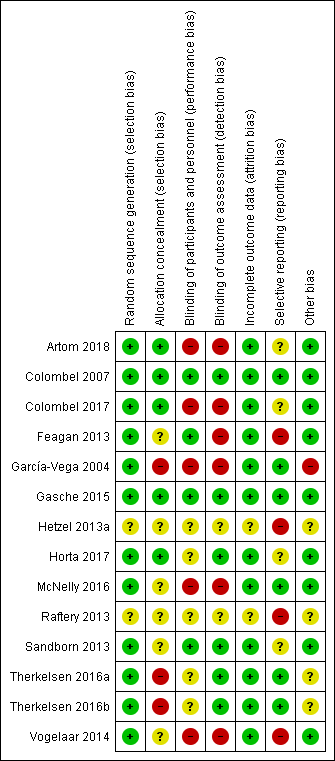
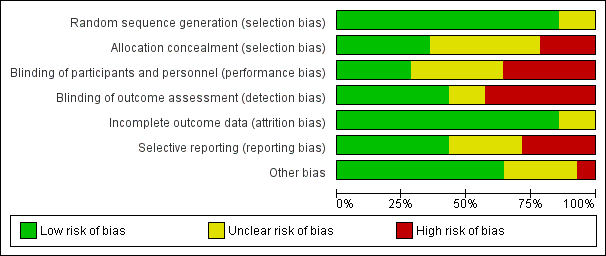
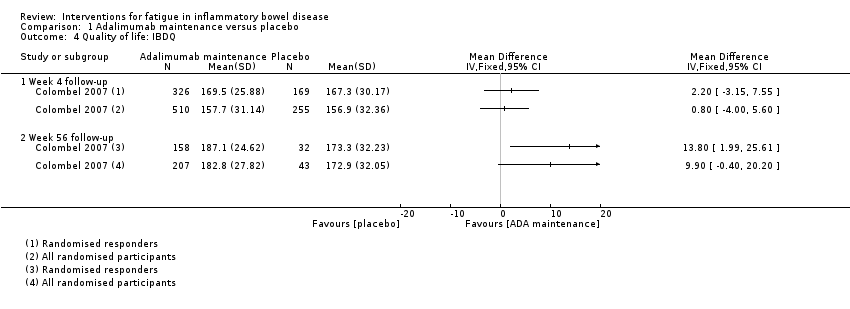
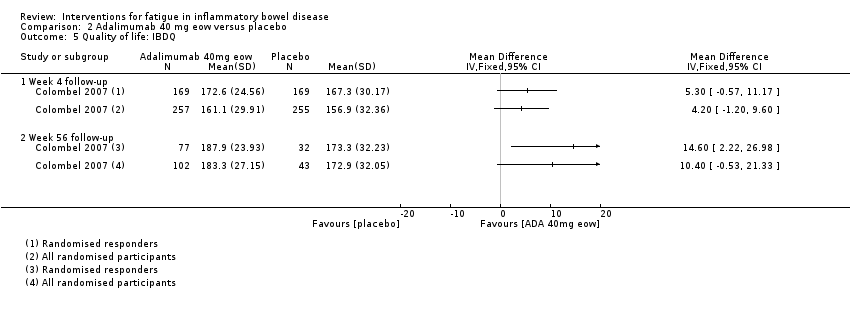
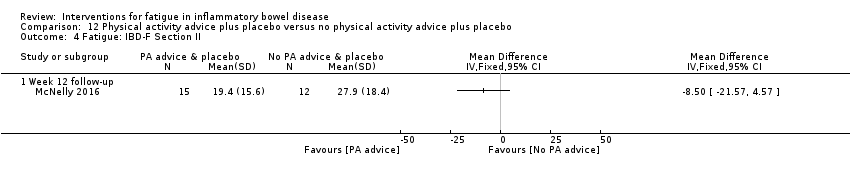
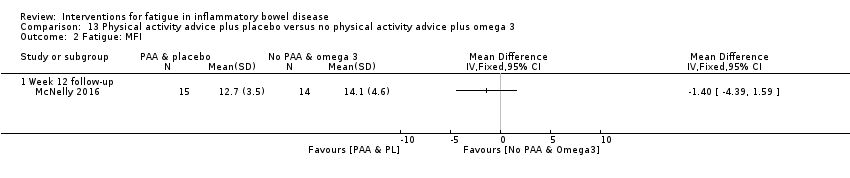
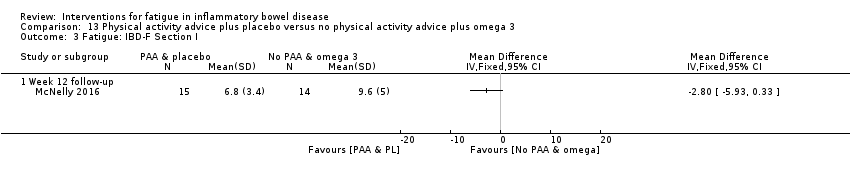
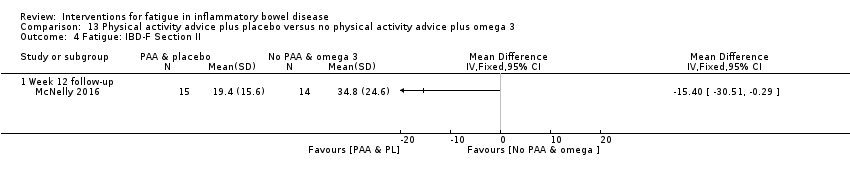
Contenido relacionado
Revisiones y protocolos relacionados
Intervenciones para el tratamiento de la anemia ferropénica en la enfermedad intestinal inflamatoria
Morris Gordon, Vassiliki Sinopoulou, Zipporah Iheozor-Ejiofor, Tariq Iqbal, Patrick Allen, Sami Hoque, Jaina Engineer, Anthony K Akobeng | 20 enero 2021
Nilesh Chande, Yongjun Wang, John K MacDonald, John WD McDonald | 27 agosto 2014
Claire E Parker, Tran M Nguyen, Dan Segal, John K MacDonald, Nilesh Chande | 1 abril 2018
Xin‐Pu Miao, Jian‐Sheng Li, Qin Ouyang, Ren‐Wei Hu, Yan Zhang, Hui‐Yan Li | 23 octubre 2014
John K Marshall, Marroon Thabane, A Hillary Steinhart, Jamie R Newman, Anju Anand, E Jan Irvine | 14 noviembre 2012
Ali Rezaie, M Ellen Kuenzig, Eric I Benchimol, Anne Marie Griffiths, Anthony R Otley, A Hillary Steinhart, Gilaad G Kaplan, Cynthia H Seow | 3 junio 2015
Alistair Murray, Tran M Nguyen, Claire E Parker, Brian G Feagan, John K MacDonald | 28 agosto 2020
Mary E Sherlock, John K MacDonald, Anne Marie Griffiths, A Hillary Steinhart, Cynthia H Seow | 26 octubre 2015
Cassandra M Townsend, Tran M Nguyen, Jeremy Cepek, Mohamad Abbass, Claire E Parker, John K MacDonald, Reena Khanna, Vipul Jairath, Brian G Feagan | 16 mayo 2020
Alistair Murray, Tran M Nguyen, Claire E Parker, Brian G Feagan, John K MacDonald | 12 agosto 2020