Intervenciones alimentarias para pacientes adultos con una nefropatía crónica
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE | 1. nutritional counseling/ 2. nutrition education/ 3. nutritional health/ 4. nutritional assessment/ 5. nutrition/ 6. exp diet/ 7. exp diet therapy/ 8. exp dietary intake/ 9. exp diet restriction/ 10. or/1‐9 11. exp renal replacement therapy/ 12. kidney disease/ 13. chronic kidney disease/ 14. kidney failure/ 15. chronic kidney failure/ 16. mild renal impairment/ 17. stage 1 kidney disease/ 18. moderate renal impairment/ 19. severe renal impairment/ 20. end stage renal disease/ 21. renal replacement therapy‐dependent renal disease/ 22. kidney transplantation/ 23. (hemodialysis or haemodialysis).tw. 24. (hemofiltration or haemofiltration).tw. 25. (hemodiafiltration or haemodiafiltration).tw. 26. dialysis.tw. 27. (CAPD or CCPD or APD).tw. 28. (kidney disease* or renal disease* or kidney failure or renal failure).tw. 29. (CKF or CKD or CRF or CRD).tw. 30. (ESRF or ESKF or ESRD or ESKD).tw. 31. (predialysis or pre‐dialysis).tw. 32. ((kidney or renal) adj (transplant* or graft* or allograft*)).tw. 33. Diabetic Nephropathies/ 34. diabetic nephropath$.tw. 35. diabetic kidney disease$.tw. 36. or/11‐35 37. and/10,36 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
| Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. | Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
| Selective reporting Reporting bias due to selective outcome reporting | Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
| Other bias Bias due to problems not covered elsewhere in the table | Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |

Flow diagram of study selection

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Dietary intervention versus control, Outcome 1 All‐cause mortality.

Comparison 1 Dietary intervention versus control, Outcome 2 Cardiovascular mortality.
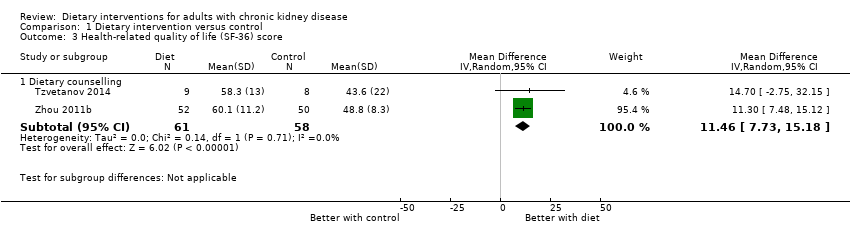
Comparison 1 Dietary intervention versus control, Outcome 3 Health‐related quality of life (SF‐36) score.

Comparison 1 Dietary intervention versus control, Outcome 4 End‐stage kidney disease.
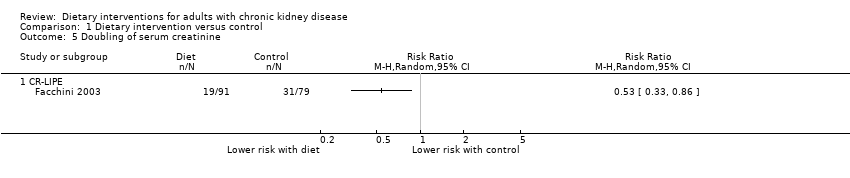
Comparison 1 Dietary intervention versus control, Outcome 5 Doubling of serum creatinine.

Comparison 1 Dietary intervention versus control, Outcome 6 Employment.

Comparison 1 Dietary intervention versus control, Outcome 7 Dietary adherence.

Comparison 1 Dietary intervention versus control, Outcome 8 Worsening nutrition.
![Comparison 1 Dietary intervention versus control, Outcome 9 eGFR [mL/min/1.73 m2].](/es/cdsr/doi/10.1002/14651858.CD011998.pub2/media/CDSR/CD011998/image_n/nCD011998-CMP-001-09.png)
Comparison 1 Dietary intervention versus control, Outcome 9 eGFR [mL/min/1.73 m2].

Comparison 1 Dietary intervention versus control, Outcome 10 Serum creatinine.

Comparison 1 Dietary intervention versus control, Outcome 11 Systolic blood pressure.
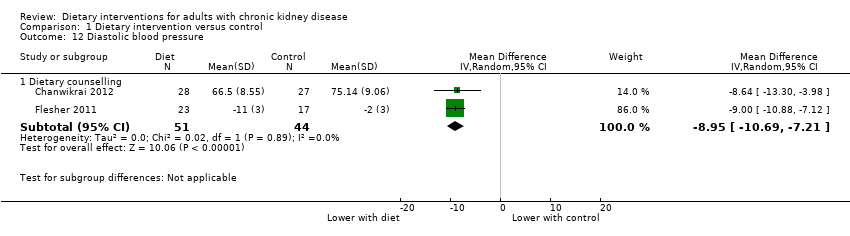
Comparison 1 Dietary intervention versus control, Outcome 12 Diastolic blood pressure.

Comparison 1 Dietary intervention versus control, Outcome 13 Energy intake.
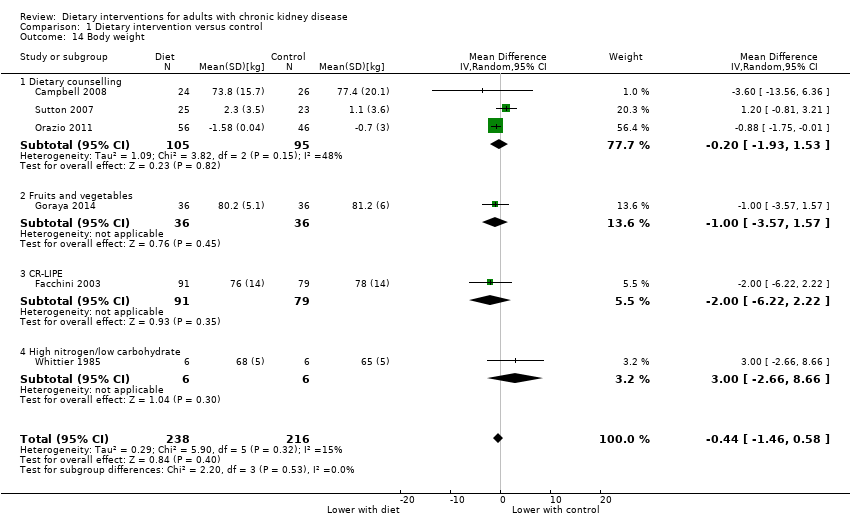
Comparison 1 Dietary intervention versus control, Outcome 14 Body weight.

Comparison 1 Dietary intervention versus control, Outcome 15 BMI.

Comparison 1 Dietary intervention versus control, Outcome 16 Waist‐hip ratio.

Comparison 1 Dietary intervention versus control, Outcome 17 Waist circumference, cm.

Comparison 1 Dietary intervention versus control, Outcome 18 Arm circumference.

Comparison 1 Dietary intervention versus control, Outcome 19 Serum albumin.

Comparison 1 Dietary intervention versus control, Outcome 20 Serum LDL cholesterol.

Comparison 2 Mediterranean diet versus low fat, Outcome 1 Serum LDL cholesterol.
![Comparison 3 Fruits and vegetables versus bicarbonate, Outcome 1 eGFR [mL/min/1.73 m2].](/es/cdsr/doi/10.1002/14651858.CD011998.pub2/media/CDSR/CD011998/image_n/nCD011998-CMP-003-01.png)
Comparison 3 Fruits and vegetables versus bicarbonate, Outcome 1 eGFR [mL/min/1.73 m2].
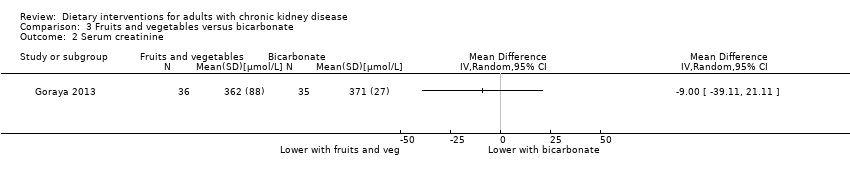
Comparison 3 Fruits and vegetables versus bicarbonate, Outcome 2 Serum creatinine.

Comparison 3 Fruits and vegetables versus bicarbonate, Outcome 3 Systolic blood pressure.
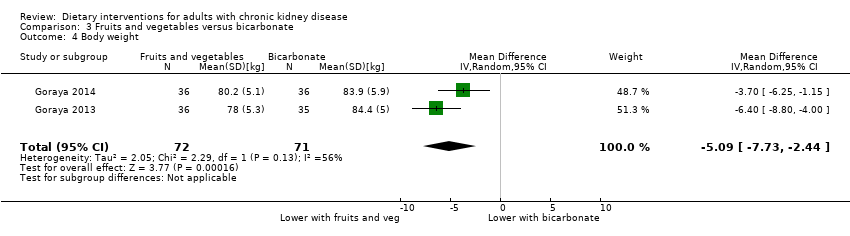
Comparison 3 Fruits and vegetables versus bicarbonate, Outcome 4 Body weight.
| Dietary modifications (counselling or dietary change) versus control for CKD | ||||||
| Patient or population: people with CKD Intervention: dietary modifications Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Dietary intervention | |||||
| Death | High risk population | Not estimable | 539 (5) | ⊕⊖⊖⊖ | Studies were not designed to measure effects of dietary interventions on mortality | |
| 150 per 1000 | Not estimable | |||||
| Medium risk population | ||||||
| 25 per 1000 | Not estimable | |||||
| Major cardiovascular event | High risk population | Not estimable | Insufficient data observations | No studies were available for this outcome | Studies were not designed to measure effects of dietary interventions on cardiovascular events. 0 studies reported major cardiovascular events | |
| 150 per 1000 | Not estimable | |||||
| Medium risk population | ||||||
| 45 per 1000 | Not estimable | |||||
| Progression to ESKD Measured as requiring dialysis treatment in people with CKD | 0.6 per 1000 | 0.3 per 1000 | RR 0.53 (0.26 to 1.07) | 242 (2) | ⊕⊖⊖⊖ | 29 participants developed ESKD in these studies. No studies included recipients of a kidney transplant |
| Health‐related quality of life Measured using the Short Form‐36 scale from 0 to 100 | The mean SF‐36 score ranged across control groups from 43.6 to 48.8 | The mean SF‐36 score in the intervention groups was 11.46 higher (95% CI 7.73 to 15.18) | 119 (2) | ⊕⊕⊖⊖ | 0 studies included recipients of a kidney transplant. None of the studies were blinded | |
| *The basis for the assumed risk of mortality (e.g. the median control group risk across studies) was obtained from the absolute population risk estimated from previously published cohort studies or data registries (Johnson 2011; Weiner 2006). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations were due to high or unclear risks of bias 2 Confidence interval includes range of plausible values that include substantial benefit or harm 3 Based on few events and/or participants across all studies 4 Data not available for recipients of a kidney transplant | ||||||
| Study ID | Treatment | Control | CKD stage | GFR (mL/min) | Mean age | % men | Mean GFR (mL/min) | Mean BMI (kg/m2) | Detailed inclusion criteria |
| Counselling | |||||||||
| Dietary counselling | Written material | 4‐5 | ≤ 30 | 69.5 (11.7) 70.9 (11.6) | 61 | 23.1 (7.2) 21.6 (6.1) | 26.8 (4.7) 27.6 (5.2) | > 18 years; eGFR < 30 mL/min/1.73 m2; CKD not previously seen by a dietitian for stage 4 CKD; absence of communication or intellectual impairment; absence of malnutrition from a cause other than CKD; not expected to require RRT within 6 months | |
| Dietary counselling | Standard care | 3‐5 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | CKD stage 3‐5 | |
| Dietary counselling + exercise | Standard care | 3‐4 | 20‐60 | 63.4 (12.1) 63.4 (11.8) | 53 | 37.2 (3.2) 38.4 (3.0) | ‐‐ | eGFR 20 to 60 mL/min for ≥3 months; presence of urinary protein; adult (≥ 19 years); hypertension or taking at least 1 antihypertensive medication; physician approval to exercise | |
| Dietary counselling and targeting nutritional barriers | Standard care | 5 (HD) | Dialysis | 62 60 | 42 | ‐‐ | 29.0 27.9 | 18 to 85 years; receiving dialysis for at least 9 months; mean serum albumin level for previous 3 months < 3.70 g/dL (bromcresol green method) or < 3.40 g/dL (bromcresol purple method) | |
| Dietary counselling | Standard care | Transplant | Transplant | 54.9 (9.9) 54.7 (11.8) | 61 | 54 (20) 48 (17) | 29 (5) 29 (6) | Kidney transplant > 6 months | |
| Dietary counselling | Low protein diet | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | CKD not requiring dialysis | |
| Dietary counselling + physical activity | Standard care | 5 (PD) | Dialysis | 60.7 (15.5) 58.5 (15.4) | 55 | ‐‐ | 25.4 (3.8) 25.7 (3.4) | Treatment with CAPD for 3 months or longer; not diabetic | |
| Dietary counselling + exercise | Standard care | 1‐3 | ‐‐ | 62.1 (14.0) 65.7 (11.2) | 71 | 53.7 (18.3) 49.5 (13.3) | 24.4 (3.9) 25.3 (3.1) | 20 years or older; communicate in Mandarin or Taiwanese; aware of CKD diagnosis; GFR range 30 to 106.7 mL/min/1.73 m2 | |
| Dietary counselling + exercise | Standard care | Transplant | Transplant | 46 (6.9) 45 (19) | 47 | ‐‐ | ‐‐ | Kidney transplant; obese | |
| Dietary counselling | Standard care | 5 (PD) | Dialysis | 57.8 (12.8) 59.9 (13.6) | 71 | ‐‐ | 23.3 (4.5) 22.8 (6.2) | 18 to 70 years; receiving long‐term dialysis > 3 months | |
| Mediterranean diet | |||||||||
| Mediterranean diet (restricted calorie) | Low‐fat (restricted calorie) diet Low‐carbohydrate (unrestricted calorie) diet | 3 | 30‐60 | 52.5 (6.2) | 99 | 52.6 (5.9) | 30.9 (3.4) | 40 to 65 years with BMI ≥ 27 kg/m2; individuals with type 2 diabetes or coronary heart disease were eligible regardless of age. Post‐hoc analysis among participants with eGFR 30 to 60 mL/min/1.73 m2 | |
| Mediterranean diet | Standard care | 2‐3 | 60‐89 | 60 (10) 59 (12) | 53 | 70 (10) 75 (15) | 26.9 (3.9) 25.1 (4.2) | eGFR 60 to 89 mL/min/1.73 m2; dyslipidaemia | |
| Modified Mediterranean diet | Low fat diet | Transplant | Transplant | 41 (12.5) 46 (9.5) | 68 | ‐‐ | 25.0 (4.1) 26.2 (4.2) | Stable transplant function | |
| Increased fruit and vegetables | |||||||||
| Increased fruit and vegetable intake | Oral bicarbonate | 4 | 15‐29 | 53.9 (6.9) 54.2 (5.3) | 54 | 22.8 (4.9) 23.0 (3.5) | ‐‐ | Non‐malignant hypertension; eGFR 15 to 29 mL/min/1.73 m2; plasma TCO2< 22 mM; no diabetes or cardiovascular disease; two or more primary care physician visits in previous year; age ≥ 18 years | |
| Increased fruit and vegetable intake | Oral bicarbonate Standard care | 3 | 30‐59 | 53.5 (5.2) 53.9 (4.8) | 44 | 42.3 (7.1) 42.6 (7.6) | ‐‐ | Non‐malignant hypertension, eGFR 30 to 59 mL/min/1.73 m2; plasma TCO2< 25 mM; macroalbuminuria; able to tolerate angiotensin‐converting inhibition; non‐smoking for ≥ 1 year; no diabetes or cardiovascular disease; 2 or more primary care physician visits in previous year; ≥ 18 years | |
| Carbohydrate‐restricted, low‐iron, polyphenol enriched (CR‐LIPE) diet | |||||||||
| CR‐LIPE diet | Protein restriction | 2‐5 | 15‐75 | 59 (10) 60 (12) | 51 | 64 (28) 62 (32) | 28 (5) 28 (5) | Type 2 diabetes; referred to nephrology clinic for kidney failure (15 ± 75 mL/min); otherwise unexplained proteinuria (350 ± 12,000 mg/d); kidney disease attributed to diabetes | |
| High‐nitrogen, low‐carbohydrate diet | |||||||||
| High‐nitrogen, low carbohydrate diet | Standard care | Transplant | Transplant | 33 32 | 75 | ‐‐ | ‐‐ | Kidney transplant; no diabetes | |
| BMI ‐ body mass index; CAPD ‐ continuous ambulatory peritoneal dialysis; CKD ‐ chronic kidney disease; eGFR ‐ estimated glomerular filtration rate; HD ‐ haemodialysis; PD ‐ peritoneal dialysis; RRT ‐ renal replacement therapy; TCO2 ‐ total carbon dioxide | |||||||||
| Study ID | Materials | Dietary intervention | Adherence | |||||||
| Why | What | Who | How | Where | When and how much | Tailoring | Modification | Planned | Actual | |
| Counselling | ||||||||||
| To determine whether individual nutrition counselling improves body composition, energy intake, and nutritional status | Individualised dietary prescription (including energy (125 to 146 kJ/kg/d) and protein (0.75 to 1.0 g/kg/d)) incorporating KDOQI recommendations to provide intensive nutritional counselling with regular monitoring | Dietitian | Face‐to‐face, telephone, individualised | ‐‐ | Baseline for 60 min; then biweekly for 1st month (15 to 30 min); then weekly till end of study period | Depending on dietary requirements, diet was tailored following clinical data and initial interview. Delivery was guided by the medical nutrition therapy framework from the American Dietetic Association | Self‐management principles: goal‐setting, menu planning, label reading, and identification of foods containing protein, sodium, and so on, depending on requirements | Dietary intake assessed using 3‐day food record, verified by the dietitian. Strategies to improve adherence or fidelity not reported | No patient voluntarily withdrew from the study | |
| Changes of diet and lifestyle can slow progression of CKD | Dietary modification with or without exercise by an empowerment approach (including low protein 0.6 to 0.8 g/kg/d) and low salt (5 g/d). | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐ | 81 (96%) completed the study program | |
| To determine whether additional of cooking and exercise classes would slow progression of CKD | Individual nutrition counselling on moderate protein and low sodium, with individualised modification of potassium and/or phosphate plus a group nutrition class, cooking classes with a dietitian and cook education, CKD cookbook, shopping tour, and 12‐week exercise program led by a Certified Exercise Physiologist and nurse. Exercise program started after 6 months | Cooking class ‐ dietitian and cook educator; Exercise ‐ exercise physiologist and nurse | Face‐to‐face; individual and group sessions | Exercise class took place in well‐equipped gym at Garatt Wellness Centre: details regarding cooking class not provided | Cooking classes over 4 weeks for 2 hour session, shopping tour; Exercise class at Garratt Wellness centre, 3 x 1 hour session/week with strength training, flexibility components, resistance training | Skills for tailoring and modifying diet and lifestyle were provided. Diet history was discussed in detail at the individual appointments | Self‐management focus in using goal‐setting and building confidence in the management of disease | Adherence to exercise was assessed by physical activity readiness questionnaire and 6 minute submaximal walk test; biochemical and clinical parameters related to cardiovascular health; monitored at baseline, 6 months and 12 months | Overall, the experimental group showed ‘improvement’’ | |
| Whether targeting specific nutritional barriers will improve albumin levels | Study coordinators abstracted medical records and interviewed participants to determine the presence of 10 specific nutritional barriers (nutritional knowledge, appetite, help needed with cooking and shopping, low fluid intake, dialysis dose, depression, difficulty chewing, difficulty swallowing, gastrointestinal symptoms, acidosis). Study coordinators educated all intervention patients about | Study coordinators; dietitians | Face‐to‐face; individualised | During dialysis sessions | During the next 12 months, study coordinators met monthly with patients to reinforce recommendations, | Tailored to specific nutritional barriers identified during interviews | Specific to nutritional barriers | ‐‐ | ‐‐ | |
| To investigate the effect of dietitian involvement in a multidisciplinary lifestyle intervention comparing risk factor modification for cardiovascular disease with standard post‐transplant care in kidney transplant recipients with abnormal glucose tolerance | Individualised dietary advice was provided to participants for the duration of the study. Achievement and/or maintenance of a healthy weight (BMI), 20 to 25 kg/m2) was the primary goal of nutrition therapy using a Mediterranean‐style (< 30% total energy from The long‐term goal of physical activity advice was to achieve 150 min of accumulated physical activity/week, in accordance with current National Physical Activity Recommendations. To help achieve this, goals The Transtheoretical Model of Health Behavior Change or Stage of Change Model underpinned the lifestyle intervention to provide a framework for goal‐setting throughout the study | Multidisciplinary team (nephrologist, dietitian, nurse, endocrinologist) | Individualised advice from nephrologist, dietitian, nurse and endocrinologist (individual or group) | Multiple locations and settings including during routine transplant care, outpatient dietetic and nursing care, and routine diabetes management | Bimonthly reviews for 2 years by nephrologist; 4‐week initial program from dietitian with bimonthly reviews for 2 years and 6 monthly group meetings; bimonthly reviews by nurse and endocrinologist | Dietitian delivery of individual diet initially and then individualist dietetic reviews including weight, waist circumference and hip circumference measurements | Specific to patient and anthropomorphic measurements during follow‐up | ‐‐ | 8/96 participants chose to withdraw | |
| To determine if a simplified dietary approach self‐managed by patients had beneficial impact on nutritional and metabolic control of CKD, to be acceptable and safe | List of recommendations to modify dietary habits (do not add salt at table or for cooking; foods to avoid; replacing noodles or bread; meat, fish and egg intake; 4‐5 servings of fruit or vegetables; replacement of noodles with legumes | Nephrologist | Face‐to‐face; individualised | ‐‐ | ‐‐ | The goal of the study was to tailor and modify diet for participants in intervention group (not otherwise specified) | ‐‐ | Adherence to diet was assessed at regular intervals (1, 3 and 6th), method for assessing adherence was not reported | 19/27 in intervention group were adherent with protein prescription whereas 12/27 in control group were adherent with protein prescription | |
| To determine whether offering dietary advice was effective in supporting patients in adjusting energy intake | The intervention group was offered follow‐up dietary advice that would encourage them to match energy intake with their estimated energy expenditure allowing for dialysate calories and with a protein intake of not < 0.8 to 1.0 g/kg IBW | Dietitian | Face‐to‐face | ‐‐ | Face‐to‐face contact at baseline and 4 months. Suggested snack ideas, alterations in food preparation, or modification of portion sizes | ‐‐ | ‐‐ | ‐ | 49/59 participants completed the study | |
| To examine effects of a targeted Lifestyle Modification Program on lifestyle behaviours, knowledge, and physical indicators of CKD | The Trans Theoretical model using the stage‐of‐change construct was used to assess the patient's readiness stage to promote behaviour change. Targeted interventions were given according to the stage of change about diet and exercise. Patients were encouraged to find individual methods of overcoming barriers to regular exercise. Written materials were provided to encourage adherence to a CKD diet. An information booklet on protecting kidney function was provided and reviewed with patient. Discussion provided information about kidney function and disease, and dietary and lifestyle management | Registered nurse research assistants | Face‐to‐face; individualised | Clinic | Counselling provided with each clinic visit | The goal of the study to tailor and modify diet for participants in intervention group | ‐‐ | To ensure the fidelity of the Lifestyle Modification Program, all provided counselling and information were recorded, and the interventions were reviewed by the investigators at random | There was a 64.4% retention rate at 12 months | |
| Examine the effectiveness of a physical exercise program including behaviour modification interventions and nutritional training for obese recipients of a kidney transplant | Individual physical training (one‐to‐one sessions with a coach) using low‐impact, low‐repetition, resistance‐based weight training with 2 x 1‐hour sessions each week in | Coach | Individual training | Private environment | 2 x 1‐hour sessions each week for 12 months | Standardised process | Response to participants muscle strength, empowerment, and identifying most impactful behaviour/lifestyle changes for each patient | ‐ | Only 4/8 people allocated to the control returned to the 6 month follow up appointment and 2 for the 12 month appointment. Adherence with the supervised rehabilitation program and follow up was 100% in people allocated to the intervention | |
| To investigate the effects of nutrition intervention and individualised nursing care on nutritional status and quality of life in people with ESKD receiving peritoneal dialysis | An individualised nutrition intervention developed by dietitian with regard to the patient's nutritional status, clinical condition, and characteristics. The study group received the following intervention: energy 125 kJ/kg/d, protein 1.2 to 1.3 g/kg/d, and 70% to 75% proportion of protein as of high biological value. Oral enteral nutrition supplements were used for patients who did not receive enough nutrients from food. The volume of water intake was equivalent to the urine volume plus 500 mL/d and sodium was 3 g/d. In addition, nurse practitioners provided psychological care, an individualised exercise program, and blood pressure treatment | Dietitian and nurses | Individual face‐to‐face | ‐‐ | Psychological support was given for 30 min once‐monthly over 6 months | Individualised according to nutritional and clinical status | ‐‐ | ‐‐ | Not reported | |
| Mediterranean diet | ||||||||||
| To investigate the long‐term effect of Mediterranean diet on kidney function | Mediterranean diet: moderate‐fat, restricted calorie, rich in vegetables and low in red meat, with poultry and fish replacing beef and lamb. Energy intake was restricted to 1500 kcal/d for women and 1800 kcal/d for men, with a goal of no more than 35% of calories from fat; the main sources of added fat were 30 to 45 g of olive oil and a handful of nuts (5 to 7 nuts, < 20 g)/d. Low carbohydrate diet: low‐carbohydrate, non‐restricted‐calorie diet aimed to provide 20 g of carbohydrates/d for the 2‐month induction phase and immediately after religious holidays, with a gradual increase to a maximum of 120 g/d to maintain weight loss. Low fat diet: Low‐fat calorie restricted diet based on American Heart Association guidelines, with an energy intake of 1500 kcal/d for women and 1800 kcal/d for men with 30% of calories from fat, 10% of calories from saturated fat, and an intake of 300 mg of cholesterol/d. Patients were counselled to consume low‐fat grains, vegetables, fruits, and legumes and to limit consumption of additional fats, sweets, and high‐fat snacks. This study was included as a post‐hoc analysis of the main study including people with CKD (eGFR < 60 mL/min/1.73 m2) | Dietitian | Members of each treatment group were assigned to subgroups of between 17 and 19 participants, with 6 groups for each dietary treatment group. Each group was assigned to a registered dietitian who led all 6 subgroups of that dietary group. Self‐service cafeterias in workplaces worked closely with dietitians to adjust specific food items to specific diet groups. Each food item was provided with a label showing the number of calories and the number of grams of carbohydrates, fat and saturated fat | ‐‐ | Dietitians met with groups in weeks 1, 3, 5, and 7, and thereafter at 6‐week intervals, for a total of 18 sessions of 90 min each. The Israeli version of the diabetes prevention program was adapted including additional themes for each dietary change. In addition, a group of spouses received education. | 6 times during the 2‐year intervention, another dietitian conducted 10 to 15 min motivational telephone calls with patients who were having difficulty adhering to the diet. | ‐‐ | Adherence with the diets was evaluated by a validated food‐frequency questionnaire that included 127 food items and three portion‐size pictures for 17 items. A subgroup of participants completed two repeated 24‐hour dietary recalls to verify absolute intake. We used a validated questionnaire to assess physical activity. At baseline, and at 6, 12, and 24 months of follow‐up, the questionnaires were self‐administered electronically through the workplace intranet. The 15% of patients who request aid in completing the questionnaires were assisted by the study nurse | Adherence with study intervention was 95.4% at first year and 84.6% at second year | |
| To evaluate effect of nutritional advice on dyslipidaemia and biomarkers | Nutritional advice based on the National Kidney Foundation—Kidney Disease Outcomes Quality Initiative guideline (energy intake 0.12 MJ/kg BW/d, protein 0.75 g/kg BW/d, lipid intake 35%, and carbohydrates 55% of total energy intake). Dietary recommendations were modified and adapted to a Mediterranean diet with increased intake of mono‐unsaturated fatty acids (MUFA), poly‐unsaturated fatty acids (PUFA), and fibres. Patients were asked to consume olive oil and nuts for seasonings, whole grains (50 g bread at each meal, 250 g cereal or starch once a day), fruits (once a day), vegetables (200 g twice a day) and fish (twice a week). A list of foods rich in salt, potassium and phosphorus was provided. In addition, patients received advice about cooking methods best suited to adherence | ‐‐ | Face‐to‐face | Nephrology ward | ‐‐ | ‐‐ | ‐‐ | Recall and record every 4 days, patients interviewed by trained interviewers using adapted and structures questionnaire regarding 24 hour dietary intake. Serving sizes were estimated by the use of the food portion model handbook. Dimensions of dishes, utensils and | By 90 days, the qualitative distribution of nutrients had a tendency to be closer to the recommended diet | |
| To verify the effect of the Mediterranean diet on risk factors of atherosclerosis in people with a kidney transplant | This diet featured carbohydrates with a low GI (poor in glucose, simple carbohydrates, and amylose, rich in cellulose). Approved diet constituents included cereals, pulse, whole‐rye bread, vegetables (cooked or fresh), oat flakes (cooked), and noodles prepared al dente. Amylose‐rich foods, sweets, and sweet drinks were prohibited. Breakfast was the main meal, providing 39% 2% of daily calorie intake, whereas supper provided the least (16% 3%). In the study group, daily energy intake was attributed as follows: 47% carbohydrates, 38% fatty acids (including 10% saturated, 22% monounsaturated, and 6% polyunsaturated species), and 15% protein. Cholesterol and fibre supply was 165 ± 17 mg/d and 47 ± 9 g/d, respectively. The significant content of fibre in the diet was attributed to the use of fresh, unprocessed food, elimination of semi processed products, and daily intake of pulse/cereal (e.g. buckwheat, barley)/vegetables/whole‐meal rye bread. The dominating fatty acid was oleic acid from olive oil and erucic acid‐poor rapeseed oil. Patients consumed 30 mL cold‐pressed | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | Dietary adherence was ascertained every 4 weeks using questionnaires (24‐h food diaries) and monitoring oleic acid content in plasma triglycerides | The content of oleic acid in triglycerides | |
| Increased fruit and vegetables | ||||||||||
| To evaluate increased intake of base‐producing fruits and vegetables on kidney function and metabolic acidosis | Patients received fruits and vegetables free of charge, distributed from the food bank in amounts to reduce potential renal acid load by half. Prescriptions emphasised base‐producing fruits and vegetables such as apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini | Dietitian prescribed | Individuals were not given specific dietary instructions and they integrated the prescribed fruits and vegetables into their diets as they wished. To better assure that each patient ate all the prescribed fruits and vegetables, the prescribed amount was given for each household person | ‐‐ | ‐‐ | ‐‐ | ‐‐ | Formal assessment methods was not employed; however to ensure participants consumed required amount of fruit and vegetables, fruit and vegetables were distributed for whole family/household | ‐‐ | |
| To evaluate increased intake of base‐producing fruits and vegetables on kidney function and metabolic acidosis | Patients received fruits and vegetables free of charge, distributed from the food bank in amounts to reduce potential renal acid load by half. Prescriptions emphasised base‐producing fruits and vegetables such as apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini | Dietitian prescribed | Individuals were not given specific dietary instructions and they integrated the prescribed fruits and vegetables into their diets as they wished. To better assure that each patient ate all the prescribed fruits and vegetables, the prescribed amount was given for each household person | ‐‐ | ‐‐ | ‐‐ | ‐‐ | Formal assessment methods was not employed; however to ensure participants consumed required amount of fruit and vegetables, fruit and vegetables were distributed for whole family/household | ‐‐ | |
| Carbohydrate‐restricted, low‐iron, polyphenol enriched (CR‐LIPE) diet | ||||||||||
| To evaluate whether dietary modification had effect on progression of CKD | CR‐LIPE diet; 50% reduction in carbohydrate intake; substitution of iron‐enriched meats (beef and pork) with iron‐poor white meats (poultry and fish) and with protein‐enriched food items known to inhibit iron absorption (dairy; eggs; soy); elimination of all beverages other than tea, water and red wine; exclusive use of polyphenol‐enriched extra‐virgin olive oil | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | Serum ferritin level; to assess adherence with low iron diet | Serum ferritin level decreased in group on CR‐LIPE diet | |
| High‐nitrogen, low carbohydrate diet | ||||||||||
| Whether a high‐nitrogen, low carbohydrate diet could result in a positive nitrogen balance and fewer cushingoid side effects in the immediate post‐transplant period | On the morning of the 4th postoperative day, the patients were randomly assigned to receive either the control or the experimental diet. A general daily diet order was prescribed for all patients; it consisted of 800 mL fluid restriction plus an amount equal to the urine volume/d, 2 g sodium, 80 mEq potassium, 800 to 1200 mg of calcium, and 30 calories/kg. Total calories and content of the diet, in identical proportions, were adjusted up or down per kilogram to the nearest 10 kg for patients who weighed more or less than 70 kg since the ideal body weight of these patients varied from 50 to 90 kg prior to transplantation | Dietitian | Diets were prepared in batches in the metabolic kitchen by a research dietician. One meal from each batch was slurried and analysed for nitrogen and electrolyte | Inpatient General Clinical Research Centre for 4‐week duration of study. | Continuous assessment | The composition of the diet was determined according | ‐‐ | Uneaten food from each tray was weighed and subtracted from the daily | Both groups ingested a similar amount of total calories, and when factored by weight, intakes per kg of body weight were very close to the objective of 28 to 30 | |
| BMI ‐ body mass index; (I)BW ‐ (individual) body weight‐ CKD ‐ chronic kidney disease; eGFR ‐ estimated glomerular filtration rate; GI ‐ glycaemic index | ||||||||||
| Study ID | Tool | Description |
| Dietary counselling | ||
| Kidney Disease Quality of Life Short Form Version 1.3 (combining the SF‐36 with a kidney‐disease specific module) | "There was a clear trend for a mean increase in ratings from the intervention group with a clinically significant mean improvement in 13 of the 18 sub‐scales from baseline to week 12, indicated by an effect size of 0.2 or greater...". There was a statistically significant difference in mean change for scores of symptoms of kidney disease (7.1 (0.1‐14.1) P = 0.047); cognitive functioning (14.6 (5.4‐23.7) P = 0.003); and vitality (12.0 (4.6‐19.5) P = 0.002) in favour of the intervention." | |
| ‐‐ | Not reported | |
| Self‐Management Questionnaire | "Overall, the experimental group showed 'improvement' in exercise frequency, concern over health condition, and frequency of visits to health providers or hospitalisation. Overall the control group answers indicated an improvement in their communication with health providers in asking question and discussing personal issues." | |
| Kidney Disease Quality of Life questionnaire (combining the SF‐36 with a kidney‐disease specific module) | "There were no differences between intervention and control patients in quality‐of‐life subscales, including general health, physical functioning, emotional well‐being, social function, pain, and dialysis‐related symptoms." | |
| ‐‐ | Not reported | |
| ‐‐ | Not reported | |
| ‐‐ | Not reported | |
| 52‐item HPLP‐IIC questionnaire | Intervention had a significant effect on health responsibility and physical activity, but not stress management, interpersonal relations, spiritual growth or nutrition | |
| SF‐36 | "The mean SF‐36 score at 6 months was significantly higher in the intervention group compared with the control group (583±13 vs 436±22, P = 0.008), reflecting an improved perception of health status. ... The intervention group had improvements compared with the control group in the domains of vitality and general health." | |
| Kidney Disease Quality of Life Short Form Version 1.3 (combining the SF‐36 with a kidney‐disease specific module) | "Prior to intervention, the differences in KDTA and SF‐36 scores were not statistically significant in both groups (P >0.05 for all). After intervention, both KDTA and SF‐36 scores were improved in the study group, but decreased in the control group. The difference in KDTA (P = 0.001) and SF‐36 scores (P = 0.001) before and after intervention were statistically significant in both groups (Table 2)." | |
| Mediterranean diet | ||
| ‐‐ | Not reported | |
| ‐‐ | Not reported | |
| ‐‐ | Not reported | |
| Increased fruit and vegetables | ||
| ‐‐ | Not reported | |
| ‐‐ | Not reported | |
| Carbohydrate‐restricted, low‐iron‐available, polyphenol‐enriched diet | ||
| ‐‐ | Not reported | |
| High‐protein, low carbohydrate diet | ||
| ‐‐ | Not reported | |
| Study | Adverse events reported in study |
| Campbell 2008a | Mortality; need for dialysis |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| No participants meeting eGFR and plasma potassium criteria developed plasma potassium concentration >5.0 mEq/L | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| Mortality; transfer from PD to HD | |
| Not reported | |
| Not reported | |
| Dialysis due to elevated blood urea and potassium concentrations | |
| Not reported | |
| eGFR ‐ estimated glomerular filtration rate; HD ‐ haemodialysis; PD ‐ peritoneal dialysis | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Dietary counselling | 4 | 371 | Risk Ratio (IV, Random, 95% CI) | 1.59 [0.60, 4.21] |
| 1.2 CR‐LIPE | 1 | 170 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.22, 1.12] |
| 2 Cardiovascular mortality Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Dietary counselling | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Health‐related quality of life (SF‐36) score Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Dietary counselling | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 11.46 [7.73, 15.18] |
| 4 End‐stage kidney disease Show forest plot | 2 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.07] |
| 4.1 Dietary counselling | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.33] |
| 4.2 CR‐LIPE | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.25, 1.05] |
| 5 Doubling of serum creatinine Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 CR‐LIPE | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Employment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Dietary counselling | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Dietary adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Dietary counselling | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Worsening nutrition Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Dietary counselling | 2 | 230 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.05, 3.37] |
| 9 eGFR [mL/min/1.73 m2] Show forest plot | 5 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 1.08 [0.20, 1.97] |
| 9.1 Dietary counselling | 3 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 1.41 [‐0.40, 3.23] |
| 9.2 Mediterranean | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.39, 0.85] |
| 9.3 Fruits and vegetables | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 1.14 [0.64, 1.64] |
| 10 Serum creatinine Show forest plot | 3 | 112 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐16.57, 18.23] |
| 10.1 Dietary counselling | 2 | 72 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐24.47, 28.05] |
| 10.2 Mediterranean | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐26.17, 24.17] |
| 11 Systolic blood pressure Show forest plot | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐9.26 [‐13.48, ‐5.04] |
| 11.1 Dietary counselling | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐11.83 [‐13.67, ‐9.98] |
| 11.2 Fruits and vegetables | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐7.10 [‐9.60, ‐4.60] |
| 12 Diastolic blood pressure Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Dietary counselling | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐8.95 [‐10.69, ‐7.21] |
| 13 Energy intake Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Dietary counselling | 4 | 340 | Std. Mean Difference (IV, Random, 95% CI) | 1.54 [‐0.87, 3.95] |
| 13.2 Mediterranean diet | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.86 [1.11, 2.61] |
| 13.3 High nitrogen/low carbohydrate | 1 | 12 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.82, 0.53] |
| 14 Body weight Show forest plot | 6 | 454 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.46, 0.58] |
| 14.1 Dietary counselling | 3 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.93, 1.53] |
| 14.2 Fruits and vegetables | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.57, 1.57] |
| 14.3 CR‐LIPE | 1 | 170 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐6.22, 2.22] |
| 14.4 High nitrogen/low carbohydrate | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.0 [‐2.66, 8.66] |
| 15 BMI Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Dietary counselling | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐5.23, 1.82] |
| 16 Waist‐hip ratio Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Dietary counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Waist circumference, cm Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Dietary counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Arm circumference Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Dietary counselling | 2 | 149 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.39, 1.12] |
| 19 Serum albumin Show forest plot | 6 | 541 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.07, 0.24] |
| 19.1 Dietary counselling | 4 | 331 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.14, 0.16] |
| 19.2 Mediterranean | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.11, 1.09] |
| 19.3 CR‐LIPE | 1 | 170 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 20 Serum LDL cholesterol Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Mediterranean diet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 CR‐LIPE | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum LDL cholesterol Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 eGFR [mL/min/1.73 m2] Show forest plot | 2 | 143 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐0.84, 2.53] |
| 2 Serum creatinine Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Systolic blood pressure Show forest plot | 2 | 143 | Mean Difference (IV, Random, 95% CI) | ‐5.81 [‐8.84, ‐2.77] |
| 4 Body weight Show forest plot | 2 | 143 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐7.73, ‐2.44] |

