Corticosteroides para pacientes adultos con cáncer avanzado que presentan náuseas y vómitos (no relacionados con la quimioterapia, la radioterapia ni la cirugía)
Appendices
Appendix 1. CENTRAL search strategy
#1 (corticosteroid* OR glucocorticoid* OR corticoid* OR betamethasone OR fludrocortisone OR cortisone OR deflazacort OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone OR triamcinolone):TI,AB,KY
#2 (adrenal cortex hormones):MH 1534
#3 #1 OR #2
#4 (nause* OR vomit* OR emesis ):TI,AB,KY
#5 anti*eme*:TI,AB,KY
#6 MESH DESCRIPTOR Vomiting, Anticipatory
#7 MESH DESCRIPTOR Vomiting
#8 MESH DESCRIPTOR Nausea
#10 MESH DESCRIPTOR neoplasms EXPLODE ALL TREES
#11 (malignant* OR malignancy OR tumor* OR tumour* OR cancer* OR carcinoma* OR sarcoma* OR melanoma* OR glioma* OR glioblastoma* OR medulloblastoma*):TI,AB,KY
#12 #10 OR #11
#13 #3 AND #9 AND #12
Appendix 2. MEDLINE Ovid search strategy
1 exp Adrenal Cortex Hormones/
2 (corticoid* or corticosteroid* or glucocorticoid*).tw.
3 (adrenal adj2 hormone*).tw.
4 Betamethasone/
5 betamethasone.tw.
6 Fludrocortisone/
7 fludrocortisone.tw.
8 Cortisone/
9 cortisone.tw.
10 deflazacort.tw.
11 Dexamethasone/
12 dexamethasone.tw.
13 Hydrocortisone/
14 hydrocortisone.tw.
15 Methylprednisolone/
16 methylprednisolone.tw.
17 Prednisolone/
18 prednisolone.tw.
19 Triamcinolone/
20 triamcinolone.tw.
21 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 Nausea/
23 Vomiting/
24 Vomiting, Anticipatory/
25 Antiemetics/
26 "nause*".tw.
27 "vomit*".tw.
28 "anti*eme*".tw.
29 emesis.tw.
30 "emetic*".tw.
31 "retch*".tw.
32 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31
33 malignant.tw.
34 malignancy.tw.
35 "tumor*".tw.
36 "tumour*".tw.
37 "cancer*".tw.
38 "carcinoma*".tw.
39 "adenocarcinoma*".tw.
40 exp Neoplasms/
41 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40
42 21 and 32 and 41
43 randomized controlled trial.pt.
44 controlled clinical trial.pt.
45 randomized.ab.
46 randomised.ab.
47 placebo.ab.
48 randomly.ab.
49 trial.ab.
50 groups.ab.
51 drug therapy.fs.
52 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
53 exp animals/ not humans.sh.
54 52 not 53
55 42 and 54
Appendix 3. MEDLINE PubMed in Process search strategy
Search (nause* OR vomit* OR emesis) AND (betamethasone OR fludrocortisone OR cortison* OR corticosteroid* OR methylpred* OR dexamethasone OR prednisone OR prednisolone OR hydrocortisone) AND (neoplas* OR tumour* OR tumor OR tumors OR cancer OR malignan* OR oncol* OR carcinoma*)
Appendix 4. Embase Ovid search strategy
#35 #5 AND #23 AND #26 AND #34
#34 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #14 OR #16
#26 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR (cross NEXT/1 over*):de,ab,ti OR placebo*:de,ab,ti OR (doubl* NEAR/1 blind*):de,ab,ti OR (singl* NEAR/1 blind*):de,ab,ti OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti
#23 #17 OR #19
#19 cancer*:ab,ti OR malignan*:ab,ti OR tumo*r*:ab,ti OR carcinoma*:ab,ti OR adenocarcinoma*:ab,ti OR melanoma*:ab,ti OR glioma*:ab,ti OR glioblastoma*:ab,ti OR medulloblastoma*:ab,ti OR sarcoma*:ab,ti
#17 'neoplasm'/exp
#16 'antiemetic agent'/de
#14 nause*:ab,ti OR vomit*:ab,ti OR anti*eme*:ab,ti OR emesis:ab,ti
#11 'vomiting'/exp
#10 'serotonin syndrome'/de
#9 'opioid induced emesis'/de
#8 'nausea'/de
#7 'anticipatory nausea and vomiting'/de
#6 'nausea and vomiting'/de
#5 #1 OR #3
#3 corticoid*:ab,ti OR corticosteroid*:ab,ti OR glucocorticoid*:ab,ti OR betamethasone:ab,ti OR fludrocortisone:ab,ti OR cortisone:ab,ti OR deflazacort:ab,ti OR dexamethasone:ab,ti OR hydrocortisone:ab,ti OR methylprednisolone:ab,ti OR prednisolone:ab,ti OR triamcinolone:ab,ti
#1 'corticosteroid'/exp
Appendix 5. CINAHL EBSCO search strategy
S49 S36 AND S48
S48 S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S46 OR S47
S47 TX allocat* random*
S46 (MH "Quantitative Studies")
S45 (MH "Placebos")
S44 TX placebo*
S43 TX random* allocat*
S42 (MH "Random Assignment")
S41 TX random* control* trial*
S40 TX (singl* OR doubl* OR tripl* OR trebl*) N1 (blind* OR mask*)
S39 TX clinic* n1 trial*
S38 PT Clinical Trial
S37 (MH "Clinical Trials+")
S36 S15 AND S23 AND S35
S35 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34
S34 TX medulloblastoma
S33 TX glioblastoma
S32 TX glioma*
S31 TX melanoma*
S30 TX sarcoma*
S29 TX adenocarcinoma*
S28 TX carcinoma*
S27 TX tumo#r*
S26 TX malignan*
S25 TX cancer*
S24 (MH "Neoplasms+")
S23 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S22 TX anti#eme*
S21 TX emesis
S20 TX nause*
S19 TX vomit*
S18 vomit*
S17 (MH "Antiemetics")
S16 (MH "Vomiting") OR (MH "Nausea") OR (MH "Anticipatory Nausea and Vomiting") OR (MH "Nausea and Vomiting")
S15 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14
S14 TX triamcinolone
S13 TX prednisolone
S12 TX methylprednisolone
S11 TX hydrocortisone
S10 TX dexamethasone
S9 TX deflazacort
S8 TX cortisone
S7 TX fludrocortisone
S6 TX corticoid* OR corticosteroid* OR glucocorticoid*
S5 TX betamethasone
S4 (MH "Dexamethasone") OR (MH "Hydrocortisone") OR (MH "Prednisolone+") OR (MH "Prednisone")
S3 (MH "Cortisone")
S2 (MH "Betamethasone")
S1 (MH "Adrenal Cortex Hormones+")
Appendix 6. Web of Science search strategy (Science Citation Index AND Conference Proceedings Citation Index – Science)
TOPIC: (corticosteroid* OR betamethasone OR fludrocortisone OR corticoid* OR glucocorticoid* OR cortisone OR deflazacort OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone OR triamcinolone) AND
TOPIC: (nause* OR vomit* OR emesis OR emet* OR anti$eme*)AND
TOPIC: (cancer* OR neoplas* OR carcinoma* OR tumo$r* OR adenocarcinoma* OR malignan* OR sarcoma* OR melanoma* OR glioma* OR glioblastoma* OR medulloblastoma*) AND
TOPIC: (clinical* OR trial* OR random* OR mask* OR blind* OR allocate* OR assign* OR cross$over* OR control* OR singl* OR doubl* OR trebl* OR tripl*)
Appendix 7. LILACS search strategy
tw:(betamethasone OR cortisone OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone)) AND (tw:(cancer* OR malignan* OR tumour* OR tumor* OR adenocarcinoma* OR carcinoma*)) AND (tw:(nause* OR vomit* OR emesis OR anti*emetic)

PRISMA Study flow diagram
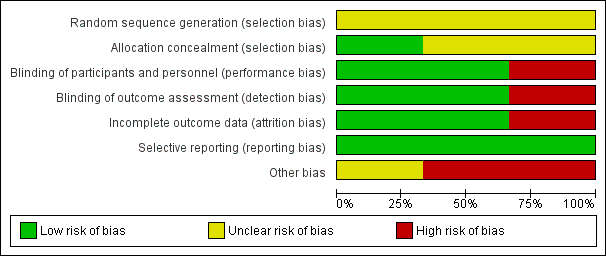
Risk of bias graph: review authors' judgements about each 'Risk of bias' domain, presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each 'Risk of bias' domain for each included study.

Forest plot of comparison: 1 Nausea, outcome: 1.1 Nausea at 8 days.

Comparison 1 Nausea, Outcome 1 Nausea at 8 days.
| Dexamethasone compared to placebo for adult patients with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery | ||||||
| Patient or population: participants with advanced cancer who have nausea and vomiting not related to chemotherapy, radiotherapy, or surgery Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dexamethasone | |||||
| Nausea at 8 days | The mean difference in the intensity of nausea at day 8 in the control groups ranged from ‐0.45 to 5.7 | The mean difference in the intensity of nausea at day 8 in the intervention groups was, on average, ‐0.48 (from ‐1.53 lower to 0.57 higher) | 127 | ⊕⊝⊝⊝ | ||
| Number of vomiting episodes | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Adverse events | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Quality of life | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Patient satisfaction | No data | No data | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by three levels due to imprecision, likely selection bias, attrition bias, and the small number of participants in the included studies. | ||||||
| Breast | Head, neck, and lung | Gastrointestinal | Gynaecological | Genitourinary | Sarcoma | Other | |
| x | x | x | x | x | x | ||
| x | x | x | x | ||||
| x | x | x | x | x | x | x |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea at 8 days Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.53, 0.57] |

