고빌리루빈혈증이 있는 만삭 및 미숙아의 광선 요법 시 신체 위치의 주기적인 변화
초록
배경
광선 요법은 신생아 고빌리루빈혈증 치료의 기본입니다. 광선 요법에서 신생아의 위치를 주기적으로 변경하면(누운 자세에서 엎드린 자세 또는 옆 자세로) 피부와 피하 조직의 다른 부분에 침착된 빌리루빈에 광선 요법 빛의 접근을 촉진하여 광선 요법의 효율성을 향상시킬 수 있습니다.
목적
생후 첫 28일 동안 비접합 고빌리루빈혈증이 있는 신생아에서 처방된 체위 변화가 없는 경우와 비교하여 광선 요법 중 체위의 주기적인 변화가 혈청 총 빌리루빈 수치 및 치료 기간에 미치는 영향을 평가합니다.
검토의 2차 목적에는 교환 수혈의 필요성 또는 횟수, 빌리루빈 유발 신경 손상(BIND)의 발생률, 광선 요법의 부작용 및 영아 돌연사 증후군(SIDS)에 대한 주기적인 체위 변경의 효능 평가가 포함되었습니다. .
검색 전략
Cochrane Neonatal의 표준 검색 전략을 사용하여 Cochrane Central Register of Controlled Trials(CENTRAL; 2021, Issue 3)와 Ovid MEDLINE 및 Epub Ahead of Print, In‐Process & Other Non‐Indexed에서 포괄적인 검색을 실행했습니다. 2021년 3월 5일 인용, 일간 및 버전. 우리는 또한 임상 시험 데이터베이스와 포함된 연구의 참조 목록과 무작위 대조 시험(RCT) 및 준 RCT에 대한 관련 리뷰를 검색했습니다.
선정 기준
광선 요법이 필요한 비접합 고빌리루빈혈증이 있는 성별의 신생아(만삭 및 미숙아)를 등록한 RCT 및 준 RCT를 포함하고 신체 위치에 처방된 변화가 없는 광선 요법을 받는 유아의 신체 위치의 주기적인 변화를 비교했습니다.
자료 수집 및 분석
2명의 리뷰 작성자가 독립적으로 시험 품질을 평가하고 데이터를 추출했으며, 불일치하는 경우 세 번째 리뷰 작성자와 상의했습니다. 포함된 연구의 비뚤림 위험 평가를 포함하여 표준 Cochrane 방법론 절차를 사용했습니다. 그리고 근거의 확실성을 평가하기 위해 GRADE 접근법을 사용했습니다. 1차 결과는 광선 요법의 기간과 24시간에서의 혈청 빌리루빈 감소율이었습니다. 이차 결과에는 교환 수혈의 필요성, 교환 수혈 횟수, BIND 발생률, SIDS가 포함되었습니다.
주요 결과
우리는 검토에서 비뚤림 위험이 전반적으로 높은 5건의 연구(343명의 신생아)를 포함했습니다. 광선 요법에서 신체 위치는 각각 2건의 연구에서 2시간마다 또는 2시간 30분마다 변경되었고, 1건의 연구에서는 3시간마다 변경되었습니다. 5개 연구 중 3개에는 건강한 만삭 신생아가 포함된 반면, 다른 2개 연구에는 미숙아(임신 33주 이상)도 포함되었습니다. 그러나 미숙아의 검토 결과에 대한 별도의 데이터는 사용할 수 없었습니다.
신체 위치의 주기적인 변화는 광선 요법 기간의 차이를 거의 또는 전혀 야기하지 않을 수 있습니다(평균 차이(MD) 1.71시간, 95% 신뢰 구간(CI) ‐3.17~6.59시간, I² = 58%, 4건의 연구, 231명의 참가자, 231명의 참가자). 낮은 확실성 증거). 한 연구만이 광선요법 시작 24시간에 혈청 총 빌리루빈의 감소율을 보고했습니다. 신체 위치의 주기적인 변화는 24시간에 혈청 총 빌리루빈의 감소 속도에 거의 또는 전혀 차이가 없을 수 있습니다(MD 0.02 mg/dL/h, 95% CI ‐0.02 ~ 0.06 mg/dL/h, 연구 1건, 100 참가자, 낮은 확실성 증거). 우리는 편견과 부정확성의 위험 때문에 증거의 확실성을 낮음으로 하향 조정했습니다. 포함된 연구 중 교환 수혈의 필요성이나 횟수, BIND 또는 SIDS의 발생률을 보고한 연구는 없습니다. 별도의 데이터가 부족하여 하위 그룹 분석이 불가능했습니다.
연구진 결론
이용 가능한 증거는 광선 요법에서 규정된 체위 변화가 없는 것과 비교하여 체위의 주기적인 변화의 효과를 결정하기에는 불충분합니다. 만삭아 및 미숙아에서 광선 요법을 받는 동안 신체 위치의 주기적인 변화와 처방된 신체 위치의 변화가 없는 사이에 광선 요법 기간과 광선 요법 시작 24시간 후 빌리루빈 감소율에 차이가 거의 없거나 전혀 없을 수 있다는 확실성이 낮다는 증거가 있습니다. . 포함된 연구 중 어떤 것도 교환 수혈의 필요성이나 횟수, BIND 또는 SIDS의 발생률에 대한 위치 변경의 영향을 보고하지 않았습니다. 한 연구가 분류 대기 중이며 검토에 포함될 수 없습니다. 특히 용혈성 고빌리루빈혈증이 있는 신생아와 미숙아 신생아에서 광선 요법 하에서 신체 위치의 주기적인 변화의 효과를 평가하기 위해서는 추가 연구가 필요합니다. 이 체계적인 검토의 결과는 주로 비용혈성 고빌리루빈혈증에 대한 광선요법을 받는 후기 조산 또는 만삭임신 신생아에게 적용됩니다.
PICOs
쉬운 말 요약
고빌리루빈혈증이 있는 만삭 및 미숙아의 광선 요법 시 신체 위치의 주기적인 변화
연구 질문
아기의 자세를 바꾸면 황달이 있는 만삭 및 미숙아의 광선 요법 결과가 향상됩니까?
주요 결과
이 체계적인 검토에서 우리는 황달에 대한 광선 요법을받는 만삭 및 미숙아의 예정된 위치 변경과 예정된 위치 변경을 비교하지 않는 연구를 평가했습니다. 이 두 가지 포지셔닝 전략은 광선 요법이 지속되는 시간이나 빌리루빈 수치가 떨어지는 비율에 거의 또는 전혀 차이가 없었습니다. 빌리루빈은 피부와 눈 흰자위가 노랗게 변하는 황달을 유발하는 물질입니다.
검토에 포함된 연구 중 어떤 것도 영아 돌연사 증후군을 포함하여 원치 않는 영향에 대한 아기의 자세 변경의 영향을 보고하지 않았습니다.
미래의 연구에서는 모든 유형의 황달을 경험하는 초미숙아와 아기를 포함하여 광선 요법을 받는 아기의 예정된 위치 변경을 조사해야 합니다.
황달이란 무엇입니까?
황달(고빌리루빈혈증이라고도 함)은 피부와 눈의 흰자위가 노랗게 변하는 신생아의 흔한 상태이며, 이는 혈액에 너무 많은 빌리루빈이 존재하기 때문입니다. 빌리루빈은 적혈구가 분해될 때 생성되는 노란색 물질입니다. 신생아의 간은 혈액에서 빌리루빈을 효과적으로 제거할 수 없기 때문에 수치가 높아집니다. 어떤 경우에는 아기의 혈액 내 빌리루빈 수치가 매우 높아 뇌 손상을 일으킬 수 있습니다. 아기가 태어난 지 약 2주가 되면 간에서 빌리루빈을 처리할 수 있으며 황달은 저절로 좋아집니다.
황달은 어떻게 치료됩니까?
황달에 대한 가장 일반적인 치료법은 광선 요법(광 요법)입니다. 아기는 특수 조명 아래에 두어 눈을 가리고 기저귀(기저귀)만 착용하여 가능한 한 많은 피부가 빛에 노출되도록 합니다. 광선 요법은 빌리루빈을 분해하여 몸 밖으로 전달할 수 있습니다. 일부 아기는 광선 요법으로 발진이나 설사를 겪을 수 있지만 일반적으로 원치 않는 영향을 일으키지 않습니다. 대부분의 아기에서 빌리루빈을 안전한 수준으로 낮추려면 일반적으로 약 48시간의 광선 요법이 필요합니다.
무엇을 알아보고 싶었는가?
광선 요법 중에 아기를 등으로 돌린 다음 옆으로 돌리면 피부의 다른 부위가 빛에 노출되어 빌리루빈 수치를 더 빨리 감소시킬 수 있습니다. 아기를 한 자세로 유지하는 것보다 정해진 시간에 뒤집어 놓는 것이 광선 요법이 더 효과적인지 알고 싶었습니다.
우리는 특히 생후 첫 28일 동안 만삭(만기 3주 전까지 출생) 및 미숙아에 미치는 영향에 관심이 있었습니다. 우리는 아기의 자세를 바꾸면 광선 요법을 받는 데 필요한 시간이 단축되는지 알고 싶었습니다. 빌리루빈 수치가 떨어지는 속도를 변경합니다. 또는 원치 않는 영향, 특히 영아 돌연사 증후군(SIDS 또는 '침대 사망')을 유발합니다.
무엇을 했습니까?
우리는 황달에 대한 광선 요법을받는 신생아의 체위 변화가없는 경우와 체위의 변화를 조사한 연구를 검색했습니다. 아기의 신체 위치에 대한 변경과 변경 사이의 시간은 정해진 일정을 따라야 했습니다. 아기는 황달이 있어야 하고 만삭 또는 미숙아로 태어나야 하며 남성 또는 여성일 수 있습니다.
무엇을 찾았습니까?
총 343명의 아기를 포함하여 5개의 연구를 찾았습니다. 3개의 연구에는 건강한 만삭 신생아가 포함되었고 다른 2개 연구에는 만삭 및 미숙아가 모두 포함되었습니다. 모든 연구는 광선 요법 동안 신체 위치의 변화가 없는 아기의 신체 위치의 예정된 변화를 비교했습니다. 아기는 등, 앞 또는 옆으로 눕힐 수 있으며 연구 계획에 따라 위치가 변경됩니다. 위치 변경은 시간(30분에서 6시간마다)을 기반으로 했습니다. 아기 다루기(예: 각 모유 수유 후); 또는 각 간호 교대에서 변경(예: 교대 중 한 번).
주요 결과
아기의 자세를 바꾸지 않은 것과 비교하여 광선 요법에서 아기의 자세를 바꾸면 광선 요법이 지속되는 시간(4건의 연구, 231명의 아기)과 광선 요법을 시작한 후 24시간 후에 빌리루빈 수치가 떨어지는 속도(1 연구, 100명의 아기). 포함된 어떤 연구에서도 보고되지 않았기 때문에 신체 위치를 변경하는 것이 원치 않는 효과를 유발하는지 여부는 알 수 없습니다.
근거의 한계는 무엇입니까?
증거에 대한 우리의 확신은 우리가 발견한 연구가 거의 없고 우리가 찾은 연구는 결과를 분석하고 보고하는 데 최상의 방법을 사용하지 않았기 때문에 제한적입니다. 포함된 연구 중 원치 않는 영향에 대해 보고한 연구는 없습니다.
이 근거는 얼마나 최신인가?
증거는 2021년 3월 5일까지입니다.
Authors' conclusions
Summary of findings
| Turning position versus supine under phototherapy in term and preterm neonates with hyperbilirubinaemia | ||||||
| Patient or population: term and preterm neonates with hyperbilirubinaemia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with turning position under phototherapy | Risk with supine position under phototherapy | |||||
| Duration of phototherapy (hours) | The mean duration of phototherapy ranged from 24.8 to 52.8 hours. | The mean duration of phototherapy ranged from 25.5 to 53.3 hours. | Mean duration of phototherapy was on average 1.71 hours longer in the turning group (95% CI −3.17 to 6.59). | 231 | ⊕⊕⊝⊝ | Periodic change in body position may lead to little or no difference in the duration of phototherapy. |
| Rate of fall of serum total bilirubin at 24 hours of starting phototherapy (mg/dL/h)b | The mean rate of fall of serum total bilirubin at 24 hours of starting phototherapy was 0.22 mg/dL/h. | The mean rate of fall of serum total bilirubin at 24 hours of starting phototherapy was 0.2 mg/dL/h. | MD in the rate of fall of serum total bilirubin was an average of 0.02 mg/dL/h higher in the turning group | 100 | ⊕⊕⊝⊝ Lowc | Periodic change in body position may lead to little or no effect on the rate of fall of serum total bilirubin at 24 hours of starting phototherapy. |
| Need for exchange transfusion | None of the studies reported this outcome. | |||||
| Bilirubin‐induced neurological damage (BIND) | None of the studies reported this outcome. | |||||
| Sudden infant death syndrome (SIDS) | None of the studies reported this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels because of statistical heterogeneity and unclear risk of selection bias. (One study has attrition bias. Three studies have unclear reporting. Blinding of outcome assessors was not possible in any of the studies.) | ||||||
Background
Description of the condition
'Hyperbilirubinaemia' is a term used to describe elevated levels of bilirubin in the blood. Nearly 97% of full‐term and preterm babies demonstrate biochemical hyperbilirubinaemia (defined as serum bilirubin level > 1 mg/dL), and more than two‐thirds appear clinically icteric (serum bilirubin > 5 mg/dL) (Maisels 1986). In the majority of cases, hyperbilirubinaemia is physiological due to increased breakdown of red blood cells, decreased uptake and metabolism of bilirubin in the liver, and increased enterohepatic circulation (Wong 2019). However, the presence of conditions like blood group incompatibility, glucose‐6‐phosphate dehydrogenase enzyme deficiency, the presence of extravasated blood, and suboptimal feeding can cause an excessive rise of bilirubin (Wells 2013). Bilirubin is a potential neurotoxin, and severe hyperbilirubinaemia can result in neurological damage (Bhutani 2013; Cashore 1990).
The acute phase of bilirubin‐induced neurological damage (BIND) manifests clinically with poor feeding, lethargy, a high‐pitched cry, hypertonia or hypotonia, opisthotonos, and seizures. The chronic manifestations include athetoid cerebral palsy, motor delay, gaze palsy, dental dysplasia, mental retardation, and sensorineural hearing loss. Prematurity, lower postnatal age, ongoing haemolysis, low albumin levels, and other comorbidities like asphyxia and sepsis may increase the risk of BIND. Threshold bilirubin levels for initiating treatment for hyperbilirubinaemia are therefore based on gestation at birth, postnatal age, and the presence of risk factors (AAP 2004).
Description of the intervention
Phototherapy is the standard treatment of neonatal hyperbilirubinaemia (Maisels 2008; Polin 1990). Phototherapy is provided by placing a light source over the baby bassinet. Bilirubin present in the skin, subcutaneous tissue, and peripheral capillaries absorbs the light energy emitted by the phototherapy unit and as a result of different photochemical reactions, is converted into more easily excreted photo‐isomers. If phototherapy fails to reduce bilirubin concentration below the neurotoxic levels, exchange transfusion is performed. However, exchange transfusion is associated with a risk of death (nearly 2%) or severe complications (12%) (Jackson 1997).
Various strategies employed to improve the efficacy of phototherapy include using a high‐intensity light source, decreasing the distance between the baby and the light source, using double‐surface phototherapy (application of another panel angulated to the first panel), and increasing the surface area of skin exposed to phototherapy (Pratesi 1989; Vreman 2004). The posture of the baby under phototherapy is usually governed by nursing preferences and the hospital policy to decrease the risk of sudden infant death syndrome (SIDS) (Jeffery 1999). As a result, neonates are most likely to be nursed in a supine posture under phototherapy. Parts of the skin under phototherapy are in a state of continuous equilibrium with the actual 'bilirubin pool' of the body such that the bilirubin photo‐isomers (formed after phototherapy) are constantly being removed from the skin and added to the circulation pool, and fresh unconjugated bilirubin circulating in the blood pool is being added to the skin (both along their respective concentration gradients). It can be hypothesised that periodically changing the body position from supine to prone or lateral positions may improve the efficiency of phototherapy by hastening the access of phototherapy light to bilirubin deposited in various parts of the skin and subcutaneous tissue.
How the intervention might work
The principal site of action of phototherapy is the outermost 2 mm of the skin (Vogl 1974). First, bilirubin in the skin is converted to its photo‐isomers, a process that occurs in nanoseconds (Cremer 1958). The photo‐isomer conversion includes configurational isomerisation, structural isomerisation, and photo‐oxidation. Configurational isomerisation is a very rapid process that changes bilirubin isomers to water‐soluble isomers; however, this isomerisation is not significantly influenced by light intensity (Dennery 2001; Maisels 2008). On the other hand, structural isomerisation, which consists of intramolecular cyclisation resulting in the formation of lumirubin, is enhanced by increasing the intensity of light. The second step is the migration of these photo‐isomers into the circulation and simultaneously, the reverse migration of non‐isomerised serum bilirubin into the skin (Cremer 1958; Maisels 2001). This second step is considered rate‐limiting, and the time required is estimated at between one and three hours (Lau 1984; Vogl 1974). Assuming that this migration occurs only through skin‐blood‐skin bilirubin transfer, and there is no lateral diffusion of bilirubin from unexposed skin to the adjacent exposed skin, phototherapy becomes less effective once the exposed skin is blanched. Hence, turning the infant over from the blanched side to the bilirubin‐loaded side (the side not receiving phototherapy) would seem to be a logical therapeutic manoeuvre for increasing the rate of bilirubin degradation and thereby improving the efficacy of phototherapy (Lau 1984; Vogl 1978).
Why it is important to do this review
The American Academy of Pediatrics (AAP) Subcommittee on Hyperbilirubinemia guidelines, AAP 2004, and the National Institute for Health and Care Excellence (NICE) guidelines on neonatal jaundice, NICE 2016, do not provide any recommendation for or against changing the infant's position under phototherapy (AAP 2004; Rennie 2010). The NICE guidelines recommend supine positioning, and the AAP guidelines do not recommend any specific position for infants. In view of conflicting evidence from studies and uncertain clinical practice regarding the optimal position of infants under phototherapy, the search for the optimal position during phototherapy remains an important research priority. The authors of a systematic review on the effect of turning versus supine position for neonates under phototherapy suggested that keeping the jaundiced newborns in the supine position throughout phototherapy is as effective as turning them (Lee Wan Fei 2015). However, this systematic review excluded studies not available in the English language and studies with position changes more frequent than two‐ to three‐hourly. Lee Wan Fei 2015 did not consider patient‐important outcomes such as BIND, need or the number of exchange transfusions, kernicterus, or SIDS. Any recommendation on change in body position during phototherapy also needs to consider a possible association with the risk of SIDS (Hunt 2003; Mitchell 1999).
Objectives
To evaluate the effects of periodic change of body position during phototherapy as compared to no prescribed change in body position, on serum total bilirubin level and duration of treatment in neonates with unconjugated hyperbilirubinaemia during the first 28 days of life.
Secondary objectives of the review included evaluation of the efficacy of periodic change of body position on the need for or number of exchange transfusions, incidence of bilirubin‐induced neurological damage (BIND), side effects of phototherapy, and sudden infant death syndrome (SIDS).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing periodic change in body position of the infant under phototherapy to no prescribed change in body position. We did not apply any restrictions on language or sex. Trials reported in abstract form were eligible for inclusion if methods were reported that allowed assessment of eligibility for inclusion and risk of bias.
We did not plan to include cross‐over trials, as this is not an appropriate study design to answer the review question. We also planned to exclude cluster‐RCTs, as this study design, although theoretically feasible, is unlikely to be used for the review question.
Types of participants
We included studies enrolling neonates (term (≥ 37 weeks' gestation) and preterm (< 37 weeks' gestation)) of either gender with unconjugated hyperbilirubinaemia (irrespective of aetiology and defined as hyperbilirubinaemia with direct‐reacting component bilirubin level > 1 mg/dL or > 20% of the total bilirubin (Feldman 2014)) within the first 28 days of life.
Types of interventions
We included studies comparing the periodic change in body position of the infant under phototherapy with no prescribed change in body position. The infant’s position may include supine, prone, or lateral body posture with the intervention prescribing a periodic change in position. Periodicity for change in position may be based on time (every 30 minutes to 6 hours), handling of the baby (e.g. after each breastfeeding session), or nursing shift (e.g. once during a shift).
Types of outcome measures
We included the following outcomes in this review.
Primary outcomes
-
Duration of phototherapy (hours)
-
Rate of fall of serum total bilirubin (mg/dL/h) at 24 hours
Secondary outcomes
-
Need for exchange transfusion (proportion)
-
Number of exchange transfusions (mean number)
-
Incidence of bilirubin‐induced neurological damage (BIND) (proportion). BIND or subtle encephalopathy was defined as neurological, cognitive, learning, movement disorders, isolated hearing loss or auditory dysfunction in the presence of hyperbilirubinaemia (Bergman 1985; Hyman 1969; Johnson 1974; Rubin 1979; Scheldt 1977)
-
Phototherapy side effects, including skin rash
-
Kernicterus (Dennery 2001; Gkoltsiou 2008; Shapiro 2005)
-
Sudden infant death syndrome (SIDS) (proportion): SIDS was defined as “the sudden death of an infant under one year of age, which remains unexplained after a thorough case investigation, including the performance of a complete autopsy, examination of the death scene, and review of the clinical history” (Willinger 1991).
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search without date, language, or publication type limits in March 2021. We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 3) in the Cochrane Library and Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1 January 2016 to 5 March 2021). The strategy combined terms for hyperbilirubinaemia and phototherapy combined with standard neonatal terms and a filter for randomised controlled trials. Search strategies are provided in Appendix 1.
We searched clinical trial registries for ongoing or recently completed trials on 5 March 2021. We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. We also searched the ISRCTN registry (www.isrctn.com/), for any unique trials not found through the CENTRAL search.
Our previous search details (up to May 2016) are listed in Appendix 2.
Searching other resources
We cross‐referenced relevant literature including identified trials and existing review articles. We searched the following additional resources:
-
reference lists of articles retrieved from the sources cited under Electronic searches and from review articles;
-
personal communication with primary authors of studies to retrieve unpublished data related to published articles.
Data collection and analysis
We used the standard methods of Cochrane and Cochrane Neonatal.
Selection of studies
Two review authors (AT and DC) independently assessed the studies identified by the search for inclusion in the review using the Covidence interface (Covidence). Any disagreements were resolved through mutual discussion.
Data extraction and management
Two review authors (AT and DC) independently extracted data using a pre‐tested data extraction form. Any disagreements were resolved through mutual discussion or by consulting a third review author (AD) if necessary.
Assessment of risk of bias in included studies
Two review authors (AT and DC) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool (Higgins 2011), for the following domains:
-
sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective reporting (reporting bias);
-
any other bias.
Any disagreements were resolved through mutual discussion or by consulting a third review author if necessary. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed continuous data using mean difference (MD). We calculated the risk ratio (RR), risk difference (RD), the number needed to treat for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH) with 95% confidence intervals (CI) for dichotomous data.
Unit of analysis issues
We compared periodic change in body position of the infant under phototherapy with no prescribed change in body position. The infant’s position could include supine, prone, or lateral body posture, with the intervention prescribing a periodic change in position. Periodicity for change in position may be based on time (every 30 minutes to 6 hours), handling of the baby (e.g. after each breastfeeding session), or nursing shift (e.g. once during a shift).
In the current review, the interval between changes in body position varied from two to three hours. All of the included studies compared 'change' versus 'no prescribed change' in body position, not different protocols for body position. If in future updates of the review we find multi‐arm studies comparing different protocols for change in body position versus no prescribed change, we will combine the intervention groups to create a single pair‐wise comparison of intervention (any prescribed change) with the control group (no prescribed change).
Dealing with missing data
We contacted the authors of studies awaiting classification for any published or presented version of the study results (IRCT201111063250N4); however, the results of the study have not been published. The authors of the five studies included in the review were not contacted for any missing data.
Assessment of heterogeneity
We examined heterogeneity between trials by first assessing differences in trial methodologies and clinical heterogeneity. We used the following cut‐offs and labels for the results of the I² test:
-
< 25%: no heterogeneity;
-
25% to 49%: low heterogeneity;
-
50% to 74%: moderate heterogeneity;
-
> 74%: high heterogeneity.
We planned to inspect forest plots and quantify the impact of heterogeneity using the I²statistic. If we found heterogeneity, we would explore the possible causes of statistical heterogeneity using prespecified subgroup analysis (e.g. term versus preterm neonates and haemolytic versus non‐haemolytic jaundice). We used a fixed‐effect model for meta‐analyses.
Assessment of reporting biases
We planned to use funnel plots to assess for publication bias if there were a sufficient number (> 10) of trials reporting the same outcome. However, we included only five trials in the review, which precluded an assessment of publication bias.
Data synthesis
We tabulated studies that met the inclusion criteria to enable the comparison of trial characteristics and individual components of the quality assessment. We tabulated the bibliographic details of trials excluded from the review with documentation of the specific reasons for exclusion. We reviewed the summary tables of included trials to identify clinical heterogeneity amongst trials. We implemented a meta‐analysis with a random‐effects model using Review Manager 5 software (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis to consider:
-
term (≥ 37 weeks') versus preterm (< 37 weeks') neonates;
-
haemolytic versus non‐haemolytic hyperbilirubinaemia.
In addition, we planned to carry out subgroup analysis if studies reported different regimens of change in position (e.g. frequency of change in position or change in position under a single‐surface versus double‐surface phototherapy). However, subgroup analysis was not possible, as separate data for the subgroups were not reported in any of the included studies.
Sensitivity analysis
We planned to perform sensitivity analyses to test the robustness of our decisions if a sufficient number of trials were found. We planned to perform a sensitivity analysis if the findings were affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and outcome measurement, and less than 10% loss to follow‐up. However, we did not perform sensitivity analysis, as the five studies included in the review had similar methodological rigour.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach as outlined in the GRADE Handbook to assess the certainty of the evidence for the following outcomes (Schünemann 2013):
-
duration of phototherapy;
-
per cent fall in bilirubin at 24 hours of starting phototherapy;
-
rate of fall of serum bilirubin at 24 hours of starting phototherapy;
-
serum total bilirubin at 24 hours of starting phototherapy.
Two review authors (AT and DC) independently assessed the certainty of evidence for each of the listed outcomes above. We considered evidence from RCTs as high certainty, downgrading one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of results, and presence of publication bias. We used GRADEpro GDT software to create summary of findings Table 1 to report the certainty of the evidence (GRADEpro GDT). The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
-
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
Our literature search in March 2021 identified a total of 1472 records. After the removal of duplicates, we screened 1228 records, of which 1216 records were excluded after title and abstract screening. We screened the full texts of 12 studies, excluding six studies for a variety of reasons. One record from a trial registry has not been published and was categorised as awaiting classification (IRCT201111063250N4). We included five studies in the review.
See Figure 1.
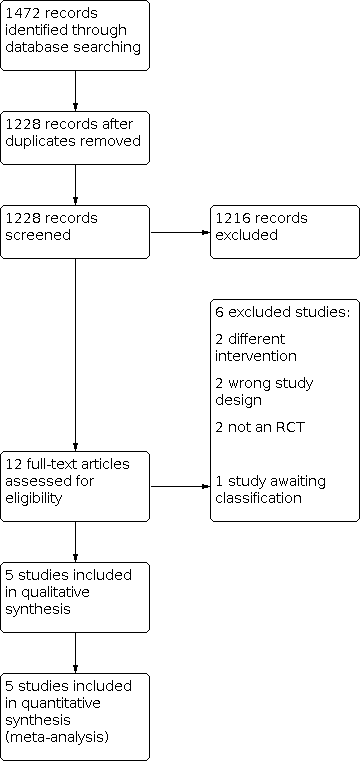
Study flow diagram.
Included studies
We included five studies (343 neonates) in the review.
Bhethanabhotla 2013 enrolled neonates born at 35 to 42 weeks of gestation, with age > 24 hours and < 14 days and hyperbilirubinaemia requiring phototherapy. Enrolled neonates were randomised to either no change in position (n = 54) or alternating supine and prone position change every two hours (n = 46) whilst under phototherapy. Both groups received single‐surface phototherapy with four blue and two white compact fluorescent tube lights. The light source was kept at a 25‐centimetre distance from the neonate. Neonates in both groups received exclusive breastfeeding. Baseline variables like gestation, birth weight, and bilirubin at the onset of phototherapy were comparable in the two groups. Reported outcomes included the duration of phototherapy, absolute levels of serum total bilirubin (STB), and rate of fall of STB at 24 hours of starting phototherapy.
Chen 2002 enrolled clinically well, term infants with non‐haemolytic hyperbilirubinaemia (bilirubin level greater than 15 mg/dL). Criteria for the diagnosis of non‐haemolytic hyperbilirubinaemia included: absence of blood group isoimmunisation, a negative Coombs' test, haemoglobin concentration greater than 14 g/dL, and a normal glucose‐6‐phosphate dehydrogenase status. Neonates were randomised to a "fixed" supine position group (n = 24) or the "position‐changing" turning group (n = 27). In the supine group, neonates were kept in supine posture whilst under phototherapy. In the turning group, the position of neonate under phototherapy was rotated from a supine to a prone position or vice versa every two hours. Both groups received single‐surface phototherapy with six white compact fluorescent tube lights. The light source was kept at a 35‐centimetre distance from the neonate. Phototherapy was interrupted for feeding. Baseline variables like birth weight, gestational age, gender, feeding type, age at phototherapy, haemoglobin at the start of phototherapy, and measured spectral irradiance were comparable in the two groups. Outcomes reported by the study included the duration of phototherapy, per cent fall in STB at 24 hours and 48 hours of phototherapy, and the rate of fall of STB (time not specified).
Donneborg 2010 enrolled healthy neonates with non‐haemolytic hyperbilirubinaemia, gestation ≥ 33 weeks, fulfilling the indications for phototherapy, postnatal age > 24 hours, not having received phototherapy for the last 48 hours, and who were able to be treated in a cradle. Enrolled neonates were randomised to supine (n = 53) or alternating position (turning, n = 59) groups. In the supine group, neonates were kept in supine posture whilst under phototherapy. In the turning group, the position was changed every third hour from supine to prone and vice versa. Both groups received single‐surface phototherapy with a light‐emitting device (LED) emitting blue light with an emission peak at 470 nm and a bandwidth of 455 to 485 nm. The light source was kept at a 20‐centimetre distance from the neonate. Neonates in both groups received feeding for 30 minutes every third hour. The outcomes reported included absolute levels of STB and its per cent fall at 24 hours and 48 hours of starting phototherapy.
Mohammadzadeh 2004 enrolled healthy, exclusively breastfed neonates more than 48 hours of postnatal age, with birth weight > 2500 g, born at 38 to 41 weeks of gestation after an uncomplicated pregnancy, and who had indirect hyperbilirubinaemia with STB ≥ 15 mg/dL at 49 to 72 hours of age or ≥ 17 mg/dL at ≥ 72 hours of age. Neonates with haemolytic disease, infection, congenital anomaly, closed haemorrhage (cephalohaematoma), and metabolic diseases were excluded. Enrolled neonates were randomised to supine (n = 25) or turning (n = 25) groups. In the supine group, neonates were kept in supine posture whilst under phototherapy. In the turning group, the position of the neonate under phototherapy was changed from supine to prone every 150 minutes, followed by a break of 30 minutes for feeding and routine nursing care. Both groups received single‐surface phototherapy with four blue compact fluorescent tube lights. The light source was kept at a 20‐centimetre distance from the neonate. Neonates in both groups received exclusive breastfeeding. The study describes the feeding schedule of the turning group in the description of the intervention. However, the feeding schedule of the supine group is not described. Baseline characteristics like gestational age, birth weight, spectral irradiance (microwatt/cm²/nm), breastfeeding, Apgar score, weight loss, and STB at the start of phototherapy were comparable in the two groups. Outcomes reported included duration of phototherapy and absolute levels of STB at 12 hours of starting phototherapy.
Shinwell 2002 enrolled healthy term infants with birth weight above 2500 g and STB concentration above 18 mg/dL at the start of phototherapy. Of note, this study also included neonates with haemolytic jaundice. Neonates were randomised to supine (n = 16) or turned (n = 16) position. However, two neonates were later excluded from the turning group because of protocol violations: one received double‐surface phototherapy, and another underwent exchange transfusion. Both the study groups received single‐surface phototherapy with two blue and two white compact fluorescent tube lights. The light source was kept at a distance of 23 to 25 cm from the neonate. In the supine group, neonates were positioned supine only and received a 30‐minute break for feeding and nursing care every 150 minutes. In the turning group, neonates were positioned alternately supine or prone every 180 minutes (150 minutes of phototherapy and 30 minutes for feeding and nursing care). Baseline variables like birth weight, gestational age, gender, feeding type, age at initiation of phototherapy, haematocrit, and bilirubin at the start of phototherapy, and incidence of positive Coombs' test, were comparable in the two groups. Reported outcomes included the duration of phototherapy, absolute levels and fall of STB at 24 hours of starting phototherapy and at the end of phototherapy.
Further details of the included studies are provided in the Characteristics of included studies section.
Excluded studies
We excluded six identified articles from the review (Caldera 1984; Fakhraee 2011; Lee Wan Fei 2015; Pritchard 2004; Stanley 2004; Yamauchi 1989). Two studies had non‐random participant allocation (Fakhraee 2011; Yamauchi 1989); one study was a review (Lee Wan Fei 2015); and one study did not fit into inclusion criteria and covered other issues related to neonatal hyperbilirubinaemia (Stanley 2004). Another study tested a different intervention (partly clothed versus naked neonates) and was excluded (Pritchard 2004). The study by Caldera 1984 reported the results of two prospective surveys on phototherapy for neonatal jaundice and evaluated the influence of the intensity of the irradiance, mode and continuous or discontinuous phototherapy, and hence was excluded.
Further details are provided in the Characteristics of excluded studies section.
Studies awaiting classification
One study is awaiting classification (IRCT201111063250N4).
Further details are provided in the Characteristics of studies awaiting classification section.
Risk of bias in included studies
Ratings of the methodological quality of the included studies and risk of bias assessments are presented in the Characteristics of included studies section and are summarised in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Two studies did not describe the method of sequence generation and were therefore at unclear risk of selection bias (Chen 2002; Mohammadzadeh 2004). Bhethanabhotla 2013 used a computer‐generated random sequence and therefore also had a low risk of selection bias. The other two studies described the use of random picking up of a sealed envelope to decide the study group allocation, which is likely to have been an acceptable method of sequence generation.
Allocation concealment
Three studies described the use of sealed envelopes. Bhethanabhotla 2013 used serially numbered, sealed envelopes, whilst Donneborg 2010 and Shinwell 2002 describe random picking up of a sealed envelope. The other two studies did not describe the use of any method to ensure allocation concealment and were therefore at unclear risk of selection bias.
Blinding
Blinding of participants and personnel
Given the nature of the intervention, blinding of healthcare providers or parents was not possible in any of the included studies.
Blinding of outcome assessors
None of the studies described any method to ensure blinding of outcome assessors. However, given the nature of the intervention and the role of the clinical care team in deciding important outcomes like duration of phototherapy, blinding of outcome assessors would not have been possible in any of the included studies.
Incomplete outcome data
Four studies described all the study outcomes for all the randomised neonates (Bhethanabhotla 2013; Chen 2002; Donneborg 2010; Mohammadzadeh 2004). However, in the study by Shinwell 2002, two enrolled neonates received a higher level of intervention (double‐surface phototherapy instead of single‐surface phototherapy) and were excluded from the analysis. The use of more effective co‐intervention could have been due to the failure of the treatment being investigated, therefore we assessed this study as at high risk of attrition bias.
Selective reporting
Bhethanabhotla 2013 reported all of the outcomes mentioned in the clinical trial registry and was therefore at low risk of reporting bias. The trial registry information was not available for the study by Chen 2002; however, all important outcomes were reported, therefore this study was also assessed as at low risk of reporting bias. In the study by Donneborg 2010, only information about the per cent fall of serum bilirubin and absolute values of serum bilirubin was provided, with no information provided regarding the duration of hospital stay and the need for an exchange transfusion. Due to the lack of a published protocol in a trial registry, it cannot be deduced whether all the predefined outcomes have been reported, therefore the study was assessed as having an unclear risk of reporting bias. For the two remaining studies, the trial registration information or study protocol was not available, and some important outcomes have not been reported, therefore these two studies were also assessed as having an unclear risk of reporting bias.
Other potential sources of bias
We could not assess the likelihood of potential publication bias using funnel plots, as at least 10 trials were not available.
Effects of interventions
See summary of findings Table 1 for the main comparison.
Periodic change in body position compared to only supine body position for neonates under phototherapy for the treatment of hyperbilirubinaemia
Duration of phototherapy
Four studies reported this outcome. Pooled results showed no significant difference was observed in the duration of phototherapy (mean difference (MD) 1.71 hours, 95% confidence interval (CI) −3.17 to 6.59 hours; 4 studies, 231 participants; Analysis 1.1). Statistical heterogeneity was significant (Chi² = 7.10, df = 3 (P = 0.07); I² = 58%). Overall, we judged that a periodic change in body position results in little to no difference in duration of phototherapy, based on low certainty evidence due to risk of bias in the included studies and significant heterogeneity.
Per cent fall in bilirubin at 24 hours of starting phototherapy
Three studies reported this outcome. Shinwell 2002 reported a significantly lower per cent fall of bilirubin at 24 hours of starting phototherapy (MD −8%, 95% CI −14.5% to −1.5%) in the supine study group. The remaining two studies did not report any significant difference between groups (Chen 2002; Donneborg 2010). Of the five included studies, only the study by Shinwell 2002 included neonates with haemolytic jaundice. However, the serum bilirubin levels at initiation of phototherapy were not higher than observed in the other included studies.
On pooling of the results, no significant difference was observed in the per cent fall in bilirubin at 24 hours of starting phototherapy (MD −1.17%, 95% CI −6.44% to 4.10%; 3 studies, 193 participants; Analysis 1.2). Statistical heterogeneity was significant (Chi² = 6.55, df = 2 (P = 0.04); I² = 69%). We downgraded the certainty of evidence for this outcome to low due to risk of bias in the included studies and significant heterogeneity.
Rate of fall of serum total bilirubin at 24 hours of starting phototherapy
Only one study reported this outcome (Bhethanabhotla 2013). The rate of fall of STB at 24 hours of starting phototherapy measured was not significantly different between groups (MD 0.02 mg/dL/h, 95% CI −0.02 to 0.06 mg/dL/h; 1 study, 100 participants; Analysis 1.3). This outcome is related to the per cent fall in serum bilirubin (Analysis 1.2). We downgraded the certainty of evidence for this outcome to low due to risk of bias in the included studies and imprecision.
Serum total bilirubin at 24 hours of starting phototherapy
Four studies reported this outcome. On pooling of the results, no significant difference was observed in STB at 24 hours of starting phototherapy (MD 0.22 mg/dL, 95% CI −0.43 to 0.86 mg/dL; 4 studies, 292 participants; Analysis 1.4). Statistical heterogeneity was not significant (Chi² = 3.95, df = 3 (P = 0.27); I² = 24%). Overall, we judged the certainty of evidence that a periodic change in body position results in little to no difference in STB 24 hours after starting phototherapy to be moderate due to risk of bias in the included studies.
Other outcomes
None of the studies reported important secondary outcomes like the need for or number of exchange transfusions, the incidence of bilirubin‐induced neurological damage (BIND), side effects of phototherapy, kernicterus, and sudden infant death syndrome (SIDS).
Subgroup analysis
Only one study included neonates with haemolytic jaundice (Shinwell 2002). However, separate data for neonates with haemolytic jaundice were not reported. In two studies, preterm neonates were eligible to be enrolled (Bhethanabhotla 2013; Donneborg 2010). However, separate data for preterm neonates were not reported. We therefore did not conduct the planned subgroup analyses comparing haemolytic versus non‐haemolytic jaundice and term versus preterm neonates.
Discussion
Summary of main results
Evidence from five studies in 343 term and preterm neonates indicates that a periodic change of body position under phototherapy as compared to no prescribed change may result in little or no difference in the duration of phototherapy and rate of fall in bilirubin at 24 hours of starting phototherapy. The available evidence is insufficient to conclusively answer if a periodic change in comparison to no prescribed change of body position improves the efficacy of phototherapy. The certainty of the evidence for the review outcomes was low due to risk of bias in the included studies, imprecision, or heterogeneity. For important review outcomes such as duration of phototherapy, per cent fall in bilirubin at 24 hours, and rate of fall of bilirubin, the effect estimates were too imprecise to determine their clinical importance. The 95% CIs of absolute measures of effectiveness cross the boundaries of clinically important effect size. The duration of phototherapy could be 3.17 hours shorter to 6.59 hours longer when a periodic change in body position is compared with no prescribed change in position. Similarly, the rate of fall of bilirubin at 24 hours of starting phototherapy could be 0.02 mg/dL/h lower to 0.06 mg/dL/h higher. None of the included studies reported on the need for or number of exchange transfusions, incidence of BIND, and SIDS.
Overall completeness and applicability of evidence
Periodic change in body position probably has little or no effect on the rate of fall of serum total bilirubin at 24 hours of starting phototherapy. Results of this systematic review apply mainly to neonates born at late‐preterm or term gestation receiving phototherapy for non‐haemolytic hyperbilirubinaemia. Only one included study enrolled neonates with haemolytic jaundice (Shinwell 2002). The results of these infants were not reported separately; however, the study results by Shinwell 2002 suggest that turning of term infants with hyperbilirubinaemia does not increase the efficacy of phototherapy, rather that infants nursed supine had a significantly greater drop in bilirubin and shorter duration of phototherapy. We did not find any specific elements in study design or conduct that could account for this, although this was the only included study that enrolled neonates with haemolytic hyperbilirubinaemia. It is possible that repeated interruptions in the phototherapy to change position of the baby could have interfered with efficacy of the phototherapy. Preterm neonates (≥ 35 weeks' and ≥ 33 weeks' gestation, respectively) were eligible to be enrolled in two of the five studies included in the review (Bhethanabhotla 2013; Donneborg 2010). However, separate data for preterm neonates were not reported. Of note, the effect of frequent change in body position is relevant only if the neonate is receiving single‐surface phototherapy. The potential effect of change in body position may be reduced if double‐surface phototherapy is being delivered with two light sources ‐ one over the head and the second underneath with neonate lying on a fibreoptic phototherapy blanket or on a glass surface covering the phototherapy lights underneath the neonate. Lastly, the rationale for changing position during phototherapy rests on the assumption that the effects of phototherapy are largely limited to bilirubin residing in the superficial and connective tissues of the skin, whereas it may be that the effects of phototherapy on bilirubin in the capillary circulation are also important (Hansen 2010).
Any recommendation regarding change in body position of neonates needs to take into account the association of prone positioning with increased risk of SIDS. Supine position is recommended for neonates when put to sleep at home. However, in hospitals, preterm neonates, especially those with respiratory distress, are frequently nursed in a prone position. Whilst during intensive care and continuous monitoring prone positioning is practised for benefits in oxygenation and as part of developmentally supportive care, its safety when providing phototherapy in less intensive areas of the hospital including postnatal wards and rooms or during home phototherapy has not been evaluated. None of the five studies included in this review reported the outcome of SIDS. It is unlikely that a study would ever be conducted to evaluate this outcome during phototherapy, as the potential benefits to improve the effectiveness of phototherapy are likely to be small, and such a trial would need to enrol thousands of neonates.
Quality of the evidence
We judged the included studies to be at high risk of bias. The overall certainty of the evidence was low to very low due to statistical heterogeneity and unclear risk of selection bias (summary of findings Table 1). The main reasons for downgrading the evidence included unclear reporting, statistical heterogeneity, imprecision, and unclear risk of selection bias (two studies). Blinding of outcome assessors was not possible in any of the included studies.
Potential biases in the review process
We did not search Embase or ClinicalTrials.gov separately. Therefore, the retrieval of available studies on phototherapy may have been reduced. While trial records from Embase and ClinicalTrials.gov are included in CENTRAL, we acknowledge a publishing delay from when study records are first available in their original sources and when they are available in CENTRAL. This publishing delay may have prevented recent trial records and reports from being identified. Further, searching only CENTRAL for records from these sources may have lessened the likelihood of retrieving eligible studies due to the limited number of fields that are published to CENTRAL compared to the original source databases. For future updates of this review, we will search Embase and ClinicalTrials.gov separately to ensure maximum retrieval of eligible study records.
It is unlikely that the literature search strategy used in this review would have missed any relevant randomised controlled trials. One trial is awaiting classification (IRCT201111063250N4); although completed, the results have not been published in the literature or presented at any conference. We did not exclude any other potentially relevant study, and there were no marginal decisions affecting the inclusion of any study in the review. However, due to the limited number of eligible studies, we were not able to evaluate publication bias. We have used the standard methods recommended by Cochrane Neonatal to minimise the risk of bias in conducting this review. Two review authors independently assessed the eligibility of studies for inclusion in the review, extracted data, and assessed risk of bias. We could not perform subgroup analysis due to a lack of separate and adequate data in haemolytic hyperbilirubinaemia and in preterm neonates.
Agreements and disagreements with other studies or reviews
Lee Wan Fei and Abdullah performed a systematic review on the effect of turning versus supine position under phototherapy on neonates with hyperbilirubinaemia (Lee Wan Fei 2015). They included five studies identical to the current review, although meta‐analysis was not performed. The authors presented the findings of individual studies and concluded that both supine and turning position were equally effective during phototherapy. Lee Wan Fei 2015 excluded studies not available in the English language and studies with position changes more frequent than two‐ to three‐hourly. We did not apply any language restriction in our search strategy. However, we did not find any non‐English study in our search. We did not apply any time restriction in the change of position in selecting studies for inclusion in the current review.

Risk of bias graph: review authors' judgements about each risk of bias item.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Turning position versus supine under phototherapy, Outcome 1: Duration of phototherapy

Comparison 1: Turning position versus supine under phototherapy, Outcome 2: Per cent fall in bilirubin at 24 hours of starting phototherapy

Comparison 1: Turning position versus supine under phototherapy, Outcome 3: Rate of fall of serum total bilirubin at 24 hours of starting phototherapy

Comparison 1: Turning position versus supine under phototherapy, Outcome 4: Serum total bilirubin at 24 hours of starting phototherapy
| Turning position versus supine under phototherapy in term and preterm neonates with hyperbilirubinaemia | ||||||
| Patient or population: term and preterm neonates with hyperbilirubinaemia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with turning position under phototherapy | Risk with supine position under phototherapy | |||||
| Duration of phototherapy (hours) | The mean duration of phototherapy ranged from 24.8 to 52.8 hours. | The mean duration of phototherapy ranged from 25.5 to 53.3 hours. | Mean duration of phototherapy was on average 1.71 hours longer in the turning group (95% CI −3.17 to 6.59). | 231 | ⊕⊕⊝⊝ | Periodic change in body position may lead to little or no difference in the duration of phototherapy. |
| Rate of fall of serum total bilirubin at 24 hours of starting phototherapy (mg/dL/h)b | The mean rate of fall of serum total bilirubin at 24 hours of starting phototherapy was 0.22 mg/dL/h. | The mean rate of fall of serum total bilirubin at 24 hours of starting phototherapy was 0.2 mg/dL/h. | MD in the rate of fall of serum total bilirubin was an average of 0.02 mg/dL/h higher in the turning group | 100 | ⊕⊕⊝⊝ Lowc | Periodic change in body position may lead to little or no effect on the rate of fall of serum total bilirubin at 24 hours of starting phototherapy. |
| Need for exchange transfusion | None of the studies reported this outcome. | |||||
| Bilirubin‐induced neurological damage (BIND) | None of the studies reported this outcome. | |||||
| Sudden infant death syndrome (SIDS) | None of the studies reported this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels because of statistical heterogeneity and unclear risk of selection bias. (One study has attrition bias. Three studies have unclear reporting. Blinding of outcome assessors was not possible in any of the studies.) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Duration of phototherapy Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | 1.71 [‐3.17, 6.59] |
| 1.2 Per cent fall in bilirubin at 24 hours of starting phototherapy Show forest plot | 3 | 193 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐6.44, 4.10] |
| 1.3 Rate of fall of serum total bilirubin at 24 hours of starting phototherapy Show forest plot | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 1.4 Serum total bilirubin at 24 hours of starting phototherapy Show forest plot | 4 | 292 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.43, 0.86] |

