درمان رینوسینوزیت مزمن با استفاده از استروئیدهای خوراکی به عنوان درمان کمکی طی یک دوره درمانی کوتاه مدت
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011992.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 abril 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Karen Head: reviewed and edited the protocol, screened abstracts and the full text of papers, extracted data from the included studies, completed the data analysis and drafted the text of the review report.
Lee Yee Chong: scoped, designed and wrote the protocol, screened abstracts and the full text of papers, extracted data from the included studies and helped to draft and review the text of the report.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Martin J Burton: helped to draft the protocol; clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Anne GM Schilder: clinical input into data analysis, reviewing and editing the report.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Apr 26 | Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis | Review | Karen Head, Lee Yee Chong, Claire Hopkins, Carl Philpott, Anne GM Schilder, Martin J Burton | |
| 2015 Dec 18 | Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis | Protocol | Lee Yee Chong, Karen Head, Claire Hopkins, Carl Philpott, Martin J Burton | |
Differences between protocol and review
As part of the discussions about the use of a total symptom score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Intranasal;
- Administration, Oral;
- Adrenal Cortex Hormones [*administration & dosage];
- Chemotherapy, Adjuvant;
- Chronic Disease;
- Methylprednisolone [*administration & dosage];
- Nasal Polyps [*drug therapy];
- Randomized Controlled Trials as Topic;
- Rhinitis [*drug therapy];
- Sinusitis [*drug therapy];
- Steroids [*administration & dosage];
- Time Factors;
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
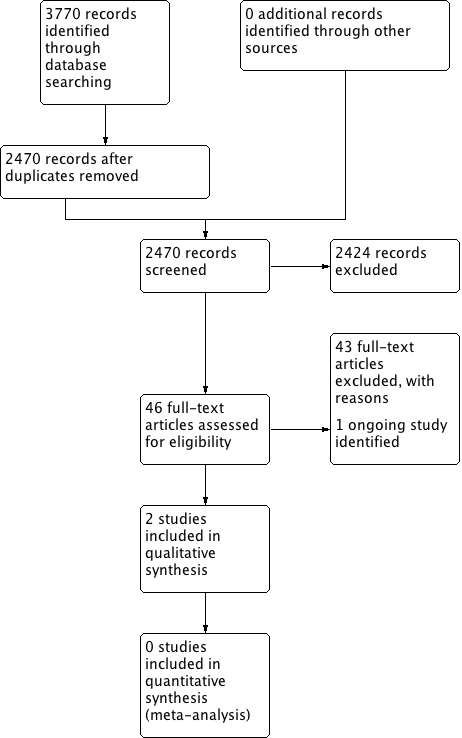
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
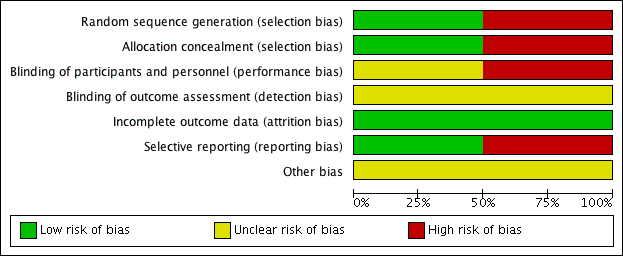
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
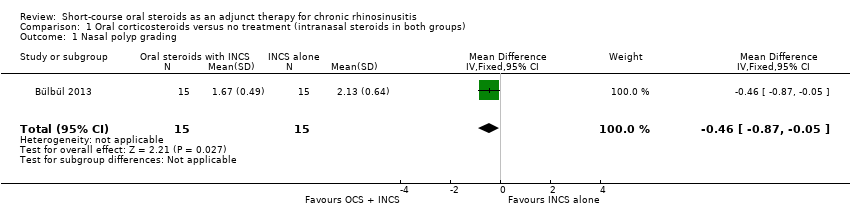
Comparison 1 Oral corticosteroids versus no treatment (intranasal steroids in both groups), Outcome 1 Nasal polyp grading.
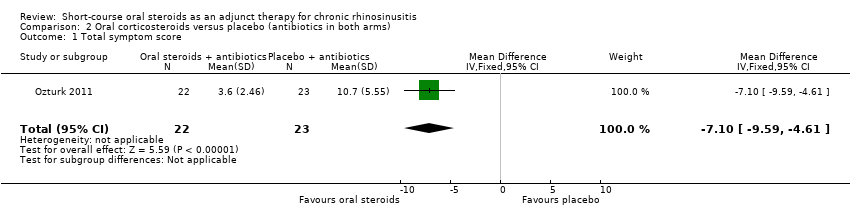
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 1 Total symptom score.
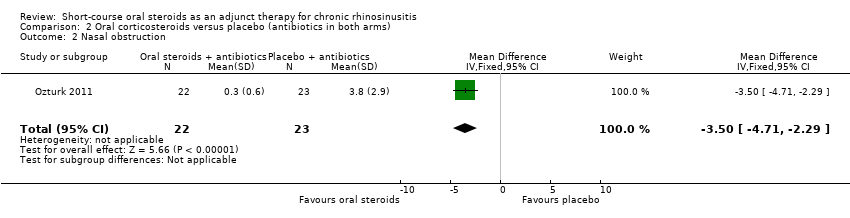
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 2 Nasal obstruction.
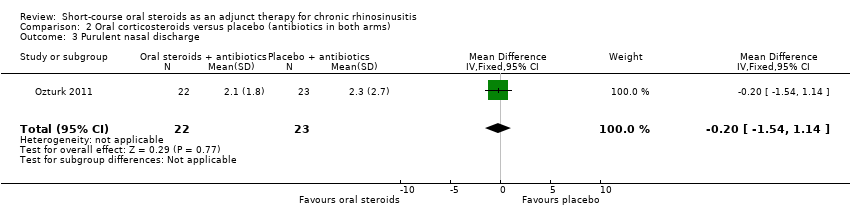
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 3 Purulent nasal discharge.
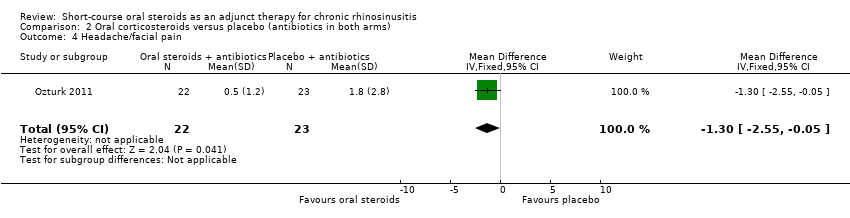
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 4 Headache/facial pain.
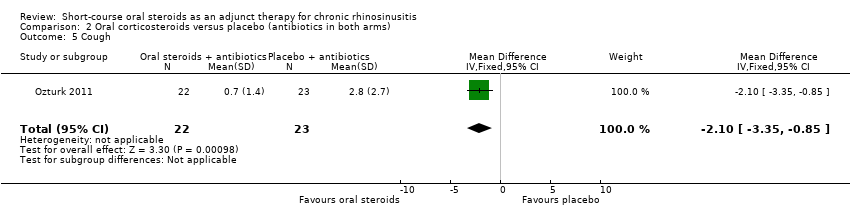
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 5 Cough.
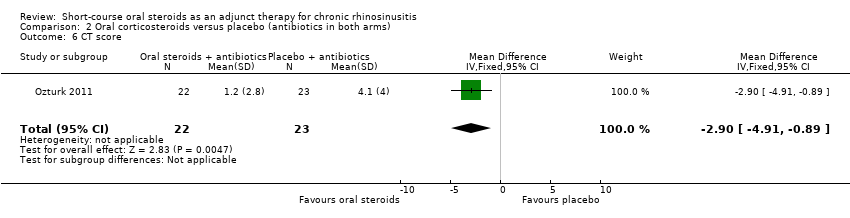
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 6 CT score.
| Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both arms) for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis | ||||||
| Outcomes No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life | — | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score | — | No RCT reported this outcome | ||||
| Adverse effect: mood or behavioural disturbances | — | No RCT reported this outcome | ||||
| Health‐related quality of life | — | No RCT reported this outcome | ||||
| Adverse effect: insomnia | — | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances ‐ not measured | — | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Short‐course oral corticosteroids compared to placebo (antibiotics in both groups) for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis | |||||
| Outcomes No of participants (studies) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | |||
| Disease‐specific health‐related quality of life | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score, assessed with: 4 individual symptoms measured on 0 to 10 visual analogue scale summed to provide a range of 0 to 40 No. of participants: 45 | The mean disease severity score without oral steroids was 15.2 | The mean disease severity score with oral steroids was 3.6 | The mean disease severity score in the intervention group was 7.10 lower (9.59 lower to 4.61 lower) | ⊕⊕⊝⊝ | A lower score indicates less severe symptoms. The results relate to a standardised mean difference of 1.61 standard deviations lower (‐2.29 to 0.93 lower), corresponding to a large difference. |
| Adverse effect: mood or behavioural disturbances | No RCT reported this outcome | ||||
| Health‐related quality of life | No RCT reported this outcome | ||||
| Adverse effect: insomnia | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Study completed only in children (mean age 8 years old). Study follow‐up time was less than 3 months (1 month). Scales were not validated and were completed by "parents and children". 2Symptoms included in this score were: purulent nasal discharge, nasal obstruction, cough and facial pain/headache. | |||||
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare; appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent, occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
| References: Da Silva 2006; Naber 1996; Stanbury 1998 NSAIDs: non‐steroidal anti‐inflammatory drugs | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nasal polyp grading Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total symptom score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.59, ‐4.61] |
| 2 Nasal obstruction Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐4.71, ‐2.29] |
| 3 Purulent nasal discharge Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.54, 1.14] |
| 4 Headache/facial pain Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐2.55, ‐0.05] |
| 5 Cough Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.35, ‐0.85] |
| 6 CT score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.91, ‐0.89] |

