Corticosteroides para pacientes adultos con cáncer avanzado que presentan náuseas y vómitos (no relacionados con la quimioterapia, la radioterapia ni la cirugía)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012002.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 julio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors initiated and designed the study and drafted the protocol. KR developed the search strategy in collaboration with the Cochrane PaPaS Group. PVB, JH, PG, and KR assessed the titles and abstracts of the studies identified by the search for potential inclusion. AH, SK, JH, and PG extracted the data and conducted the 'Risk of bias' assessment. AH and SK conducted the statistical analyses and GRADE assessment. JH and PG commented on and revised the review, checked the data extraction, and arbitrated in the event of disagreement between other authors.
Sources of support
Internal sources
-
Mater Research ‐ The University of Queensland, School of Pharmacy and Menzies Health Institute Queensland, Griffith University, The Mater Palliative Care Research Fund and St Vincent’s Hospital Brisbane, Australia.
Inkind and operational funds
External sources
-
Travel fellowship for Petra Vayne‐Bossert, Switzerland.
Sponsored by the University Hospital of Geneva
Declarations of interest
JH: none known; JH is a specialist palliative medicine physician and manages patients with nausea and vomiting due to advanced cancer. JH has authored a book “Opioids in cancer pain".
PVB: none known; PVB is a specialist palliative medicine physician and manages patients with nausea and vomiting due to advanced cancer.
PG: none known; PG is a specialist palliative medicine physician and manages patients with nausea and vomiting due to advanced cancer.
AH: none known.
SK: none known.
KR: none known.
Acknowledgements
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jul 03 | Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemotherapy, radiotherapy, or surgery) | Review | Petra Vayne‐Bossert, Alison Haywood, Phillip Good, Sohil Khan, Kirsty Rickett, Janet R Hardy | |
| 2015 Dec 18 | Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemo‐ or radiotherapy, or surgery) | Protocol | Petra Vayne‐Bossert, Alison Haywood, Phillip Good, Sohil Khan, Kirsty Rickett, Sue Jenkins‐Marsh, Janet R Hardy | |
Differences between protocol and review
We expanded the description of our approach to GRADE and moved it to the data synthesis section in line with current PaPaS guidance. Blinding of participants and personnel and selective reporting have been added to the assessment of risk of bias in included studies. Four of the review authors (JH, PG, PVB, KR) independently assessed the titles and abstracts due to the large number of results obtained in the literature search.
Notes
2019
An updated search in January 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in 2020. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
2020
An updated restricted search in February 2020 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in 2022. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adrenal Cortex Hormones [adverse effects, *therapeutic use];
- Chlorpromazine [adverse effects, therapeutic use];
- Dexamethasone [adverse effects, therapeutic use];
- Indoles [adverse effects, therapeutic use];
- Metoclopramide [adverse effects, therapeutic use];
- Nausea [*drug therapy, etiology];
- Neoplasms [*complications];
- Time Factors;
- Tropisetron;
- Vomiting [*drug therapy, etiology];
Medical Subject Headings Check Words
Adult; Humans;
PICO

PRISMA Study flow diagram
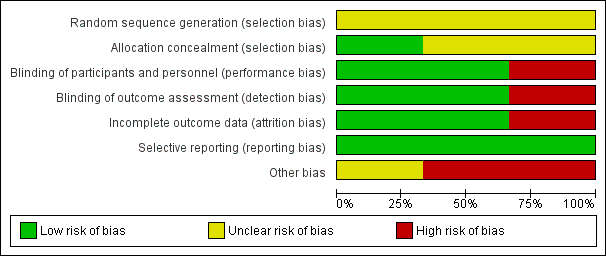
Risk of bias graph: review authors' judgements about each 'Risk of bias' domain, presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each 'Risk of bias' domain for each included study.

Forest plot of comparison: 1 Nausea, outcome: 1.1 Nausea at 8 days.

Comparison 1 Nausea, Outcome 1 Nausea at 8 days.
| Dexamethasone compared to placebo for adult patients with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery | ||||||
| Patient or population: participants with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dexamethasone | |||||
| Nausea at 8 days | The mean difference in the intensity of nausea at day 8 in the control groups ranged from ‐0.45 to 5.7 | The mean difference in the intensity of nausea at day 8 in the intervention groups was, on average, ‐0.48 (from ‐1.53 lower to 0.57 higher) | 127 | ⊕⊝⊝⊝ | ||
| Number of vomiting episodes | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Adverse events | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Quality of life | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Patient satisfaction | No data | No data | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by three levels due to imprecision, likely selection bias, attrition bias, and the small number of participants in the included studies. | ||||||
| Breast | Head, neck, and lung | Gastrointestinal | Gynaecological | Genitourinary | Sarcoma | Other | |
| x | x | x | x | x | x | ||
| x | x | x | x | ||||
| x | x | x | x | x | x | x |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea at 8 days Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.53, 0.57] |

