours. Application of Triple P to eating and activity behaviours was supported by provision of a general healthy lifestyle pamphlet The Triple P Selected Seminar Series consisted of 3 x 2‐hour seminars covering: 1. positive parenting; 2. raising confident and competent‐children, and; 3. raising resilient‐children Learning outcomes ‐ to practitioners (which some is applicable to parents)
Triple P Discussion Groups topics are:
Intervention delivered using standard protocols and a single, trained facilitator to limit site bias and enhance internal study validity. Sessions taken by a dietician Parenting‐skills training with intensive lifestyle education: While parents attended the lifestyle sessions, children in the group attended structured, supervised activity sessions developed by physical activity experts. The sessions consisted of fun, non‐competitive games designed around aerobic activity and development of fundamental motor skills. Sessions were designed as play rather than exercise and were diversional rather than interventional. The activities required minimal equipment and were deliverable by non‐expert staff and easily replicated at home. Sessions taken by a dietician Waiting list control: | |
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist circumference z score | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "Our goal was to evaluate the relative effectiveness of parenting‐skills training as a key strategy for the treatment of overweight children. The aim of this study was to evaluate the relative effectiveness of parenting‐skills training as a key strategy for the treatment of overweight children" | |
| Notes | There were 3 comparisons of relevance to this review: the parent‐only intensive education group vs. the parent‐only group; the parent‐only + intensive education group vs. the waiting list control; the parent‐only group vs. the waiting list control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomization schedules were computer generated using a 3‐block design stratified for gender and site of recruitment..." Comment: researchers involved in recruitment, participant allocation and intervention delivery or data collection were not involved in the randomisation process |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Individual group allocations were sealed in opaque envelopes, with the next envelope opened on a child's completion of baseline measurements..." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: carers and participants masked to allocation to treatment assignment but unclear if this also related to blinding throughout the trial |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "...Data collection was performed by the same trained assessor who was blinded to participant group allocation" Comment: outcome assessment was masked |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote from publication: "Intention‐to‐treat analysis was performed, with all participants included in the analysis according to original group allocation, and follow‐up was maximized regardless of program attendance" Comment: states ITT but the total number did not match |
| Selective reporting (reporting bias) | High risk | Comment: health‐related quality of life and satisfaction stated as outcomes but not reported |
| Other bias | Unclear risk | Comment: not enough detail to judge |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design | |
| Participants | Inclusion criteria: children > 20% overweight (BMI for age and sex > 85th percentile). Parents agreed to attend programme meetings. No current participation of any family member in a weight‐loss programme. No restriction regarding participation in a physical activity programme for children and parents. No diagnosis of psychiatric or major endocrine pathology Exclusion criteria: current participation of any family member in a weight‐loss programme; restriction regarding participation in a physical activity programme for children and parents; diagnosis of psychiatric or major endocrine pathology Diagnostic criteria: BMI for age and sex > 85th percentile | |
| Interventions | Number of study centres: 1 Treatment before study: none Titration period: none Parent‐only group: 16 x 1 hour sessions at the following intervals: weeks 1‐10 (10 sessions); bi‐weekly ‐ weeks 11‐18 (4 sessions); monthly ‐ weeks 19‐26 (2 sessions). Nurturing the child emotionally. Problem solving. The first 3 sessions focused on nutrition education and parental modelling. In the next 2 sessions, the use of an authoritative feeding style was discussed. Sessions 6 and 7 focused on eating and activity behaviour modification, reinforcing means to influence a child's food preferences, as well as employing behaviour modification. Sessions 8 and 9 focused on problem solving while implementing Physical activity goals of 4 hours per week, and decrease in sedentary behaviours (to < 3 hours/day) Theoretical basis: parents as the exclusive agent of change. Authoritative feeding style. Nurturing. Parental modelling. Behaviour change. Based on previous work Structured 12‐session programme (unclear if a manual). A clinical dietician, supervised by a family therapist, administered the programme. Training of dietician not reported Parent and children group: Similar in content; however, adapted to fit the children included | |
| Outcomes | Outcomes reported in abstract of publication: % overweight at end of programme (6 months) and 1‐year follow‐up. Food stimuli in the home (from Family Eating and Activity questionnaire). Parents' weight | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Funding not stated Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "The question remains, which is better: parents only or parent and child treatment? The present study extends this knowledge by comparing targeting parents and child versus parents alone, to address the question: Do the children need to be involved at all?..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation stratified by age groups: participants divided into age groups: 6‐7 years, 8‐9 years, 10‐11 years, then randomised. No details of randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "The process was carried out by using two concealed opaque envelopes indicating group 1, namely parents‐only, or group 2, parents and children" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "The data were gathered by an MSc [Master of Science] student who was blinded to the treatment allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from publication: "The data were gathered by an MSc student who was blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis where the missing values were replaced with baseline values. Reasonable attendance in both arms and numbers and reasons for drop‐outs given |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis where the missing values were replaced with baseline values. Reasonable attendance in both arms and numbers and reasons for drop‐outs given |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: although power calculations given, 12 participants in each arm seems a small number from the power calculation |
| Methods | Parallel randomised controlled trial Randomisation ratio: superiority Superiority design: 1 : 1 : 1 | |
| Participants | Inclusion criteria: overweight girls aged 5‐10 years. Children considered overweight if both their physician and parents recommended that they participate in the weight‐loss programme Exclusion criteria: undergoing psychotherapy, drug therapy or involved in a weight‐reduction programme Diagnostic criteria: not stated | |
| Interventions | Number of study centres: assume 1 Treatment before study: none Titration period: none Response‐cost plus reinforcement: 12‐week treatment. Parents given daily weight and calorie graphs, a calorie counter guide and an eating diary. Parents also given a weight reduction programme behavioural contract, instruction on daily exercise and an exercise programme (daily calisthenics that increased in difficulty over a 3‐week period, thereafter 30 minutes per day), nutritional information, instructions in stimulus control techniques, a book 'Living with Children' and information in reinforcement techniques, a daily reinforcement diary. At second baseline visit given a response‐cost contract to return the following week with money for deposit and a weight loss goal of between 1 and 2 pounds per week. Deposits were on a sliding scale of income vs. number of dependents and ranged between USD 12‐30. Money could be redeemed in 12 weekly instalments (25% weekly for attendance, 25% for bringing completed graphs and charts to the meeting, 50% if the child lost the agreed weight). Unearned money divided among the successful parents. Children weighed and then sent to a playroom. After the programme there was an 8‐week no contract follow‐up and following that a post follow‐up check 31 weeks later Response‐cost: Parents given the same as the response‐cost plus reinforcement group except did not receive the book, information on reinforcement techniques or the reinforcement diary Control: Informed would be able to participate at a later date (waiting list control) | |
| Outcomes | Outcomes reported in abstract of publication: weight change | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed publication | |
| Stated aim for study | Quote from publication: "The present study treated with behavioural techniques overweight children 5 to 10 year of age" | |
| Notes | There were 3 comparisons of relevance to this review: parent‐only + reinforcement vs. parent‐only; parent‐only + reinforcement vs. control; parent‐only vs. control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "subjects were randomly assigned to one of three groups" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details |
| Incomplete outcome data (attrition bias) | Low risk | Comment: numbers and reasons for drop‐outs provided, differential drop‐out between groups (small numbers) |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
Note: where the judgement is 'unclear' and the description is blank, the study did not report that particular outcome.
"‐" denotes not reported.
BMI: body mass index; CBT: cognitive behavioural therapy; ITT: intention‐to‐treat; NOURISH: Nourishing Our Understanding of Role modelling to Improve Support and Health; TV: television.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention not parent‐only | |
| Duration of intervention/follow‐up < 6 months | |
| Intervention not parent‐only | |
| Intervention not parent‐only | |
| Intervention not parent‐only | |
| Intervention not parent‐only | |
| Intervention not parent‐only | |
| Duration of intervention/follow‐up < 6 months | |
| Intervention not parent‐only | |
| Duration of intervention/follow‐up < 6 months | |
| Primary prevention study | |
| Intervention not parent‐only | |
| Duration of intervention/follow‐up < 6 months | |
| Intervention not parent‐only | |
| Duration of intervention/follow‐up < 6 months | |
| Intervention not parent‐only |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | No details |
| Participants | Obese children and adolescents |
| Interventions | No details |
| Outcomes | No details |
| Study identifier | Dissertation abstract: 1982‐72486‐001 |
| Official title | A study of weight control in paediatric obesity using mothers as behavior modifiers |
| Stated purpose of study | No details |
| Notes | We were unable to access the full publication of this study |
| Methods | No details |
| Participants | Mothers and their 6‐ to 12‐year‐old obese children |
| Interventions | No details |
| Outcomes | No details |
| Study identifier | Dissertation abstract: 1977‐13293‐001 |
| Official title | Training parents as therapists in the treatment of juvenile obesity |
| Stated purpose of study | No details |
| Notes | We were unable to access the full publication of this study |
| Methods | Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
| Participants | Inclusion criteria: aged 6‐11 years; weight > 20% over expected weight for age, height and gender; no history of psychiatric contact for children; and both parents living at home and parental agreement to meet all requirements of the study (check‐ups, questionnaire, group sessions) Exclusion criteria: the main reasons for exclusion were the children's reluctance to undergo blood sampling and the parents' denial of their children being obese or needing treatment Diagnostic criteria: weight > 20% over expected weight for age, height and gender |
| Interventions | Number of study centres: 1 Treatment before study: none Titration period: none Parent‐only: 14 x 1‐hour group sessions, conducted by clinical dietician, attended only by parents (delivered as 2 groups of 15 parents). 4 sessions ‐ weekly; 4 sessions ‐ bi‐weekly; 6 sessions ‐ every 6 weeks. Also, 5 x 15 minute individual sessions for whole family, during last 6 group sessions Apply behavioural modifications (implement lifestyle change); practice parenting skills (overlap with nutrition advice as well). All instructions were oriented to the family system. At the sessions, the parents were taught to alter the family sedentary lifestyle, provide a prudent diet (reduction of total and saturated fats, increase of mono‐unsaturated fatty acids), decrease the family's exposure to food stimuli, apply behavioural modifications and practise relevant parenting skills. Training of staff not reported, assumed delivered by a dietician Child prescribed calorie‐controlled diet. Children divided into 2 subgroups. 30 x 1‐hour group sessions, by clinical dietician; 8 sessions ‐ weekly; 22 session ‐ biweekly |
| Outcomes | Outcomes reported in abstract of publication: drop‐out; mean reduction in percentage overweight; exposure to food stimuli/changes in eating habits |
| Study identifier | Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
| Official title | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
| Stated purpose of study | Quote from publication: "This study examined the reduction in overweight and changes in eating‐related behaviours in obese children treated with a family‐based approach, in which the parents were the exclusive agents of change. Results were compared to the conventional approach in which children are responsible for their own weight loss" |
| Notes | We contacted authors to establish if the outcome of interest had been measured but have not had a response |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Acronym: PAC |
| Methods | Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind (participants) Primary purpose: weight loss intervention |
| Participants | Condition: parents of overweight children Enrolment: estimated 90 Inclusion criteria: families are eligible for this study if children are aged 8‐12 years, children present with an age‐ and sex‐specific BMI ≥ 85th percentile, at least 1 parent agrees to attend weekly PAC sessions for 16 weeks and children and parents are fluent in English (verbal and written) Exclusion criteria: not stated |
| Interventions | Intervention(s): Parents as Agents of Change (PAC) intervention (includes cognitive behavioural therapy) Comparator(s): psycho‐educational‐based intervention Both intervention arms in the trial are the same in frequency of contact (16 sessions), content (identical information is delivered), mode (group format), duration (60‐90 minutes per session), intervention goals (related to nutrition and physical activity) and the number of group leaders (2 per group). The intervention arms differ in how information is conveyed to parents, and how parents work towards attempting, achieving and maintaining healthy cognitive and behavioural changes |
| Outcomes | Primary outcome(s): BMI z score Secondary outcome(s): lifestyle behaviours, nutrition and physical activity behaviours; Parental Stress Index (PSI); blood pressure; fasting glucose; fasting insulin; HDL cholesterol; LDL cholesterol; total cholesterol; triglycerides (child only); Family Adaptability and Cohesion Scale‐IV (FACES‐IV). Other outcome(s): as above |
| Starting date | Study start date: September 2010 Study completion date: January 2014 |
| Contact information | Responsible party/principal investigator: Geoff Ball ([email protected]), University of Alberta, Canada |
| Study identifier | |
| Official title | Parents as Agents of Change (PAC) in paediatric weight management |
| Stated purpose of study | Quote: "We hypothesize that children with obesity whose parents complete a CBT‐based PAC intervention will achieve greater reductions in adiposity and improvements in cardiometabolic risk factors, lifestyle behaviours, and psychosocial outcomes than children whose parents complete a psycho‐education‐based PAC intervention (PEP)" |
| Notes |
| Trial name or title | Acronym: PLAN |
| Methods | Type of study: efficacy study Allocation: randomised Intervention model: cluster trial Masking: none Primary purpose: weight loss intervention |
| Participants | Condition: parents of overweight children Enrolment: estimated 80 Inclusion criteria: BMI ≥ the 85th percentile during the recruitment period, and 1 parent or other primary carer agrees to participate in the study, including individual visits and group sessions as well as telephone follow‐ups. Only one child per family will be included in the study Exclusion criteria: current child or parent/primary carer participation in a weight management programme; presence of a diagnosed psychiatric/psychological disorder in the child or parent/primary carer (e.g. attention deficit hyperactivity disorder, autism, eating disorder); presence of an underlying medical condition affecting weight status (e.g. hypothyroidism, Cushing's syndrome, chronic steroid use); current dietary or physical activity restrictions (e.g. such as in children with diabetes or orthopaedic problems including slipped capital femoral epiphysis); and parents/primary carers do not have telephone accessibility. Parents/primary carers will also have to understand and speak English |
| Interventions | Intervention(s): parent‐mediated childhood overweight intervention (PLAN) for healthy living and the parent handbook described below. 2 individual visits with clinic provider, 4 clinic‐based group sessions moderated by a trained clinic provider and 4 follow‐up telephone calls from the Project Co‐ordinator or research staff Comparator(s): parents will receive "Families Finding the Balance: A Parent Handbook", a health education handbook adopted by NIH We Can! during the baseline assessment |
| Outcomes | Primary outcome(s): BMI z score Secondary outcome(s): child's and family's eating and physical activity behaviours and the child's health‐related quality of life Other outcome(s): covariates; healthcare provider perceptions of treatment of child overweight and obesity. Parents and healthcare providers will also complete surveys and focus groups, respectively, on the acceptability and feasibility of this approach including provider perceptions of training |
| Starting date | Study start date: not reported Study completion date: not reported |
| Contact information | Responsible party/principal investigator: Karen E Schetzina, East Tennessee State University, Johnson City, Tennessee, USA |
| Study identifier | NCT number: NCT01729910 |
| Official title | Parent‐Led Activity and Nutrition (PLAN) for Healthy Living (published protocol) Primary Care Child Obesity Intervention Targeting Parents (trial document) |
| Stated purpose of study | Quote: "1) to establish a primary care based and parent‐mediated childhood overweight intervention program in the primary care setting, 2) to explore the efficacy of this intervention in promoting healthier weight status and health behaviours of children, 3) to examine the acceptability and feasibility of the approach among parents and primary care providers" |
| Notes |
| Trial name or title | Acronym: GO4fit |
| Methods | Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: none Primary purpose: weight loss intervention |
| Participants | Condition: parents of overweight children Enrolment: estimated 84 child‐parent triads Inclusion criteria: parents of children are eligible for participation if their child is considered overweight or obese, based on the BMI, using the international sex‐ and age‐specific cut‐off points proposed by Cole et al. Exclusion criteria: none stated |
| Interventions | Intervention(s): Lifestyle Triple P intervention for parents with active skills training methods based on self regulation principles, to provide parents with new knowledge and skills. 14‐week intervention, 8 weekly 90‐minute parental group sessions, followed by 2 weekly 15‐30 minute telephone sessions, 1 further 90‐minute group session, 2 weekly 15‐ to 30‐minute telephone sessions, and a final 90‐minute group session Comparator(s): receive 2 brochures (1 on healthy nutrition and physical activity, and 1 on positive parenting), web‐based tailored advice on setting a good example to their child, and suggestions for exercises to increase active play at home |
| Outcomes | Primary outcome(s): BMI z score, waist circumference, fat mass Secondary outcome(s): children's dietary behaviour and physical activity level, parenting practices, parental feeding style, parenting styles, parental self efficacy, and body composition of family members (parents and siblings) Other outcome(s): as above |
| Starting date | Study start date: December 2010 Study completion date: December 2012 |
| Contact information | Responsible party/principal investigator: Sanne Gerards ([email protected]), Maastricht University, The Netherlands |
| Study identifier | The Netherlands National Trial Register NTR2555 |
| Official title | Lifestyle Triple P: a parenting intervention for childhood obesity (published protocol) Effectiveness of Lifestyle Triple P: an intervention aimed at the prevention of excessive weight gain in 4‐ till 8‐year‐old overweight children. ‐ GO4fit (trial document) |
| Stated purpose of study | Quote: "The aim of the current randomised controlled trial is to assess the effectiveness of the Lifestyle Triple P intervention when applied to Dutch parents of overweight and obese children aged 4–8 years" |
| Notes |
| Trial name or title | Acronym: E‐FLIP for Kids |
| Methods | Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: not reported Primary purpose: treatment |
| Participants | Condition: overweight and obese Enrolment: estimated 240 Inclusion criteria: aged 8‐12 years, BMI ≥ 85th percentile for age and gender, living within the same dwelling in a rural county. Participating parents or legal guardians must be age ≤ 75 years Exclusion criteria: participating parent has a dietary or exercise restriction, or a medical condition that contraindicates mild energy restriction or moderate physical activity. Children or participating parents on antipsychotic agents, systemic corticosteroids, antibiotics for HIV or tuberculosis, chemotherapeutic drugs or who are currently using prescription weight‐loss drugs, child has a resting blood pressure ≥ 140/90 mmHg, not engaged in another weight control programme, exhibit conditions or behaviours that are likely to affect their participation in the trial, such as being unwilling or unable to give informed consent, parent(s) or legal guardian(s) unable to read English at approximately the 5th grade level, unwilling to accept random assignment, unable to travel to the extension office for intervention sessions, or likely to move out of the county within the next 24 months |
| Interventions | Interventions: 1. General intervention + parent‐only intervention: weekly group sessions for the first 8 weeks, then 4 biweekly sessions over the next 8 weeks for a total of 12 sessions across 16 weeks. Contact will then fade to 1 group session per month for months 5‐12, with the exception of month 9, during which participants will attend 2 group sessions. Each session will last 90 minutes. Focus on diet, physical activity and behavioural components 2. General intervention + family‐based behavioural intervention: as above but involves children and parents who will meet in simultaneous, but separate, parent‐child groups at each meeting Comparator(s): 21 group meetings on the same schedule as the intervention arms. Each session will last 90 minutes. The families in the Health Education group will not receive training in behavioural self regulation strategies, such as goal setting and self monitoring. There will be no instruction on behavioural parenting strategies and parent |
| Outcomes | Primary outcome(s): BMI z score Secondary outcome(s): child body fat, waist circumference, height, weight, dietary intake, physical activity, blood lipids, blood glucose, parental measures, health‐related quality of life, parenting skills, costs and cost effectiveness |
| Starting date | Study start date: July 2009 Study completion date: December 2014 |
| Contact information | Responsible party/principal investigator: David Janicke ([email protected]) |
| Study identifier | NCT number: NCT01820338 |
| Official title | The Extension Family Lifestyle Intervention Project (E‐FLIP for Kids) (published protocol and trial document) |
| Stated purpose of study | Quote: "assessing the effectiveness of two behavioral weight management interventions in an important and at‐risk population, overweight and obese children and their parents in rural counties..." |
| Notes |
| Trial name or title | Acronym: PAAC |
| Methods | Type of study: efficacy and cost effectiveness study Allocation: randomised Intervention model: parallel assignment Masking: none Primary purpose: weight loss intervention |
| Participants | Condition: parents of overweight children Enrolment: estimated 150 Inclusion criteria: overweight child > 95th percentile for age and gender; an overweight (BMI > 25) parent willing to participate and attend all treatment meetings; eligible parent who can read at a minimum of an 8th grade level; family willing to commit to 5 months of treatment attendance and follow‐up for 18 months post‐treatment Exclusion criteria: major child psychiatric disorder diagnoses; child diagnoses of a serious current physical disease (such as diabetes) for which physician supervision of diet and exercise prescription are needed (self report); family with restrictions on types of food, such as food allergies, religious or ethnic practices that limit the foods available in the home; child with physical difficulties that limit the ability to exercise; child with an active eating disorder (based on Eating Disorder Examination interview); families where children or parents are involved in swimming or weight training more than 5 hours per week; major parent psychiatric disorder |
| Interventions | Intervention(s): behavioural parent‐only intervention Comparator(s): behavioural parent‐child intervention The treatment length is set for 12 weekly meetings and bi‐monthly meetings during months 4 and 5. Each group session will be 60 minutes including weigh‐ins |
| Outcomes | Primary outcome(s): weight; BMI for age percentile; BMI z score Secondary outcome(s): cost effectiveness, dietary quality, exercise behaviour, quality of life, psychosocial measures, parenting adherence, parenting style, parent weight loss, compliance and changes in household food environment |
| Starting date | Study start date: November 2010 Study completion date: July 2015 |
| Contact information | Responsible party/principal investigator: Kerri Boutelle ([email protected]), University of California, San Diego, USA |
| Study identifier | NCT number: NCT01197443 |
| Official title | Parents as the Agent of Change for Childhood Obesity (PAAC) |
| Stated purpose of study | Quote: "To evaluate the efficacy of parent only treatment versus parent + child treatment in the body weight of the target child. To evaluate the cost effectiveness compared to current gold standard treatment of parent and child dual education" |
| Notes |
| Trial name or title | Acronym: Behavioral Treatment for Obese Preschoolers (LAUNCH) |
| Methods | Type of study: efficacy Allocation: randomised Intervention model: factorial assignment Masking: double blind Primary purpose: treatment |
| Participants | Condition: obesity Enrolment: estimated 168 Inclusion criteria: children aged 2 years 0 months to 5 years 11 months; BMI percentile ≥ 95th percentile for age and gender, but no more than 100% above the median BMI for age and gender; English‐speaking; Live within 50 miles of Cincinnati Children's Hospital Medical Center (CCHMC); medical clearance from the child's paediatrician to participate Exclusion criteria: medical conditions known to promote obesity (e.g. Prader‐Willi syndrome, Cushing's syndrome); already involved with another weight control programme; taking weight‐affecting medications (e.g. steroids); a disability or illness that would preclude them from engaging in at least moderate intensity physical activity; developmental disability |
| Interventions | Intervention(s): 1. Behavioural family intervention. 3 months of weekly treatment delivered via alternating group‐based clinic visits and individual home visits followed by 3 months of every other week treatment alternating between clinic and home 2. Behavioural: motivational interviewing ‐ 4 in‐person visits spaced at first visit, month 3 and month 5. Weekly telephone calls during the first 3 months and every other week during months 4‐6 Comparator(s): standard care |
| Outcomes | Primary outcomes: BMI z score change Secondary outcome(s): BMI z score; caloric intake; physical activity; home environment; parent caloric intake; parent physical activity; parent and child eating behaviours; health‐related quality of life |
| Starting date | Study start date: March 2012 Study completion date: November 2016 |
| Contact information | Responsible party/principal investigator: Lori J Stark, Children's Hospital Medical Center, Cincinnati, USA |
| Study identifier | NCT number: NCT01546727 |
| Official title | Clinic and Home Family Based Behavioral Treatment for Obese Preschoolers |
| Stated purpose of study | Quote: "to test a clinic and home family behavioral intervention (LAUNCH) against 1) motivational interviewing (attention control; MI) and 2) standard of care (true standard of care control" |
| Notes |
| Trial name or title | Acronym: none |
| Methods | Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants | Condition: obesity, overweight Enrolment: estimated 156 Inclusion criteria: 1 parent of a 3‐ to 6‐year old child with a BMI Ͱ5 85th percentile, Internet access, English speaking Exclusion criteria: children with a developmental disorder, children with a chronic underlying disease that may contribute to obesity, children taking medication that may interfere with a healthy weight |
| Interventions | Intervention: parenting behavioural intervention: 6 weekly face‐to‐face group (10‐12 parents) meetings and access to a website. Contents include authoritative parenting, using the food plan "Go, Slow, and Whoa", increasing physical activity and behaviour change strategies. Parenting skills will be discussed at every session. Website has information and links about nutrition and physical activity, an interactive group session and an ask the expert facility Comparator: no intervention |
| Outcomes | Primary outcome: feasibility Secondary outcomes: BMI z scores, healthy behaviour changes, parenting skills Other outcome(s): ‐ |
| Starting date | Study start date: February 2013 Study completion date: August 2015 |
| Contact information | Responsible party/principal investigator: Ellen R Wald, University of Wisconsin, Madison, USA |
| Study identifier | NCT number: NCT01552642 |
| Official title | An Interactive Web‐based Intervention to Achieve Healthy Weight in Young Children |
| Stated purpose of study | Quote: "to develop and implement an effective intervention program designed to prevent and treat obesity in young children" |
| Notes |
| Trial name or title | Acronym: More and Less study (M+L) |
| Methods | Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants | Condition: obesity Enrolment: estimated 180 Inclusion criteria: age 4‐6 years old; obesity as defined by international cut‐offs Exclusion criteria: weight affecting diseases |
| Interventions | Intervention(s): parent training group with 2 subgroups, 12‐week only vs. bolster sessions at 8‐week intervals over 1 year Focus on how to use positive parenting practices instead of ineffective practices. 12 sessions (1.5 hours per week), introduction to effective parenting practices, discussion and practice using role play and home practice assignments. Tailored to focus on changes in the home environment, mostly related to child food habits and physical activity Comparator(s): standard treatment with focus on lifestyle, provided by local paediatricians in outpatient paediatric departments and will be based on lifestyle modifications, as recommended in the action plan for Stockholm County |
| Outcomes | Primary outcome(s): BMI change at 1 year Secondary outcome(s): changes in: parenting practices; child's dietary intake and behaviour; child's physical activity; family functioning; child's metabolic health; parental functioning; waist circumference; child's functioning, socioeconomic status Other outcome(s): ‐ |
| Starting date | Study start date: January 2013 Study completion date: December 2017 |
| Contact information | Responsible party/principal investigator: Paulina Nowicka, Karolinska Institutet, Sweden |
| Study identifier | NCT number: NCT01792531 |
| Official title | The More & Less Study: A Trial Testing Different Treatment Approaches to Obesity in Preschoolers (M&L) |
| Stated purpose of study | Quote: "to evaluate the effectiveness of early treatment of childhood obesity" |
| Notes |
| Trial name or title | Acronym: none |
| Methods | Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants | Condition: obesity Enrolment: estimated 60 Inclusion criteria: aged 2‐5 years, BMI z score > 2 Exclusion criteria: medical conditions or receiving medications affecting weight |
| Interventions | Intervention: parent mentors using positive deviance findings to promote healthy behaviours. Using locally derived positive deviance findings to inform a behavioural intervention Comparator: education, community health workers providing health education to promote healthy behaviour. Using standardised healthy behaviour education (EatPlayGrow curriculum from NHLBI) |
| Outcomes | Primary outcome: BMI z score Secondary outcome(s): not reported Other outcome(s): not reported |
| Starting date | Study start date: January 2015 Study completion date: June 2015 |
| Contact information | Responsible party/principal investigator: The University of Texas Health Science Center at San Antonio, USA |
| Study identifier | NCT number: NCT02373670 |
| Official title | Parent Mentors Using Positive Deviance in Childhood Obesity |
| Stated purpose of study | Quote: "A feasibility study randomising participants (parents of children age 2‐5 years old) to receive either education or a parent mentor with the aim of improving health behaviours and improving their body mass index z‐score" |
| Notes |
| Trial name or title | Acronym: LOOPS |
| Methods | Type of study:interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants | Condition: overweight, obese Enrolment: estimated 240 (160 overweight, 80 obese) Inclusion criteria: 4‐ to 6‐year old children with overweight and obesity Exclusion criteria: do not understand written and spoken Swedish well enough to participate in group activities |
| Interventions | Intervention(s): All start with a 2‐hour lecture with general facts about overweight in children (GFO), performed by health professionals. Also access to a website, Healthy Children (HC), with general information about diet and exercise recommendations Obese children randomised to either:
in the parents'–everyday life will induce changes that will gradually lead to a normalisation of their children's weight. Groups meet for 13 x 2‐hour sessions over 12 months Overweight children randomised to 1 of 3 groups
Parents are invited to attend group meetings with the general purpose of supporting the parents to accomplish preferred lifestyle changes, both in the short and long term Comparator(s): as above |
| Outcomes | Primary outcome(s): change in BMI z score Secondary outcome(s): dietary and exercise patterns, waist circumference, insulin resistance, dietary hormones, fecal micro‐flora Other outcome(s): parent change in BMI, perception of their own health, parent stress, child feeding and exercise habits |
| Starting date | Study start date: August 2008 Study completion date: November 2015 |
| Contact information | Responsible party/principal investigator: Kristina Thorngren‐Jerneck (Kristina.Thorngren‐[email protected]), Lund University Children's hospital, Sweden |
| Study identifier | NCT number: NCT00916318 |
| Official title | Overweight and Obesity in Preschool Children, Prevalence and Prevention ‐ Family Based Health Interventions for Child Health (trial document) LOOPS ‐ Lund Overweight and Obesity Preschool Study (published protocol) |
| Stated purpose of study | Quote: "to evaluate if a family‐based intervention, targeting parents of preschool children with overweight and obesity, has a long‐term positive effect on weight development of the children" |
| Notes |
BMI: body mass index; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| Analysis 1.1  Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 1 BMI z score change post intervention. | ||||
| 1.1 Parent‐only vs. parent‐child | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.04] |
| 1.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| 1.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 2 BMI z score change longest follow‐up Show forest plot | 3 | 267 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.08] |
| Analysis 1.2 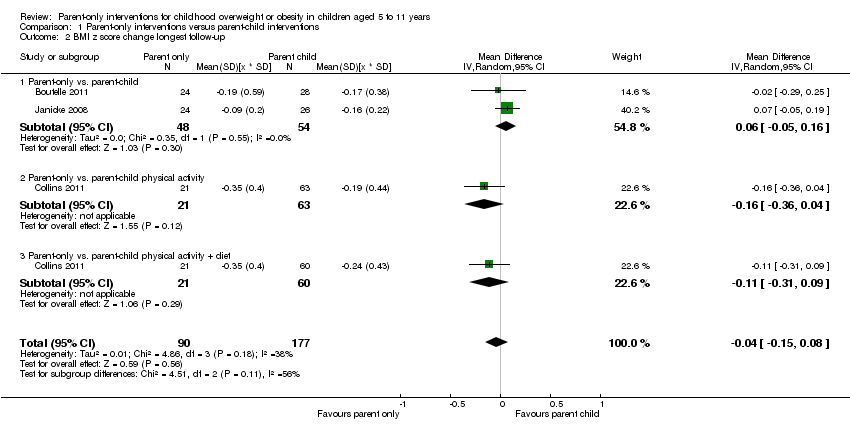 Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 2 BMI z score change longest follow‐up. | ||||
| 2.1 Parent‐only vs. parent‐child | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.16] |
| 2.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.36, 0.04] |
| 2.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.31, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.21, ‐0.04] |
| Analysis 2.1 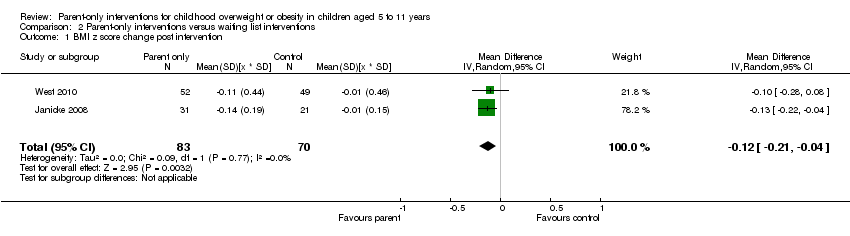 Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 1 BMI z score change post intervention. | ||||
| 2 BMI z score change longest follow‐up Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| Analysis 2.2  Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 2 BMI z score change longest follow‐up. | ||||
| 2.1 Parent‐only vs. waiting list | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.21, ‐0.01] |
| 2.2 Parent‐only intensive education vs. waiting list | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 3 BMI percentile change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 3 BMI percentile change post intervention. | ||||
| 4 BMI percentile change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 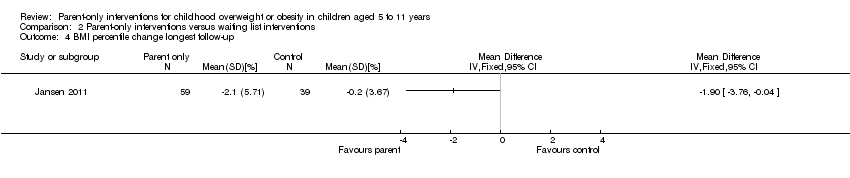 Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 4 BMI percentile change longest follow‐up. | ||||
| 5 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 5 BMI change post intervention. | ||||
| 5.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 BMI change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6 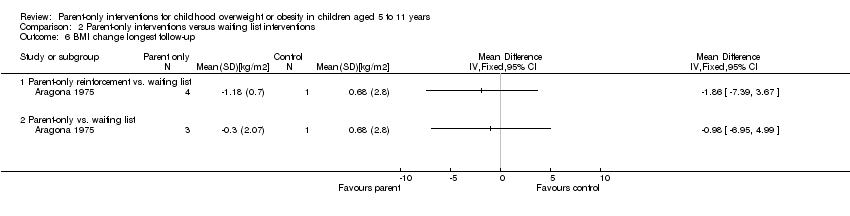 Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 6 BMI change longest follow‐up. | ||||
| 6.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 1 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| Analysis 3.1 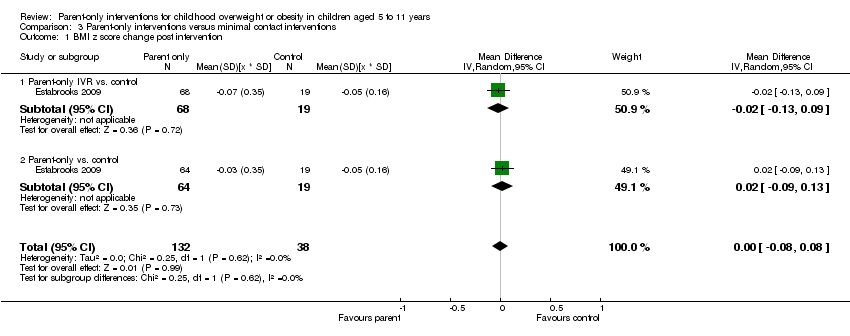 Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 1 BMI z score change post intervention. | ||||
| 1.1 Parent‐only IVR vs. control | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 1.2 Parent‐only vs. control | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 2 BMI z score change longest follow‐up Show forest plot | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| Analysis 3.2  Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 2 BMI z score change longest follow‐up. | ||||
| 2.1 Parent‐only interactive voice response vs. control | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 2.2 Parent‐only vs. control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.07, 0.15] |
| 3 BMI percentile change post intervention Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 3 BMI percentile change post intervention. | ||||
| 3.1 Parent‐only vs. minimal contact control | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Parent motivational interviewing vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Parent motivational interviewing + dietician vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI percentile change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4 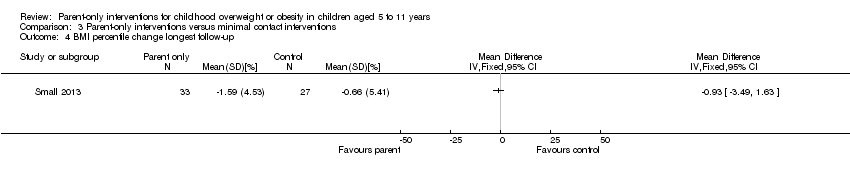 Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 4 BMI percentile change longest follow‐up. | ||||
| 5 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 5 BMI change post intervention. | ||||
| 6 BMI change longest follow‐up Show forest plot | 2 | 614 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.39, 0.15] |
| Analysis 3.6 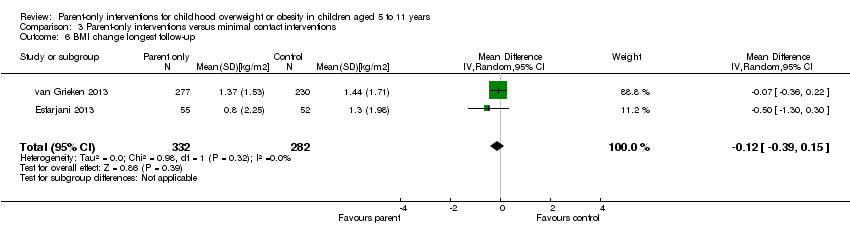 Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 6 BMI change longest follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 5 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.28, ‐0.17] |
| Analysis 4.1  Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 1 BMI z score change post intervention. | ||||
| 1.1 Parent‐only interactive voice response vs. parent‐only | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
| 1.2 Parent‐only intensive vs. parent‐only | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.38, 0.20] |
| 1.3 Parent health lifestyle vs. healthy lifestyle | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.29, 0.15] |
| 1.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 1.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 1.6 Parent‐only vs. substitute | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.86, ‐0.54] |
| 1.7 Parent‐only vs. traditional | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.83, ‐0.55] |
| 2 BMI z score change longest follow‐up Show forest plot | 5 | 467 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| Analysis 4.2 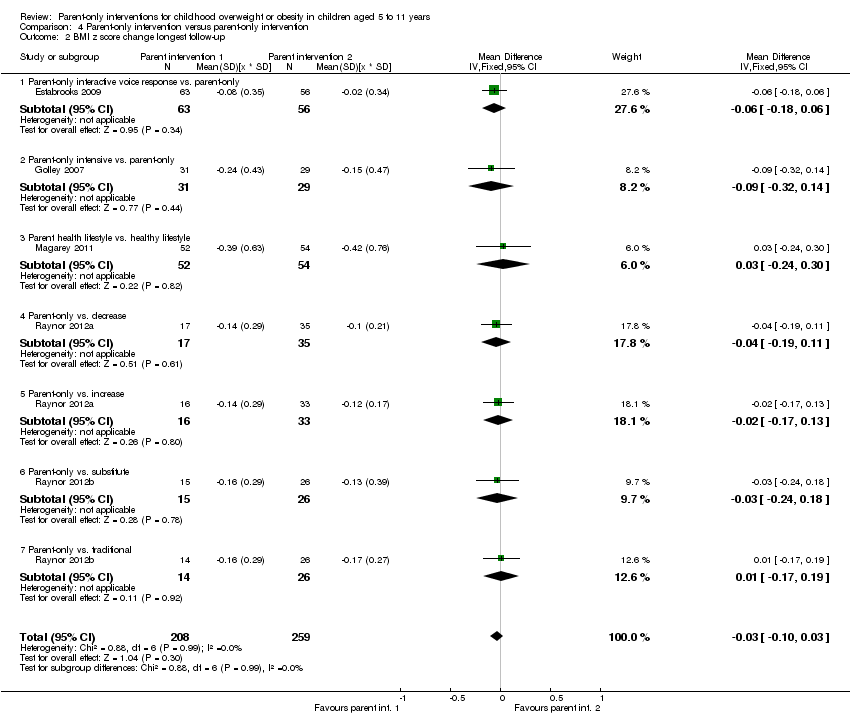 Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 2 BMI z score change longest follow‐up. | ||||
| 2.1 Parent‐only interactive voice response vs. parent‐only | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 2.2 Parent‐only intensive vs. parent‐only | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 2.3 Parent health lifestyle vs. healthy lifestyle | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.24, 0.30] |
| 2.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 2.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.17, 0.13] |
| 2.6 Parent‐only vs. substitute | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 2.7 Parent‐only vs. traditional | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.19] |
| 3 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 3 BMI change post intervention. | ||||
| 4 BMI change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 4 BMI change longest follow‐up. | ||||
| 5 BMI percentile change post intervention [%] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.5 ![Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 5 BMI percentile change post intervention [%].](/cdsr/doi/10.1002/14651858.CD012008/media/CDSR/CD012008/image_n/nCD012008-CMP-004-05.png) Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 5 BMI percentile change post intervention [%]. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not investigated in some studies).
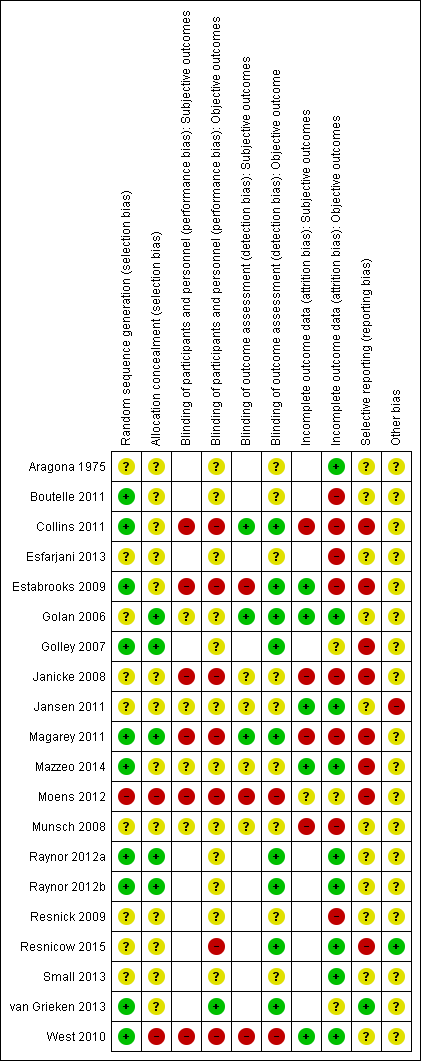
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not report that particular outcome).

Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 1 BMI z score change post intervention.
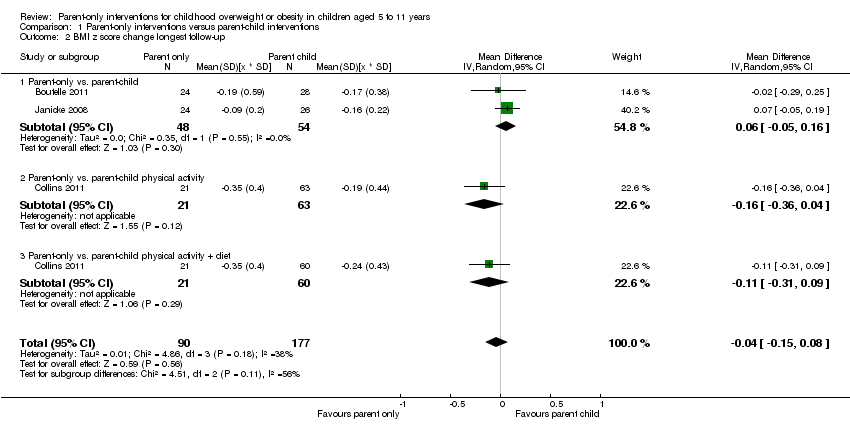
Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 2 BMI z score change longest follow‐up.

Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 1 BMI z score change post intervention.

Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 2 BMI z score change longest follow‐up.

Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 3 BMI percentile change post intervention.
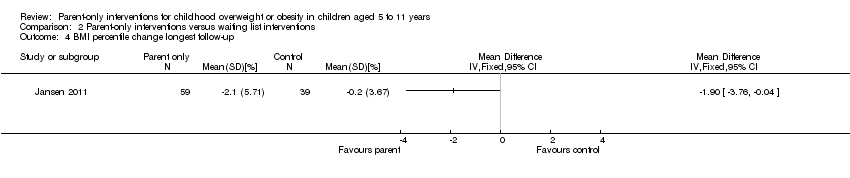
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 4 BMI percentile change longest follow‐up.
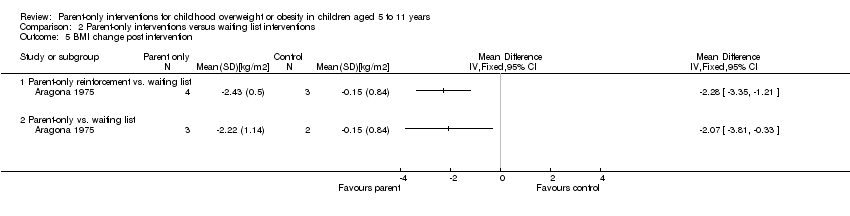
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 5 BMI change post intervention.
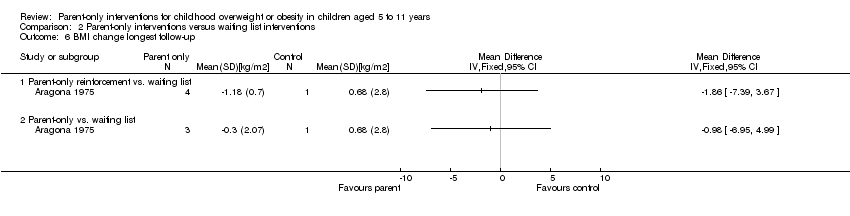
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 6 BMI change longest follow‐up.
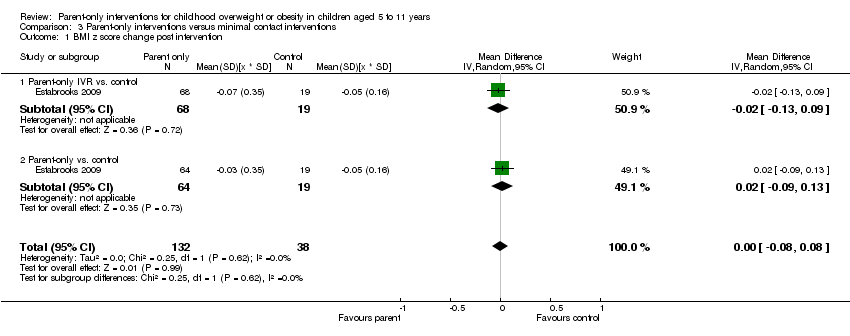
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 1 BMI z score change post intervention.

Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 2 BMI z score change longest follow‐up.

Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 3 BMI percentile change post intervention.
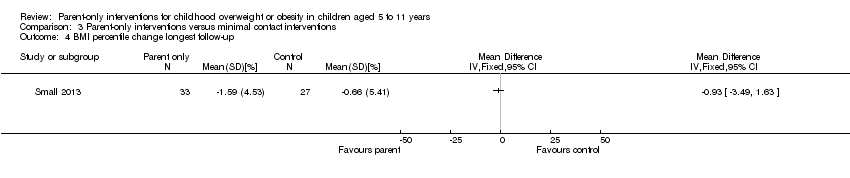
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 4 BMI percentile change longest follow‐up.
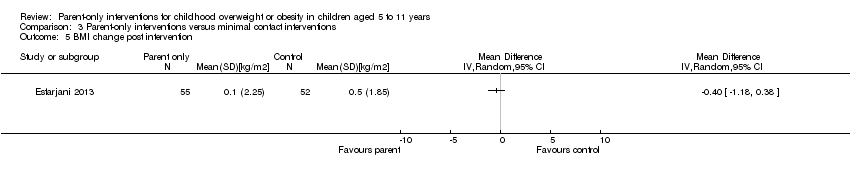
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 5 BMI change post intervention.

Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 6 BMI change longest follow‐up.
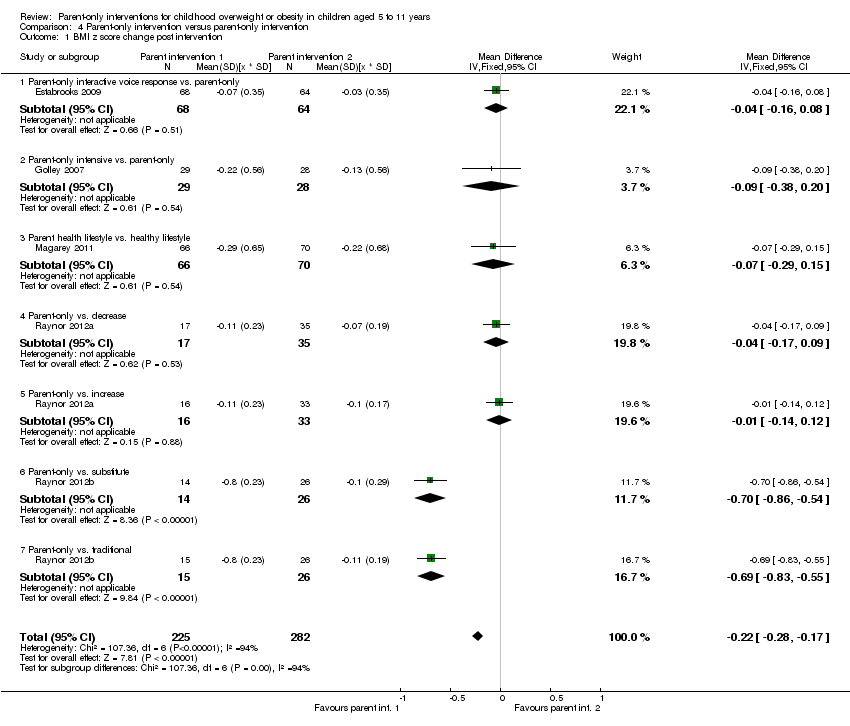
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 1 BMI z score change post intervention.

Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 2 BMI z score change longest follow‐up.

Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 3 BMI change post intervention.
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 4 BMI change longest follow‐up.
![Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 5 BMI percentile change post intervention [%].](/es/cdsr/doi/10.1002/14651858.CD012008/media/CDSR/CD012008/image_n/nCD012008-CMP-004-05.png)
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 5 BMI percentile change post intervention [%].
| Parent‐only interventions vs. parent‐child interventions for childhood overweight or obesity | ||||||
| Population: children with overweight or obesity Settings: outpatients; community/university Intervention: parent‐only interventions Comparison: parent‐child interventions | ||||||
| Outcomes | Parent‐child | Parent‐only | Relative effect | No of participants | Quality of the evidence | Comments |
| BMI z score change (x * SD) Follow‐up: 40‐104 weeks | The mean BMI z score change ranged across control groups from ‐0.16 to ‐0.24 | The mean BMI z score change in the intervention groups was 0.04 lower (0.15 lower to 0.08 higher) | ‐ | 267 (3) | ⊕⊕⊝⊝ | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
| Parent‐child relationship or assessment of parenting | See comment | See comment | See comment | See comment | See comment | No trials reported outcomes assessing parent‐child relationships or an assessment of parenting |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence | ||||||
| "A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011). aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9). | ||||||
| Parent‐only interventions vs. waiting list control for childhood overweight or obesity | ||||||
| Population: children with overweight or obesity Settings: outpatients; community Intervention: parent‐only interventions Comparison: waiting list control | ||||||
| Outcomes | Waiting list | Parent‐only | Relative effect | No of participants | Quality of the evidence | Comments |
| BMI z score change (x * SD) Follow‐up: 40‐48 weeks | The mean BMI z score change ranged across control groups from ‐0.13 to 0.02 | The mean BMI z score change in the intervention groups was 0.1 lower (0.19 lower to 0.01 lower) | ‐ | 136 (2) | ⊕⊕⊝⊝ | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
| Parent‐child relationship or assessment of parenting (parenting scale (PS), 30 items, scored from 1 to 7; lower scores indicate more effective parental discipline practices) Follow‐up: 12 weeks | The mean PS score for the control group was 3.4 | The mean PS score in the intervention group was 0.6 points lower | ‐ | 101 (1) | ⊕⊕⊝⊝ | ‐ |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence | ||||||
| "A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011). aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9). | ||||||
| Parent‐only interventions vs. minimal contact control for childhood overweight or obesity | ||||||
| Population: children with overweight or obesity Settings: outpatients Intervention: parent‐only interventions Comparison: minimal contact control | ||||||
| Outcomes | Minimal contact | Parent‐only | Relative effect | No of participants | Quality of the evidence | Comments |
| BMI z score change (x * SD) Follow‐up: 52 weeks | The mean BMI z score change ranged across control groups from ‐0.06 to ‐0.06 | The mean BMI z score change in the intervention group was 0.01 lower (‐0.07 lower to 0.09 higher) | ‐ | 165 (1) | ⊕⊕⊝⊝ | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
| Health‐related quality of life (Pediatric Health‐Related Quality of Life, scale from 0 to 100; higher scores indicate better HRQoL) Follow‐up: 24 weeks) | See comment | See comment | See comment | 93 (1) | See comment | No data were presented ('"no improvements in health‐related quality of life") |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
| Parent‐child relationship or assessment of parenting (Child Feeding Questionnaire subscale parental concern (total of 7 subscales), score range 3‐15; higher scores indicate greater parental concern) Follow‐up: 12 weeks | The mean parent concern score was 4.7 in the control group | The mean parent concern score in the intervention group was 0.1 lower. | ‐ | 93 (1) | ⊕⊕⊝⊝ | ‐ |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence | ||||||
| "A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011). aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9). | ||||||
| Parent‐only interventions vs. parent‐only interventions for childhood overweight or obesity | ||||||
| Population: children with overweight or obesity Settings: outpatients; university + primary care Intervention: parent‐only interventions Comparison: parent‐only interventions | ||||||
| Outcomes | Parent‐only | Parent‐only | Relative effect | No of participants | Quality of the evidence | Comments |
| BMI z score change (x * SD) Follow‐up: 12‐24 months | See comment | See comment | See comment | 467 (5) | ⊕⊕⊝⊝ | No meta‐analysis because of little consistency between trial interventions and comparators; there were no substantial differences between different parent‐only interventions |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Two trials reported that there were no serious adverse events (Raynor 2012a; Raynor 2012b) |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
| Parent‐child relationship or assessment of parenting (Alabama Parenting Questionnaire, 35 items; higher scores indicate improvement) Follow‐up: 24 months | See comment | See comment | See comment | 106 (1) | See comment | 1 study assessed parent‐child relationship or assessment of parenting but there were no data for comparisons between intervention groups provided |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence | ||||||
| "A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011). aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9) | ||||||
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| (20) Resnicow 2015 | I1: parent‐only PCP motivational interviewing | The study was powered to detect a 3‐point difference in BMI percentile between any pair of study groups at 2‐year follow‐up, with an assumed SD for BMI percentile between 4 and 6: power of 0.80 and 2‐tailed a of 0.05. Sample size was inflated to account for practice‐level clustering, assuming an intraclass correlation between 0.01 and 0.05. On this basis and a projected 25‐30% attrition at 2‐year follow‐up, 10‐12 practices per arm (30‐36 total) and a mean of 15‐20 children per practice at baseline were required | ‐ | 16 practices 212 participants | 145 | 145 | 145 | 68 | 2 years (2 years) |
| I2: parent‐only PCP + dietician motivational interviewing | 15 practices 235 participants | 154 | 154 | 154 | 66 | ||||
| C: usual care | 11 practices 198 participants | 158 | 158 | 158 | 80 | ||||
| total: | 645 | 457 | 457 | 457 | 71 | ||||
| (19) Mazzeo 2014 | I: parent NOURISH | ‐ | 235 | 48 | ‐ | 46 | 10 | 21 | 12 weeks post 12‐week intervention (24 weeks) |
| C: parent control | 45 | ‐ | 45 | 16 | 36 | ||||
| total: | 93 | 91 | 26 | 28 | |||||
| (18) Van Grieken 2013 | I: parent‐only | Sample size was calculated taking into account the intracluster correlation coefficient (ρ = 0.1), the number of clusters (44), the expected prevalence of overweight children in the study population, the SD, expected effect (a difference in mean), and the power of the study (80%). With a participation of 50%, an expected prevalence of overweight children of 9% and a loss‐to‐follow‐up of 30%, at least 11,301 children (and their parents) should be invited by the YHC teams to participate in the study to have a final sample of about 356 overweight children (178 in both the intervention and control group). Assuming a SD of BMI to be 1.0 kg/m2, a difference in mean BMI of 0.35 kg/m2 between the children in the intervention group and the children in the control group can be established under the assumptions mentioned above | 22 clusters 7004 participants | 349 | ‐ | 21 clusters 277 participants | 277 | 79 | 2 years post up to 12‐month intervention |
| C: usual care | 22 clusters 7004 participants | 288 | ‐ | 21 clusters 230 participants | 230 | 80 | |||
| total | 637 | ‐ | 42 (507) | 507 | 80 | ||||
| (17) Small 2013 | I: parent‐only | ‐ | ‐ | 34 | 33 | 33 | 33 | 97 | 24 weeks post 16‐week intervention (41 weeks) |
| C: parent control | 33 | 27 | 27 | 27 | 82 | ||||
| total: | 67 | 60 | 60 | 60 | 90 | ||||
| (16) Esfarjani 2013 | I: parent‐only | ‐ | 550/156 | 70 | ‐ | 55 | 58 | 83 | Intervention 6 months (not reported) |
| C: parent control | 86 | ‐ | 52 | 59 | 69 | ||||
| total: | 156 | ‐ | 107 | 117 | 75 | ||||
| (15) Moens 2012 | I: parent‐only | ‐ | 80/75 | 31 | ‐ | ‐ | 31 | 100 | Immediately following 6‐month intervention |
| C: waiting list control | 19 | ‐ | ‐ | 15 | 79 | ||||
| total: | 50 | ‐ | ‐ | 46 | 92 | ||||
| (14) Raynor 2012a | I1: parent‐only | Sample size calculations presumed 2‐sided hypothesis testing at 6‐month assessment, with type 1 error rate = 0.05. To reject with 80% power the null hypothesis of no pre‐ to post‐treatment difference between intervention conditions vs. the alternative that the pre‐ to post‐treatment difference was 0.6 or greater (effect size), 24 participants per group were needed | 549 | 33 | 33 | 33 | 29 | 88 | 24 weeks post 24‐week intervention (reported as '12 months') |
| I2: parent ‐ diet decrease | 33 | 33 | 33 | 29 | 88 | ||||
| I3: parent ‐ diet increase | 35 | 35 | 35 | 32 | 91 | ||||
| total: | 101 | 101 | 101 | 90 | 89 | ||||
| (13) Raynor 2012b | I1: parent‐only | Sample size calculations presumed 2‐sided hypothesis testing at 6‐month assessment, with type 1 error rate = 0.05. To reject with 80% power the null hypothesis of no pre‐ to post‐treatment difference between intervention conditions vs. the alternative that the pre‐ to post‐treatment difference is 0.6 or greater (effect size), 24 participants per group were needed | 549 | 29 | 29 | 29 | 26 | 90 | 24 weeks post 24‐week intervention (reported as '12 months') |
| I2: parent ‐ diet and activity traditional | 26 | 26 | 26 | 24 | 92 | ||||
| I3: parent ‐ diet and activity substitute | 26 | 26 | 26 | 24 | 92 | ||||
| total: | 81 | 81 | 81 | 74 | 91 | ||||
| (12) Margarey 2011 | I: parent healthy lifestyle | Sample size calculation was based on a reduction in BMI z score of 0.26 (SD 0.49) over 12 months (power 80%, alpha = 0.05, and drop‐out rate of 30%). This represents a 50% reduction in weight velocity over 12 months and no change in height velocity. We sought 42 children per group per site (168 children) | 398 | 85 | 85 | 85 | 52 | 61 | 80 weeks post 24‐week intervention (104 weeks) |
| C: healthy lifestyle | 84 | 84 | 84 | 54 | 64 | ||||
| total: | 169 | 169 | 169 | 106 | 63 | ||||
| (11) Jansen 2011 | I: parent CBT | ‐ | 161 | 59 | ‐ | 54 | 54 | 92 | 12 weeks post 12‐week intervention (24 weeks) |
| C: waiting list control | 39 | ‐ | 34 | 34 | 87 | ||||
| total: | 98 | 88 | 88 | 90 | |||||
| (10) Collins 2011 | I: parent‐only ‐ diet | Power: 80% chance of detecting significance (2‐sided 5% level), with a 0.26 BMI z score difference from baseline to 12 months as the initial end point, with an anticipated loss to follow‐up of 20% | 505/319 | 63 | ‐ | 42 | 22 | 35 | 80 weeks post 24‐week intervention (104 weeks) |
| C1: parent‐child (physical activity) | 73 | ‐ | 63 | 35 | 48 | ||||
| C2: parent‐child (physical activity + diet) | 70 | ‐ | 60 | 36 | 51 | ||||
| total: | 206 | ‐ | 165 | 93 | 45 | ||||
| (9) Boutelle 2011 | I: parent‐only | Sample size was determined by pragmatic factors, including budget and investigator time commitments. No interim analyses were done. The hypotheses tested related to non‐inferiority of the parent treatment to the parent‐child treatment on child and parent weight loss and child daily caloric intake and physical activity. The bound for non‐inferiority hypotheses related to BMI percentile was set to 1. This is the maximum value the parent‐child group could do better than parent‐only, below which non‐inferiority would be concluded. This bound could correspond to an mean‐aged child in this sample having a BMI of 26 in the parent‐child group and 28.5 in the parent‐only group at post‐treatment/follow‐up, assuming equivalence at baseline. For a non‐inferiority bound for child BMI, which was selected post hoc, we considered choosing a BMI that would correspond to the BMI percentile non‐inferiority bound (BMI = 2.5), but instead chose a more rigorous value of BMI = 1 | 157 | 40 | ‐ | 24 | 24 | 60 | 24 weeks post 20‐week intervention (week 44) |
| C: parent‐child | 40 | ‐ | 28 | 28 | 70 | ||||
| total: | 80 | 52 | 52 | 65 | |||||
| (8) West 2010 | I: parent‐only | ‐ | 205 | 52 | 52 | 52 | 34 | 65 | 40 weeks post 12‐week intervention (52 weeks) |
| C: waiting list control | 49 | 49 | 49 | 46 | 94 | ||||
| total: | 101 | 101 | 101 | 80 | 79 | ||||
| (7) Resnick 2009 | I: educational material + personal encounters | ‐ | 84/46 | 22 | ‐ | 18 | 18 | 82 | Unclear (41 weeks between start and last mail out) |
| C: educational material | 24 | ‐ | 24 | 24 | 100 | ||||
| total: | 46 | 42 | 42 | 91 | |||||
| (6) Estabrooks 2009 | I1: parent group + IVR | Sample size calculations were completed, varying the detectable effect sizes from small to medium with a power of 0.8. The result was a need for 42 participants per intervention to detect a medium effect and 64 participants to detect a small effect | 1487/656 | 85 | ‐ | 63 | 63 | 74 | 28‐40 weeks post 12‐ to 24‐week intervention (52 weeks) |
| I2: parent group | 85 | ‐ | 56 | 56 | 66 | ||||
| C: parent workbook | 50 | ‐ | 36 | 36 | 72 | ||||
| total: | 220 | 155 | 155 | 70 | |||||
| (5) Munsch 2008 | I: mother‐only CBT | Trial authors did not reach the necessary sample size of 68 families with obese children within the given time span (the target sample size of 68 was based on a repeated‐measures analysis with alpha = 0.05, 1 ‐ beta = 0.8, and a medium effect size for the linear term of the interaction between treatment and time, assuming a drop‐out rate of 20% | 181/60 | 25 | ‐ | 7 | 7 | 28 | 24 weeks post 10‐week intervention (34 weeks) |
| C: mother‐child CBT | 31 | ‐ | 20 | 20 | 65 | ||||
| total: | 56 | 27 | 27 | 48 | |||||
| (4) Janicke 2008 | I: parent‐only | Post hoc power analyses were used to determine the detectable change in BMI z score from 0 to 10 months for the family based and parent‐only interventions relative to the waiting list control condition. Effect sizes (standardised BMI index) detectable with 80% power and 2‐sided level 0.05 tests were used. Standard deviations and sample sizes were set equal to their observed values. For comparing the family‐based and waiting list control conditions, trial authors reported 80% power to detect a shift from 0.022 to ‐0.145. For comparing the parent‐only and waiting list control conditions, trial authors reported 80% power to detect a shift from 0.022 to ‐0.135 | 111 | 34 | ‐ | 26 | 26 | 76 | 14 weeks post 16‐week intervention (40 weeks) |
| C1: parent‐child | 33 | ‐ | 24 | 24 | 73 | ||||
| C2: waiting list control | 26 | ‐ | 21 | 21 | 81 | ||||
| total: | 93 | 71 | 71 | 76 | |||||
| (3) Golley 2007 | I: parent intervention + lifestyle education | Sample size calculation was based on a fall in BMI z score reflecting a weight gain of only 50% of that expected over 12 months with normal growth. A sample size of 28 per group was estimated to have 80% power to detect a 12‐month fall in mean BMI z score from a baseline of 0.26 (SD 0.49), assuming no change in the control group, at a 2‐sided significance level of 0.05. To account for a drop‐out rate of up to one‐third (commonly 20‐50% in child weight‐management studies), 42 children per study group were sought (126 children) | 262/115 | 38 | ‐ | 31 | 31 | 82 | 24 weeks post 24‐week intervention (48 weeks) |
| C1: parent intervention | 37 | ‐ | 29 | 29 | 78 | ||||
| C2: waiting list control | 36 | ‐ | 31 | 31 | 86 | ||||
| total: | 111 | 101 | 101 | ‐ | |||||
| (2) Golan 2006 | I: parent‐only | The study was designed to detect differences of 10% weight loss with a power of 90% and a significance level of 0.05, given a drop‐out rate of 10% with a sample of 12 in each group | 102 | 14 | ‐ | 10 | 10 | 71 | 1 year post 26‐week intervention (18 months) |
| C: parent‐child | 18 | ‐ | 17 | 17 | 94 | ||||
| total: | 32 | 27 | 27 | 84 | |||||
| (1) Aragona 1975 | I1: parent‐only with reinforcement | ‐ | ‐ | 5 | ‐ | 4 | 4 | 80 | 12 weeks (51 weeks' follow‐up) |
| I2: parent‐only | 5 | ‐ | 3 | 3 | 60 | ||||
| C: waiting list control | 5 | ‐ | 5 | 2 | 40 | ||||
| total: | 15 | 12 | 9 | 60 | |||||
| Grand total | All interventions | ‐ | 1773c | 1276 | |||||
| All comparators | 1284c | 942 | |||||||
| All interventions and comparators | 3057c | 2218 | |||||||
| aAccording to power calculation in trial publication or report "‐" denotes not reported BMI: body mass index; C: comparator; CBT: cognitive behavioural therapy; I: intervention; ITT: intention‐to‐treat; IVR: interactive voice response; n: number of participants; NOURISH: nourishing our understanding of role modelling to improve support and health; PCP: primary care providers; SD: standard deviation; YHC: Youth Health Care | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 1.1 Parent‐only vs. parent‐child | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.04] |
| 1.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| 1.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 2 BMI z score change longest follow‐up Show forest plot | 3 | 267 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.08] |
| 2.1 Parent‐only vs. parent‐child | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.16] |
| 2.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.36, 0.04] |
| 2.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.31, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.21, ‐0.04] |
| 2 BMI z score change longest follow‐up Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 2.1 Parent‐only vs. waiting list | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.21, ‐0.01] |
| 2.2 Parent‐only intensive education vs. waiting list | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 3 BMI percentile change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 BMI percentile change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 BMI change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 1 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| 1.1 Parent‐only IVR vs. control | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 1.2 Parent‐only vs. control | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 2 BMI z score change longest follow‐up Show forest plot | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 2.1 Parent‐only interactive voice response vs. control | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 2.2 Parent‐only vs. control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.07, 0.15] |
| 3 BMI percentile change post intervention Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Parent‐only vs. minimal contact control | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Parent motivational interviewing vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Parent motivational interviewing + dietician vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI percentile change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 BMI change longest follow‐up Show forest plot | 2 | 614 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.39, 0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI z score change post intervention Show forest plot | 5 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.28, ‐0.17] |
| 1.1 Parent‐only interactive voice response vs. parent‐only | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
| 1.2 Parent‐only intensive vs. parent‐only | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.38, 0.20] |
| 1.3 Parent health lifestyle vs. healthy lifestyle | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.29, 0.15] |
| 1.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 1.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 1.6 Parent‐only vs. substitute | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.86, ‐0.54] |
| 1.7 Parent‐only vs. traditional | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.83, ‐0.55] |
| 2 BMI z score change longest follow‐up Show forest plot | 5 | 467 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 2.1 Parent‐only interactive voice response vs. parent‐only | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 2.2 Parent‐only intensive vs. parent‐only | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 2.3 Parent health lifestyle vs. healthy lifestyle | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.24, 0.30] |
| 2.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 2.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.17, 0.13] |
| 2.6 Parent‐only vs. substitute | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 2.7 Parent‐only vs. traditional | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.19] |
| 3 BMI change post intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 BMI change longest follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI percentile change post intervention [%] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

