Kortikosteroide für erwachsene Patienten mit fortgeschrittenen Tumorerkrankungen mit Übelkeit und Erbrechen (nicht in Zusammenhang mit Chemotherapie, Strahlentherapie oder chirurgischem Eingriff)
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, parallel‐arm trial Five international centres Study duration: seven days | |
| Participants | Fifty‐one participants (25 intervention, 26 control) with advanced cancer and chronic nausea (> two weeks) resulting from advanced cancer, despite treatment with metoclopramide at a minimal daily dose of 40 to 60 mg for two days. | |
| Interventions | Participants randomised into two groups to receive dexamethasone (20 mg/day) + metoclopramide (60 mg/day), or placebo + metoclopramide (60 mg/day). All interventions administered per oral route. | |
| Outcomes | Appetite, nausea, fatigue, and pain, measured on both a 0 to 10 numerical rating scale (NRS; 0 = symptom absent, 10 = worst possible symptom) and a categorical scale of four categories for appetite, nausea, and fatigue (1 = best appetite or no nausea or fatigue, 4 = worst). The number of vomiting episodes in the preceding 24 hours was recorded. Wellbeing was estimated on a 0 to 10 numerical scale (0 = best possible well‐being, 10 = worst possible well‐being). Quality of life (physical, social and family, emotional, and functional well‐being) measured by the Functional Assessment of Cancer Therapy (FACT) instrument. Toxicity assessment: presence or absence of ankle oedema, insomnia, restlessness, or other symptoms (patient‐rated). | |
| Notes | Dexamethasone was not significantly better than placebo in the management of chronic nausea, appetite, or fatigue. Dexamethasone did improve appetite sooner (day 3), however, no significant difference in improvement in appetite by day eight compared to placebo. No significant difference between the dexamethasone and placebo groups for well‐being, quality of life, medium number of daily vomiting episodes, or adverse effects. Study funded by The Brown Foundation, Houston, Texas. Author FS was supported by a grant from Swiss Cancer Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind, capsules containing either drug or placebo were identical in appearance |
| Incomplete outcome data (attrition bias) | Low risk | Three of 25 participants receiving dexamethasone dropped out, compared to five of 26 participants receiving placebo |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 51 participants; < 50 participants per treatment arm |
| Methods | Randomised, parallel‐arm trial Single‐institution Study duration: 14 days | |
| Participants | Two hundred and eighty participants (40 metoclopramide + dexamethasone, 40 metoclopramide + tropisetron, 40 metoclopramide + tropisetron + dexamethasone) with advanced cancer who, though well controlled on antiemetic medication (metoclopramide 10 mg twice daily), suddenly presented with nausea and vomiting (≥ three vomiting or retching events/day). | |
| Interventions | Participants randomised into seven groups to receive either: (i) metoclopramide (40 mg/day) + dexamethasone (2 mg/day), (ii) tropisetron (5 mg/day), (iii) tropisetron (5 mg/day) + metoclopramide (20 mg/day), (iv) tropisetron (5 mg/day) + metoclopramide (20 mg/day) + dexamethasone (2 mg/day), (v) chlorpromazine (50 mg/day) + dexamethasone (2 mg/day), (vi) tropisetron (5 mg/day) + chlorpromazine (25 mg/day), or (vii) tropisetron (5 mg/day) + chlorpromazine (25 mg/day) + dexamethasone (2 mg/ day) for 14 days. All interventions administered per oral route. | |
| Outcomes | Nausea and vomiting measured on patient diary cards at 24 hours, days three, seven, and 15. Outcomes measured on a categorical scale of four categories. Nausea control was classified as total (no nausea), major (< 4 hrs), minor (> 4 hrs to < 8 hrs), or no control (> 8 hrs). Vomiting control was classified as total (no vomiting), major (one event), minor (two events), or no control (≥ three events). Tolerability assessment: occurrence of adverse reactions (constipation, dizziness, weakness, extrapyramidal symptoms or other upsetting symptoms) (patient‐recorded). | |
| Notes | Only duration of nausea evaluated, not intensity. All antiemetic drugs well tolerated with no significant difference between intervention groups. Study was supported by Santoz Pharma Ltd, Athens, Greece. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) | High risk | Participants received different amounts of medication, therefore blinding not possible |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “…lack of blinded experimentation” and nausea was measured incompletely |
| Incomplete outcome data (attrition bias) | Low risk | Participants withdrawing from the study due to minor emesis control was low, with similar numbers across the intervention groups |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 280 participants; < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled Three study centres Study duration: 14 days | |
| Participants | One hundred and twenty participants (62 intervention, 58 control) with advanced cancer who had ≥ three cancer‐related fatigue symptoms (fatigue, pain, nausea, loss of appetite, depression, anxiety, or sleep disturbance), ≥ 4 of 10 on the Edmonton Symptom Assessment Scale (ESAS). | |
| Interventions | Participants randomised into two groups to receive dexamethasone 4 mg or placebo orally twice per day for 14 days. | |
| Outcomes | The Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) subscale, ESAS, Hospital Anxiety and Depression Scale (HADS), and Functional Assessment of Cancer Therapy–Anorexia‐Cachexia (FAACT) instruments were used. Participants were monitored for adverse events, and a research nurse supervised their completion of the rating scales. | |
| Notes | Primary endpoint was fatigue (change in the FACIT‐F subscale from baseline to day 15). Secondary outcomes included anorexia, anxiety, depression, and symptom distress scores. Study supported by Mentored Research Scholar Grant from the American Cancer Society. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | All members of the research team except the investigational pharmacist and statistician were blinded to treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) | High risk | Nineteen of 62 participants receiving dexamethasone were not evaluable |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Sample size: 120 participants; 50 to 199 participants per treatment arm |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong study design and symptoms measured did not include nausea | |
| Wrong study design | |
| Wrong study design ‐ dexamethasone was used in both arms | |
| Wrong study design ‐ dexamethasone was used in both arms | |
| Wrong outcomes ‐ did not measure nausea | |
| Wrong study design | |
| Wrong study design | |
| Wrong outcomes ‐ nausea not reported as part of symptom distress |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea at 8 days Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.53, 0.57] |
| Analysis 1.1  Comparison 1 Nausea, Outcome 1 Nausea at 8 days. | ||||

PRISMA Study flow diagram
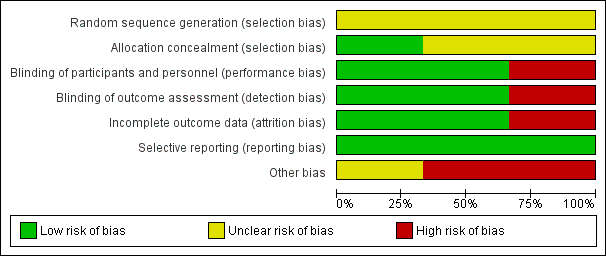
Risk of bias graph: review authors' judgements about each 'Risk of bias' domain, presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each 'Risk of bias' domain for each included study.

Forest plot of comparison: 1 Nausea, outcome: 1.1 Nausea at 8 days.

Comparison 1 Nausea, Outcome 1 Nausea at 8 days.
| Dexamethasone compared to placebo for adult patients with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery | ||||||
| Patient or population: participants with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dexamethasone | |||||
| Nausea at 8 days | The mean difference in the intensity of nausea at day 8 in the control groups ranged from ‐0.45 to 5.7 | The mean difference in the intensity of nausea at day 8 in the intervention groups was, on average, ‐0.48 (from ‐1.53 lower to 0.57 higher) | 127 | ⊕⊝⊝⊝ | ||
| Number of vomiting episodes | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Adverse events | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Quality of life | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Patient satisfaction | No data | No data | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by three levels due to imprecision, likely selection bias, attrition bias, and the small number of participants in the included studies. | ||||||
| Breast | Head, neck, and lung | Gastrointestinal | Gynaecological | Genitourinary | Sarcoma | Other | |
| x | x | x | x | x | x | ||
| x | x | x | x | ||||
| x | x | x | x | x | x | x |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea at 8 days Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.53, 0.57] |

