Učinci različitih tipova intranazalnih steroida u kroničnom rinosinuitisu
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011993.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 abril 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Lee Yee Chong: scoped, designed and wrote the protocol (Chong 2015), screened abstracts, extracted data, conducted the analysis and wrote up the review.
Karen Head: reviewed and edited the protocol, screened abstracts, extracted data, helped to check the analysis and contributed to the writing of the review.
Claire Hopkins: clinical guidance at all stages of project scoping, protocol development and data interpretation. Commented on drafts of the review.
Carl Philpott: clinical guidance at all stages of project scoping, protocol development and data interpretation. Contributed to the writing of the review.
Martin J Burton: helped to draft the protocol; clinical guidance at all stages of project scoping and protocol development, and contributed to the writing of the review.
Anne GM Schilder: commented on drafts and contributed to the writing of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Apr 26 | Different types of intranasal steroids for chronic rhinosinusitis | Review | Lee Yee Chong, Karen Head, Claire Hopkins, Carl Philpott, Martin J Burton, Anne GM Schilder | |
| 2015 Dec 15 | Different types of intranasal steroids for chronic rhinosinusitis | Protocol | Lee Yee Chong, Karen Head, Claire Hopkins, Carl Philpott, Martin J Burton | |
Differences between protocol and review
As part of the discussions about the use of a total symptoms score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Intranasal;
- Beclomethasone [administration & dosage];
- Chronic Disease;
- Fluticasone [administration & dosage];
- Mometasone Furoate [administration & dosage];
- Nasal Polyps [*drug therapy];
- Nasal Sprays;
- Randomized Controlled Trials as Topic;
- Rhinitis [*drug therapy];
- Sinusitis [*drug therapy];
- Steroids [*administration & dosage];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
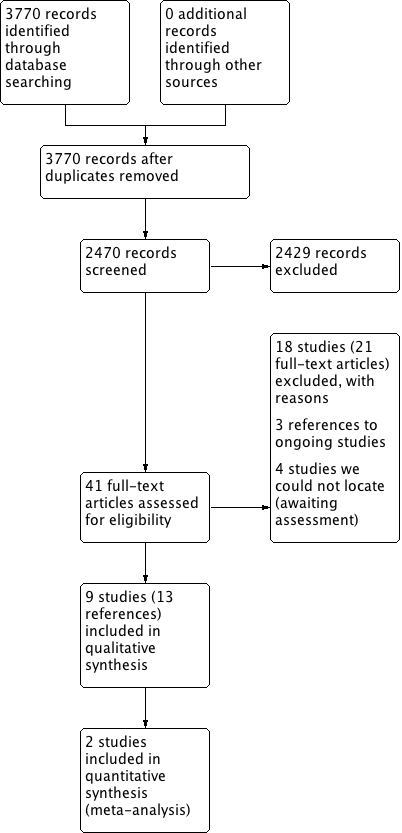
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).
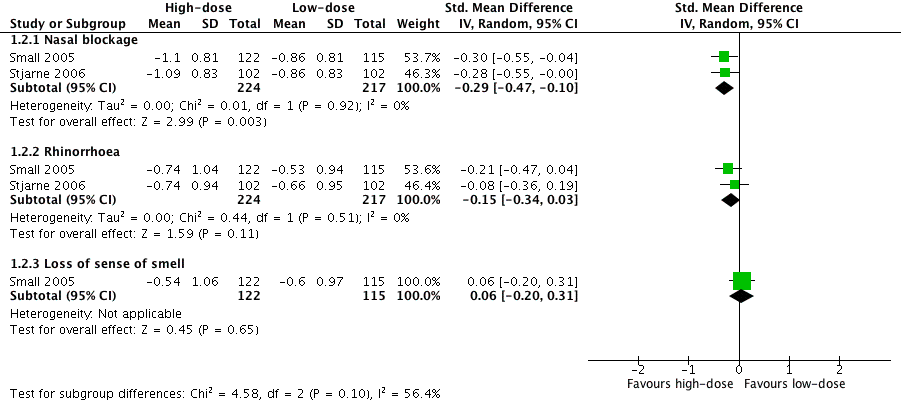
Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 3 Adverse effects: epistaxis.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 4 Adverse effects: local irritation.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 6 Nasal polyps ‐ proportion with improvement at 12 weeks.
| Different types of intranasal corticosteroid molecules for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect (95%) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms
| — |
| ⊕⊝⊝⊝ | No differences observed but evidence was too low quality to draw a conclusion | ||
| Adverse events: epistaxis
| — |
| ⊕⊝⊝⊝ | Unclear whether the risk of epistaxis varies for different types of steroid molecules | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were either very small (n = 20 and n = 26) and had important drop‐outs or were only reported as an abstract with inadequate information available (n = 100). We considered all studies to be at unclear to high risk of selective reporting and attrition bias. The evidence was very low quality due to very serious imprecision and very serious risk of bias concerns. | ||||||
| High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months | ||||||
| All 4 EPOS domains | No information available | |||||
| 3 domains (nasal blockage, rhinorrhoea, loss of sense of smell) Range 0 to 3, lower score = less severe № of participants: 237 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (3 domains) without high‐dose was ‐0.66 points | — | MD 0.13 points lower (0.37 lower to 0.11 more) than low‐dose group | ⊕⊕⊝⊝ | The average score for 3 types of symptoms seems to be similar between the high‐dose and low‐dose groups. |
| (2 domains: nasal blockage, rhinorrhoea) № of participants: 441 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (2 domains) without high‐dose was ‐0.73 points | — | MD 0.19 points lower (0.36 lower to 0.02 lower) than low‐dose group | ⊕⊕⊝⊝ | The average score for 2 types of symptoms seems to be slightly lower for the high‐dose group. The clinical significance of this reduction is unclear. |
| Disease severity ‐ measured as average change from baseline at 4 months (range 0 to 3) | ||||||
|
№ of participants: 441 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ nasal blockage without high‐dose was ‐0.86 points | — | MD 0.24 points lower (0.39 lower to 0.08 lower) than low‐dose group | ⊕⊕⊝⊝ | The nasal blockage score seems to be slightly lower in the high‐dose group. The clinical significance of this reduction is unclear. |
|
№ of participants: 441 (2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ rhinorrhoea without high‐dose was ‐0.6 points | — | MD 0.15 points lower (0.33 lower to 0.03 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for rhinorrhoea seems to be similar between the high‐dose and low‐dose groups. |
|
№ of participants: 237 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ loss of sense of smell without high‐dose was ‐0.6 points | — | MD 0.06 points higher (0.2 lower to 0.32 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for loss of sense of smell seems to be very similar between the high‐dose and low‐dose groups. |
| Adverse effects: epistaxis № of participants: 637 | RR 2.06 | Study population | ⊕⊕⊕⊝ | The risk of epistaxis is likely to be higher in the higher‐dose groups. However, the studies included very minor nosebleeds, such as blood stains in the mucus, and most of these events are not likely to be severe. | ||
| 57 per 1000 | 118 per 1000 | 61 more per 1000 (11 more to 145 more) | ||||
| Moderate | ||||||
| 60 per 1000 | 124 per 1000 | 64 more per 1000 (12 more to 153 more) | ||||
| Adverse effects: local irritation № of participants: 542 | RR 0.97 | Study population | ⊕⊕⊝⊝ | The risk of local irritation seems to be similar between groups, but the overall risks are underestimated due to the way the data were reported. | ||
| 19 per 1000 | 18 per 1000 | 10 fewer per 1000 (13 fewer to 43 more) | ||||
| Moderate | ||||||
| 17 per 1000 | 17 per 1000 | 10 fewer per 1000 (13 fewer to 40 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Scale validity, particularly discriminant validity (ability to distinguish the differences between groups), was unclear. There was a high risk of reporting bias. Studies tended to report enough information for meta‐analysis only for statistically significant results. One study, which had 101 participants, reported very similar values for both intervention arms for all disease scores but had no information related to SD. 2Small sample size ‐ evidence only from one or two relatively small studies. 3Only data from patients with bilateral nasal polyposis. We considered this to be indirectness of the evidence to patients without polyps but have not further downgraded the evidence. 4One of the studies had inadequate blinding ‐ a double dummy was not used to mask the twice daily (higher) versus once daily (lower) dose; the study had 101 participants. 5Sample size relatively small for a precise estimate of adverse events. We downgraded this outcome once, after taking into consideration the inadequate blinding in one of the studies and the relatively small sample size. 6Studies did not use consistent terminology/methods to report different types of local irritation. For analysis we only selected the most frequent types of local irritation from a list (to avoid double counting). This is a possible underestimation of overall event rates. The relatively low event rates and small sample size contributed to the large confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Average symptom score (3 domains) | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 1.2 Average symptom score (2 domains) | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.03] |
| 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Nasal blockage | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.10] |
| 2.2 Rhinorrhoea | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.03] |
| 2.3 Loss of sense of smell | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.31] |
| 3 Adverse effects: epistaxis Show forest plot | 4 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.54] |
| 4 Adverse effects: local irritation Show forest plot | 3 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.31] |
| 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months Show forest plot | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.16, 0.54] |
| 6 Nasal polyps ‐ proportion with improvement at 12 weeks Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.21] |

