Analgesia controlada por la paciente con remifentanilo versus métodos parenterales alternativos para el tratamiento del dolor del trabajo de parto
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, controlled trial. Double‐blinded. No statement on time of randomisation. The purpose of this pilot study was to compare two regimens of IV remifentanil PCA, along with continuous background infusion, for labour analgesia. The study was conducted in Mount Sinai Hospital, Toronto, Canada, from September 2005 to December 2006. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 22 Number randomised: 20 (10/10) Number receiving treatment: 20 (10/10) Number analysed: 20 (10/10) Inclusion criteria: Term pregnancy, ASA I and II women in active labour, who requested systemic analgesia with or without contraindications to epidural analgesia Exclusion criteria: Allergy or hypersensitivity to remifentanil, opioid dependence or addiction, consumption of narcotics within 24 h of the study period, FHR abnormalities, fetal compromise and/or language barrier Baseline details: Fixed bolus group (n = 10): Age (years, mean (SD)): 32.7 (5.9) Weight (kg, mean (SD)): 85 (30) ASA I/II (n/n): NA Type of delivery (n): vaginal (6), CS (4) Week of gestation: 39.2 ± 1.5 Singleton, twin, multiple pregnancy: NA Parity (n): Primipara (5) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Fixed infusion group (n = 10): Age (years, mean (SD)): 30.4 (5.8) Weight (kg, mean (SD)): 77.1 (14.1) ASA I/II (n/n): NA Type of delivery (n): vaginal (6), CS (4) Week of gestation: 39.0 ± 1.4 Singleton, twin, multiple pregnancy: NA Parity (n): Primipara (7) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Initially, all women received a standard regimen of remifentanil with an infusion of 0.025 µg/(kg*min) and a PCA bolus of 0.25 µg/kg. The PCA lockout interval was set at 2 min, and the 4 h limit was 3 mg. As labour progressed and women required additional analgesia, they received higher doses of either the infusion (group: constant bolus) or the PCA boluses (group: constant infusion). (At the woman’s request, if there was either no change or worsening of pain scores; each step was maintained for at least 15 min before progressing to the subsequent one.) Fixed bolus group (n = 10): The infusion rate was increased stepwise from 0.025 µg/(kg*min) to 0.05 µg/(kg*min), 0.075 µg/(kg*min) and 0.1 µg/(kg*min), while the bolus of 0.25 µg/kg was maintained. Fixed infusion group (n = 10): The bolus dose was increased stepwise from 0.25 µg/kg to 0.5 µg/kg, 0.75 µg/kg and 1 µg/kg, while the infusion rate of 0.025 µg/(kg*min) was kept constant. | |
| Outcomes | The primary outcome variables were maternal pain and desaturation. Continuous: ‐ overall satisfaction (VNRS 0 to 10, within 2 h after delivery) ‐ pain intensity (VNRS 0 to 10, at 0, every 30 min until delivery), overall pain score (VNRS 0 to 10, within 2 h of delivery) ‐ umbilical cord BE (artery, vein), umbilical cord pH (artery, vein) ‐ sedation score (observer, 5 to 0, at baseline and lowest sedation) Dichotomous: ‐ additional analgesia (epidural) ‐ rate of CS ‐ need for neonatal resuscitation ‐ augmented labour (no substance indicated) ‐ women: oxygen desaturation (< 95%, < 90%), hypotension, bradycardia, nausea, vomiting, pruritus, drowsiness, dizziness, confusion ‐ newborns: Apgar score ≥ 7 at 1 and 5 min, non‐reassuring FHR, need for naloxone | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VNRS pain, n = 10 per group) Concomitant medication: Nausea or vomiting was treated with dimenhydrinate, and diphenhydramine was administered for pruritus. The woman could choose to cross over to epidural analgesia at any time during labour, unless there was a contraindication to a regional technique. Funding: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomized, via a computer‐generated randomisation scheme, into one of the two study groups.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The group allocation was blinded via sealed envelopes until the time of PCA administration.” Not specifically mentioned sequentially numbered, opaque envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The patient and the obstetrician, as well as the registered nurse collecting the data, were all blinded to the study group. The group allocation was known only to the anaesthesiologist who was making changes to the pump settings when needed.” Blinding for participants and personnel adequate. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The patient and the obstetrician, as well as the registered nurse collecting the data, were all blinded to the study group. The group allocation was known only to the anaesthesiologist who was making changes to the pump settings when needed.”, “Fetal heart rate tracings were analysed by an obstetrician (P.B.) who was blinded to the study group.” Blinding for outcome assessment adequate. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation. ‐ Rate of escape (epidural): 10%/0% ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Single‐blinded. No statement on time of randomisation. The purpose of this trial was to determine the analgesic efficacy and safety of remifentanil versus pethidine via PCA for women in established uncomplicated labour. There are no details where or when the study was conducted. The authors’ origin is United Kingdom. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 40 (20/20) Number receiving treatment: 40 (20/20) Number analysed: 39 (20/19) Inclusion criteria: Women with ASA I or II, either before the onset of labour in the antenatal ward or in early labour before any analgesia had been requested Exclusion criteria: Women were excluded from the study if they planned to use epidural analgesia or had pre‐eclampsia, multiple pregnancy, premature labour or allergy to any agent under investigation. Baseline details: Remifentanil group (n = 20): Age (years, mean (SD)): 29 (5.2) Weight (kg, mean (SD)): 76 (9) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (median (IQR [range])): 1 (1 ‐ 2 [0 ‐ 5]) Duration of labour: ‐ before PCA use (min, mean (SD)): 125 (98) ‐ first stage of labour (min, mean (SD)): 260 (97) ‐ second stage of labour (min, mean (SD)): 22 (22) Pethidine group (n = 19): Age (years, mean (SD)): 29 (5.4) Weight (kg, mean (SD)): 76 (12) ASA I/II (n/n): NA Type of delivery (n): spontaneous labour (NA) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (median (IQR [range])): 1 (0 ‐ 2 [0 ‐ 3]) Duration of labour: ‐ before PCA use (min, mean (SD)): 191 (205) ‐ first stage of labour (min, mean (SD)): 296 (158) ‐ second stage of labour (min, mean (SD)): 20 (12) | |
| Interventions | Remifentanil group (n = 20): Women received a remifentanil PCA using 40 µg remifentanil with a lockout of 2 min. The bolus dose and lockout period for the remifentanil PCA were based on an average maternal weight (80 kg) and a bolus of 0.5 µg/kg. Pethidine group (n = 19): Control participants received PCA using pethidine 15 mg with a lockout of 10 min. | |
| Outcomes | The primary endpoint of the study was overall pain. Continuous: ‐ satisfaction with analgesia (VAS 0 to 10, at 0, 30 min until 120 min, median + IQR + range (symmetric)) ‐ pain intensity (VAS 0 to 10, at 0, every 30 min until 120 min, median + IQR (asymmetric)), overall pain score (VAS 0 to 10, at 2 h after delivery) ‐ umbilical cord pH (not specified) ‐ sedation score (observer, 5 to 1, and parturient score, VAS 0 to 10, at 0, 30, 60, 90, 120 min, respectively, median + IQR + range (asymmetric)) ‐ women: mean respiratory rate, mean SBP, mean HR, median nausea score (VAS 0 to 10) (at 0, 30, 60, 90, 120 min, respectively) ‐ newborns: mean FHR (at 0, 30, 60, 90, 120 min), Apgar score at 1 and 5 min (median + IQR + range (asymmetric)), NACS at 30 and 120 min (median + IQR + range (symmetric)) Dichotomous: ‐ additional analgesia (Entonox) ‐ women: oxygen desaturation (% total PCA time spent with < 94% and < 90%, diagrammed) ‐ newborns: need for naloxone | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS overall pain, n = 20 per group) Concomitant medication: All women were free to change to regional analgesia at any time if so desired. Entonox was available to all women throughout the study. Funding: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The women were randomly allocated to receive PCA using either remifentanil […] or pethidine […].” No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “[…] women, who were unaware of which treatment they were receiving.” No statement on whether key study personnel were blinded. We assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. The used method of blinding may only work for the outcome assessors (which was not mentioned in the published report for most of the relevant outcomes). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “Baseline non‐invasive blood pressure, heart rate, SpO2, respiratory rate, observer sedation score […] and fetal heart rate were recorded by a blinded investigator. Baseline visual analogue scale (VAS) measurements were also recorded for the pain of contractions, satisfaction with current analgesia, nausea, anxiety and sedation.”, “At delivery, Apgar scores at 1 and 5 min were recorded, cord blood was taken for blood gas analysis and the fetal requirement for naloxone was noted.”, "The cardiotocograph (CTG) was recorded for a minimum of 1 h after starting the PCA and was subsequently analysed by a blinded obstetrician […].” It is unclear from the description whether assessment for most of the outcomes after treatment (during study) was blinded. The study did address this issue only for the assessment of FHR patterns. We do not know who was responsible for outcome assessment and participants and attending personnel may be able to uncover group allocation. The subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. Therefore, insufficient information exists to judge "yes" or "no". |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 0%/5% Quote: “There was one protocol violation in the pethidine group and no data were included from this patient.” The protocol violation was not described. ‐ No statement on why 1 woman in the Remifentanil group was not analysed for all outcomes (satisfaction with pain relief, AE for newborn) ‐ Rate of escape (Entonox): 90%/100% (may influence data on AE, satisfaction and pain) ‐ Rate of cross‐over: NA ‐ Data‐analysis: Per‐protocol (protocol violation) |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. No statement on blinding. No statement on time of randomisation. The purpose of this trial was to evaluate the effectiveness and security of remifentanil administered by means of elastomeric infusor with PCA IV compared with IM meperidine in obstetric women with contraindication for epidural analgesia. There are no details where or when the study was conducted. The authors’ origin is Spain. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 24 (12/12) Number receiving treatment: 24 (12/12) Number analysed: 24 (12/12) Inclusion criteria: ASA I to III, aged 20 to 40 years, requesting analgesia Exclusion criteria: NA Baseline details: Remifentanil group (n = 12): Age (years, mean (SD)): 28 (5) Weight (kg, mean (SD)): 72 (8) ASA I/II (n/n): 8/2 Type of delivery (n): spontaneous (NA), instrumental (1), CS (0) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Meperidine group (n = 12): Age (years, mean (SD)): 30 (3) Weight (kg, mean (SD)): 75 (6) ASA I/II (n/n): 7/3 Type of delivery (n): spontaneous (NA), instrumental (2), CS (1) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 12): An elastomeric infusor with a capacity of 250 mL was filled with 2.5 mg of remifentanil and a 12 mL/h was started (average infusion of 0.025 µg/(kg*min) of remifentanil and boluses of 5 mL with a time of closing of 30 min). Meperidine group (n = 12): Women were given 1 mg/kg of meperidine and 2.5 mg of haloperidol every 4 h by IM route. | |
| Outcomes | The primary endpoint of the study was not defined. Continuous: ‐ overall satisfaction (VAS 0 to 10, time point unclear) ‐ pain intensity (VAS 0 to 100, at 0, every 30 min until 280 min, "expulsivo") ‐ newborns: Apgar score at 1 and 5 min Dichotomous: ‐ rate of CS, rate of assisted birth (instrumental) ‐ women: nausea + vomiting | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis not performed Concomitant medication: NA Funding: NA Intervention: Lockout time of 30 min seems too long for adequate analgesia. Contact to the authors: We contacted Dr. Torres via e‐mail (23 June 2016) to inquire the number of women who reported 'pain intensity at 2 hours'. We did not receive any answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “24 patients were randomized […].” No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The study did not address this issue. However, we assume that blinding of outcome assessment did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Double‐blinded. No statement on time of randomisation. The purpose of this trial was to compare the analgesic efficacy of remifentanil with meperidine and fentanyl in a patient‐controlled setting (PCA). There are no details where or when the study was conducted. The authors’ origin is the Netherlands. Trial Identifier: NTR543 | |
| Participants | Participant flow: Number assessed for eligibility: 180 Number randomised: 180 (60/60/60) Number receiving treatment: 159 (52/53/54) Number analysed: 159 (21 excluded, delivery within 1 h after randomisation) Inclusion criteria: ASA physical status I or II, singleton cephalic presentation in active labour Exclusion criteria: Obesity (BMI (body mass index) ≥ 40 kg/m²), opioid allergy, substance abuse history, and women at high‐risk (pre‐eclampsia, severe asthma, insulin‐dependent diabetes mellitus, hepatic insufficiency, or renal failure) Baseline details: Remifentanil group (n = 52): Age (years, mean (SD)): 33.1 (5.0) Weight (kg, mean (SD)): 81 (13) ASA I/II (n/n): NA Type of delivery: spontaneous (62%), instrumental (22%), CS (16%) Week of gestation: 40 Singleton, twin, multiple pregnancy: singleton pregnancy Parity: Primiparity 58% Duration of labour: ‐ First stage of labour (min, mean (SD)): 363 (191) ‐ Second stage of labour (min, mean (SD)): 36 (30) Meperidine group (n = 53): Age (years, mean (SD)): 33.6 (5.5) Weight (kg, mean (SD)): 84 (14) ASA I/II (n/n): NA Type of delivery: spontaneous (69%), instrumental (23%), CS (9%) Week of gestation: 40 Singleton, twin, multiple pregnancy: singleton pregnancy Parity: Primiparity 66% Duration of labour: ‐ First stage of labour (min, mean (SD)): 293 (155) ‐ Second stage of labour (min, mean (SD)): 42 (35) Fentanyl group (n = 54): Age (years, mean (SD)): 33.5 (4.1) Weight (kg, mean (SD)): 79 (12) ASA I/II (n/n): NA Type of delivery: spontaneous (85%), instrumental (13%), CS (2%) Week of gestation: 40 Singleton, twin, multiple pregnancy: just singleton pregnancy Parity: Primiparity 68% Duration of labour: ‐ First stage of labour (min, mean (SD)): 348 (175) ‐ Second stage of labour (min, mean (SD)): 38 (26) | |
| Interventions | Remifentanil group (n = 52): Women received a 40 µg loading dose and remifentanil 40 µg per bolus with a lockout of 2 min and a maximum dose limit of 1200 µg/h. Meperidine group (n = 53): Women received a 49.5 mg loading dose and 5 mg boluses with a lockout of 10 min and a maximum overall dose limit of 200 mg. Fentanyl group (n = 54): Women received a 50 µg loading dose and boluses of 20 µg with a lockout of 5 min and a maximum dose limit of 240 µg/h. | |
| Outcomes | The primary endpoint was not clearly stated but power analysis was performed for average pain score. Continuous: ‐ overall satisfaction (VRS 1 to 10, at 2 h after delivery) ‐ pain intensity (VAS 0 to 10, at 0 h, every 1 h until 6 h) ‐ umbilical cord BE (not specified), umbilical cord pH (not specified) ‐ sedation score (observer, 1 to 5, at 0, 1, 2, 3 h) ‐ newborns: Apgar score at 1 and 5 min, NACS at 15 and 120 min Dichotomous: ‐ additional analgesia (epidural) ‐ rate of CS, rate of assisted birth (instrumental) ‐ oxytocin use ‐ women: oxygen desaturation (< 95%), nausea + vomiting, pruritus ‐ newborns: CTG reactive | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (average pain score, n = 60 per group) Concomitant medication: All women were free to change to epidural analgesia at any time. The PCA device was discontinued at full cervical dilatation. Hypotension (systolic arterial pressure < 90 mmHg or > 25% below baseline) was treated with IV fluids and ephedrine 5 mg IV. When oxygen saturation decreased below 95%, oxygen 6 L/min was administered by facemask. If labour failed to progress (first or second stage), oxytocin was given, according to the hospital protocol. Funding: This work was supported by the Bronovo Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was established by using a computer‐generated random sequence […].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] random sequence in numbered envelopes.” Not specifically mentioned opaque and sealed envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “Study medication was prepared and blinded by the hospital pharmacy.”, “Observants and medical personnel attending to the parturient were unaware of the drug assignment.” We assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the 2 interventions. The used method of blinding may only work for the outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Study medication was prepared and blinded by the hospital pharmacy.”, “With the exception of baseline data, all observations and measurements were made by blinded observers. Observants entered the delivery room only after the PCA device had been connected […]. This way the observants were unable to notice time differences in the administration […], which might have jeopardized blinding. Observants had no knowledge of the differences in programming of the PCA devices.”, “Observants and medical personnel attending to the parturient were unaware of the drug assignment.”, “Fetal heart rate patterns were scored as reactive or non‐reactive at regular intervals by an obstetrician who was blinded to the treatment groups.” An attempt was made and reported in the method section to blind the outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 15%/12%/10% Quote: “[…] 21 were excluded due to delivery within 1h after randomisation.” No statement on time of randomisation. ‐ Large amount (up to 50%) of outcome data (satisfaction with pain relief, AE newborn/AE mother, rate of CS, rate of assisted birth, umbilical blood pH/base excess) not reported. No reasons declared. ‐ Rate of escape (epidural): 13%/34%/15% (may influence data on AE, satisfaction and pain) ‐ Rate of cross‐over: NA ‐ Data‐analysis: Per‐protocol (women who delivered within 1 hour were excluded) |
| Selective reporting (reporting bias) | High risk | The protocol is available (NTR543, ISRCTN12122492) and there are several deviations. In the protocol the primary outcomes were amongst others requirement for naloxone as fetal outcome and presence of opioid substances in umbilical and maternal blood samples. In the published report both outcomes were mentioned within the methods but results were not reported. The observer sedation score was not pre‐specified in the protocol. The study protocol was retrospectively registered: Study registration (NTR543): 12/2005 First enrolment: 08/2005 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. No statement on blinding. No statement on time of randomisation. The purpose of this trial was to compare the efficacy of IV remifentanil PCA with epidural ropivacaine/sufentanil during labour. There are no details where or when the study was conducted. The authors’ origin is the Netherlands. Trial Identifiers: NTR1127 or EUCTR2007‐000808‐32‐NL | |
| Participants | Participant flow: Number assessed for eligibility: 147 Number randomised: 26 (14/12) Number receiving treatment: 25 (14/11) Number analysed: 20 (10/10 at 1 h, 9/8 at 2h, 6/6 at 3 h) Inclusion criteria: singleton pregnancy, ASA I or II, without prior use of opioid analgesics Exclusion criteria: Cervical dilation > 5 cm, pre‐eclampsia, insulin‐dependent diabetes, substance abuse, opioid allergy and morbid obesity (BMI ≥ 40 kg/m²) Baseline details: Remifentanil group (n = 10): Age (years, mean (SD)): 32.7 (5.9) Weight (kg, mean (SD)): 83.3 (16.7) ASA I/II (n/n): NA Type of delivery (n): spontaneous (7), instrumental (1), CS (2) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Parity (n): Primiparity (5) Duration of labour: ‐ First stage of labour (min, mean (SD)): 488 (277) ‐ Second stage of labour (min, mean (SD)): 71 (40) Epidural group (n = 10): Age (years, mean (SD)): 31.0 (5.2) Weight (kg, mean (SD)): 78.9 (11.9) ASA I/II (n/n): NA Type of delivery (n): spontaneous (4), instrumental (4), CS (2) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Parity (n): Primiparity (7) Duration of labour: ‐ First stage of labour (min, mean (SD)): 410 (173) ‐ Second stage of labour (min, mean (SD)): 32 (14) | |
| Interventions | Remifentanil group (n = 10): Parturients randomised to the IV remifentanil group received a 40 µg loading dose and boluses of 40 µg with a 2 min lockout time and bolus duration of 36 s using a Graseby 3300 syringe pump (Smiths Medical International, Ashford, Kent, UK). Maximum dose limit was 1200 µg/h. The PCA device was discontinued when parturients reached full cervical dilation. No further analgesia was provided during the second stage. Epidural group (n = 10): Women randomised to receive epidural analgesia were pre‐hydrated with 500 mL IV crystalloid solution before an epidural catheter was placed using a midline paramedian approach with a 17‐gauge Tuohy needle and loss‐of‐resistance to saline at L2–3 or L3–4. A loading dose of 0.2% ropivacaine 12.5 mL was given through the epidural catheter, followed by a continuous infusion of 0.1% ropivacaine with sufentanil 0.5 µg/mL at 10 mL/h. If analgesia was inadequate, additional boluses of the epidural solution were given. At full cervical dilation the epidural infusion was discontinued according to local hospital policy. | |
| Outcomes | The primary outcome parameter of this study was the VAS pain score. Continuous: ‐ satisfaction with analgesia (VAS 0 to 10, at 0, 1, 2, 3 h after starting analgesia), overall satisfaction (NRS 1 to 10, at 2 h after delivery) ‐ pain intensity (VAS 0 to 10, at 0, 1, 2, 3 h after starting analgesia) ‐ umbilical cord BE (artery), umbilical cord pH (artery) ‐ sedation score (observer, 1 to 5, at 0, 1, 2, 3 h after starting analgesia) ‐ newborns: Apgar score at 1 and 5 min Dichotomous: ‐ additional analgesia (conversion remifentanil (PCA) to epidural) ‐ rate of CS, rate of assisted birth (instrumental) ‐ oxytocin use ‐ women: oxygen desaturation (< 95%), hypotension, bradycardia, nausea, vomiting, pruritus ‐ newborns: Apgar score ≤ 7 at 5 min, CTG reactive | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS pain score, n = 10 per group) Concomitant medication: If pain relief was inadequate at any time, the woman could request epidural analgesia. Hypotension (SBP < 90 mmHg or > 25% below baseline) was treated with IV fluids and IV ephedrine 5 mg or phenylephrine 100 µg. Supplemental oxygen was administered if maternal oxygen saturation (SpO2) levels remained below 95% for more than 60 s. Funding: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “[…] numbered envelopes that had been randomised using a computer‐generated random sequence […].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] numbered envelopes.” Not specifically mentioned opaque and sealed envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “Fetal heart rate patterns were scored as reactive or non‐reactive by an obstetrician who was blinded to study allocation.” The study did not address this issue for most of the relevant outcomes with exception of the assessment of FHR patterns. However, we assume that blinding of outcome assessment did not occurred due to technical reasons and at least all other subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 29%/17% Quote: “Twenty‐six parturients were enrolled of whom 20 completed the study; 10 subjects received remifentanil, 10 received epidural analgesia. Six parturients were excluded because of either delivery within one hour of randomisation (n = 5) or unsuccessful placement of the epidural catheter (n = 1).” No statement on time of randomisation. ‐ 30%/20% of outcome data were not reported for neonatal outcomes. No reasons declared. One woman in the remifentanil group required an epidural 2 h after initiation of the intervention. Outcome data of this woman were excluded from the analysis for satisfaction, pain, neonatal outcomes. Reason for exclusion may be related to true outcome. ‐ Rate of escape: NA ‐ Rate of cross‐over: 7%/NA, cross‐over participants were analysed as randomised ‐ Data‐analysis: Per‐protocol (women who delivered within 1 h were excluded) |
| Selective reporting (reporting bias) | High risk | The protocol is available (NTR1127, EUCTR2007‐000808‐32‐NL) and there are several deviations. In the protocol pain scores, woman's satisfaction, and fetal outcome (Apgar scores, umbilical cord pH, NACS, and requirement for naloxone) were defined as primary outcomes. No secondary outcomes were defined. In the published report the only primary outcome was pain score. Woman's satisfaction, sedation score, and SpO2 were defined as secondary outcomes. NACS and requirement for naloxone were not reported. Sedation, nausea and vomiting, SpO2 were not pre‐specified in the protocol. The study protocol was prospectively registered: Study registration (NTR1127): 17/11/2007 First enrolment: 26/11/2007 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | 2‐arm randomised, controlled trial with a third‐arm observational cohort. Not blinded. Randomisation after onset of labour. The purpose of this trial was to compare the incidence of maternal fever (temperature ≥ 38°C) in parturients receiving IV remifentanil by PCA, with those receiving either epidural analgesia or no analgesia. The study was conducted in Leiden University Medical Center (period unknown). Trial Identifier: NTR1498 | |
| Participants | Participant flow: Number assessed for eligibility: 250 Number randomised: 116 (57/59) Number receiving treatment: 114 (57/57) Number analysed: 98 (49/49) + 42 control group Inclusion criteria: ASA I or II parturients with a singleton pregnancy, between 37 and 42 weeks of gestation Exclusion criteria: BMI ≥ 40 kg/m², insulin‐dependent diabetes, severe pre‐eclampsia (proteinuria ≥ 5 g/24 h), use of antibiotics during delivery, initial maternal SpO2 < 98%, initial maternal temperature ≥ 38°C, cervical dilation of > 7 cm and ruptured membranes for > 24 h at the time of inclusion. If delivery occurred within 1 h of starting the study, women were excluded from analysis. Baseline details: Remifentanil group (n = 49): Age (years, mean (SD)): 32 (4.8) Weight (kg, mean (SD)): 81 (17.2) ASA I/II (n/n): NA Type of delivery (n): spontaneous (32), instrumental (9), CS (7), missing (1) Week of gestation: 39 Singleton, twin, multiple pregnancy: singleton Parity (n): Nulliparous (25) Duration of labour: ‐ First stage of labour (min, mean (SD)): 355 (179) ‐ Second stage of labour (min, mean (SD)): 35 (29.9) Epidural group (n = 49): Age (years, mean (SD)): 31 (5.6) Weight (kg, mean (SD)): 81 (12.6) ASA I/II (n/n): NA Type of delivery(n): spontaneous (29), instrumental (9), CS (10), missing (1) Week of gestation: 40 Singleton, twin, multiple pregnancy: singleton Parity (n): Nulliparous (27) Duration of labour: ‐ First stage of labour (min, mean (SD)): 434 (158) ‐ Second stage of labour (min, mean (SD)): 40 (28.9) Control group (n = 42): Age (years, mean (SD)): 33 (4.5) Weight (kg, mean (SD)): 83 (13.3) ASA I/II (n/n): NA Type of delivery (n): spontaneous (34), instrumental (3), CS (5) Week of gestation: 40 Singleton, twin, multiple pregnancy: singleton Parity (n): Nulliparous (11) Duration of labour: ‐ First stage of labour (min, mean (SD)): 224 (131) ‐ Second stage of labour (min, mean (SD)): 24 (24.1) | |
| Interventions | Remifentanil group (n = 49): Women in the remifentanil (PCA) group received a 40 µg bolus (lockout 2 min, bolus duration 36 s) using a Graseby 3300 syringe pump (Smiths Medical Int., Luton, UK). The maximum dose permitted was 1200 µg/h. No background infusion was added. Because of concerns about the potential for neonatal respiratory depression, the pump was stopped when the woman reached full cervical dilatation. Epidural group (n = 49): A catheter was inserted at the L2–3 or L3–4 interspace using a 17‐gauge Tuohy needle. Parturients received a loading dose of ropivacaine 25 mg (0.2% ropivacaine 12.5 mL), followed by a continuous infusion of 0.1% ropivacaine and sufentanil 0.5 µg/mL at 10 mL/h. In case of inadequate analgesia, additional 10 mL boluses could be given. In case of epidural catheter dislodgement, the catheter was replaced. Control group (n = 42): No intervention | |
| Outcomes | The primary outcome variable was the proportion of women who developed a temperature ≥ 38°C before delivery. Continuous: ‐ overall satisfaction (NRS 1 to 10, after delivery) ‐ pain intensity (VAS 0 to 10, at 0, 1, 2, 3, 4 h after starting analgesia, diagrammed) ‐ umbilical cord BE (venous), umbilical cord pH (venous) ‐ sedation score (observer, 1 to 5, at 0, 1, 2, 3, 4 h after starting analgesia) ‐ newborns: Apgar score at 1 and 5 min Dichotomous: ‐ additional analgesia (escape analgesia) ‐ rate of CS, rate of assisted birth (instrumental) ‐ oxytocin use ‐ women: oxygen desaturation (< 92%, < 90%, for 1, 2 or 5 min), nausea, vomiting, pruritus ‐ newborns: Apgar score ≤ 7 at 5 min, CTG reactive | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (incidence of fever, n = 175 in total) Concomitant medication: When parturients were dissatisfied with analgesia, an epidural was offered as alternative. When SpO2 dropped < 92% for more than 60 s, oxygen was administered by facemask. Intrapartum fever was defined as maternal tympanic temperature ≥ 38°C. In cases of fever, antibiotics could be administered to the parturient. Hypotension (SBP < 90 mmHg or > 25% below pre‐analgesia values) was treated with IV fluids and/or IV ephedrine or phenylephrine. Funding: NA Contact to the authors: We contacted Dr. Douma via e‐mail (15 March 2016) to inquire the number of women who reported 'satisfaction with pain relief' and 'pain intensity at 1, 2, 3, and 4 hours'. We received the missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was performed using a computer‐ generated randomisation list and treatments (RPCA or EA) […]” |
| Allocation concealment (selection bias) | Low risk | Quote: “…were presented in a numbered opaque sealed envelope that was opened upon the request for analgesia.” |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “The study was not blinded.” No blinding and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “The study was not blinded.” No blinding and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 14%/17% Quote: “We assessed the eligibility of 250 women, of whom 164 were enrolled in the study […]. After excluding women who delivered within one hour, [2 failed epidural, 2 withdrawals, 13 delivered within 1 hour, 5 exclusion criterion] 140 women were analysed, 49 received RPCA, 49 received EA and 42 were in the observational control group." No reasons declared for exclusion of the 5 women (exclusion criterion). ‐ Quote: “Due to technical difficulties, continuous saturation data were not always available and this information is reported for only 114 women.”; Data on type of delivery from one woman each group were missing with no reasons declared. ‐ Rate of escape: NA ‐ Rate of cross‐over: 16%/2%, cross‐over participants were analysed as randomised ‐ Data‐analysis: Per‐protocol (women who delivered within 1 h were excluded) |
| Selective reporting (reporting bias) | High risk | 2 protocols are available (NTR1498 and EUCTR2008‐002792‐28‐NL) and there are several deviations. In the protocol maternal temperature and maternal saturation were defined as primary outcomes. In the published report, however, maternal saturation was reported as a secondary outcome. The secondary outcomes pain and overall satisfaction were not pre‐specified in the protocol. The pre‐specified outcomes NACS and requirement for naloxone were not reported in the published report. The study protocol was prospectively registered: Study registration (NTR1498): 10/2008. First enrolment: 11/2008 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. No statement on blinding. No statement on time of randomisation. The study was planned to compare the use of remifentanil patient‐controlled IV analgesia (PCIA) to epidural bupivacaine plus fentanyl for labour analgesia in pre‐eclamptic women. There are no details where or when the study was conducted. The authors’ origin is Egypt/Saudi Arabia. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: NA Number receiving treatment: NA Number analysed: 30 (15/15) Inclusion criteria: ≥ 32 weeks of gestation, normal cephalic presentation, < 5 cm cervical dilatation, clinical diagnosis of pre‐eclampsia Exclusion criteria: Remifentanil allergy, progression to eclampsia, evidence of increased intracranial pressure or focal neurologic deficit, platelet count of less than 80*10⁹/L, evidence of pulmonary oedema, non‐reassuring FHR tracing requiring imminent delivery Baseline details: Remifentanil group (n = 15): Age (years, mean (SD)): 26 (8) Weight (kg, mean (SD)): 79 (22) ASA I/II (n/n): NA Type of delivery (n): spontaneous (10), instrumental (0), CS (3) Week of gestation: 36 ± 4 Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Epidural group (n = 15): Age (years, mean (SD)): 28 (9) Weight (kg, mean (SD)): 84 (37) ASA I/II (n/n): NA Type of delivery (n): spontaneous (8), instrumental (3), CS (4) Week of gestation: 35 ± 3 Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 15): The remifentanil hydrochloride concentration used was 50 µg/mL (3 mg diluted to 60 mL of normal saline). The PCA was set to deliver 0.5 µg/kg as a loading bolus infused over 10 s, lockout time of 5 min, PCA bolus of 0.25 µg/kg, continuous background infusion of 0.05 µg/(kg*min), and maximum dose was 3 mg in 4 h. Women were advised to start the PCA bolus when they felt the signs of a coming uterine contraction. Epidural group (n = 15): An epidural catheter was placed under complete aseptic technique at the L3‐L4 or L4‐L5 interspaces. A test dose of 0.25% bupivacaine was administered, and epidural analgesia was established with initial bolus of 10 mL to 15 mL of 0.25% bupivacaine plus 1 µg/kg fentanyl. Analgesia was maintained by continuous infusion of 0.125% bupivacaine plus 2 µg/mL fentanyl at a rate of 10 mL to 12 mL per hour aiming to obtain a T‐10 sensory level. | |
| Outcomes | The primary endpoint of the study was not defined. Continuous: ‐ overall satisfaction (NRS 1 to 4, 24 h after delivery) ‐ pain intensity (VAS 0 to 10, at 0, 1 h and after delivery) ‐ umbilical cord pH (artery, vein) ‐ sedation score (observer, 1 to 4, 0, 1 h and after delivery) ‐ women: mean respiratory rate, mean SpO2, mean HR (at 0, 1 h and after delivery, respectively) Dichotomous: ‐ rate of CS, rate of assisted birth (instrumental) ‐ need for neonatal mechanical ventilation ‐ women: hypotension (at 0, 1 h and after delivery), nausea, vomiting, pruritus ‐ newborns: Apgar score ≤ 7 at 1 and 5 min, need for naloxone, FHR abnormalities 1 h after analgesia | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis not performed ‐ Satisfactory analgesia was considered if VAS ≤ 3 Concomitant medication: If the assigned analgesia was inadequate for the woman at any time, an alternative was offered and further study recording was discontinued. Hypotension (defined as reduction of > 25% of baseline level) was treated by either additional IV crystalloid or IV bolus doses (e.g. 2.5 to 5.0 mg) of ephedrine. Funding: NA Intervention: Lockout time of 5 min seems too long for adequate analgesia. Ethics: The study did not report that the study protocol was approved by the local Ethics committee. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “30 preeclamptic patients were randomly assigned….” No method described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The study did not address this issue. However, we assume that blinding of outcome assessment did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: NA |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Double‐blinded. Randomisation after onset of labour. The purpose of this trial was to compare the analgesic effect of remifentanil in PCIA during labour and delivery with the effect of an IV infusion of meperidine. There are no details where or when the study was conducted. The authors’ origin is Israel. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 88 (43/45) Number receiving treatment: 88 (43/45) Number analysed: 88 (43/45) Inclusion criteria: Term parturients with singleton cephalic presentation requesting systemic analgesia, ASA I or II, active labour (cervical dilation of 3 cm to 6 cm) Exclusion criteria: ASA III or more, obesity (more than 100 kg or BMI ≥ 40kg/m²), history of drug (including analgesic chronic use or large doses) or alcohol abuse, smoking more than 10 cigarettes per day, and abnormal liver, renal, or haematological function Baseline details: Remifentanil group (n = 43): Age (years, mean (SD)): 29.5 (5.3) Weight (kg, mean (SD)): 75.1 (16) ASA I/II (n/n): NA Type of delivery (n): spontaneous (40), vacuum extraction (1), forceps delivery (0), CS (2) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): Primiparity (22) Duration of labour: ‐ First stage of labour (min, mean (SD)): active phase 245.2 (150.8) ‐ Second stage of labour (min, mean (SD)): 38.0 (32.2) Meperidine group (n = 45): Age (years, mean (SD)): 29.2 (5.2) Weight (kg, mean (SD)): 74.8 (11.27) ASA I/II (n/n): NA Type of delivery (n): spontaneous (38), vacuum extraction (2), forceps delivery (0), CS (5) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): Primiparity (19) Duration of labour: ‐ First stage of labour (min, mean (SD)): active phase 251.4 (118.8) ‐ Second stage of labour (min, mean (SD)): 42.2 (45.6) | |
| Interventions | Remifentanil group (n = 43): Participants randomised to receive remifentanil were connected to PCIA by a 1‐way infusion line (PCAM Syringe Pump Model P500; IVAC Medical Systems, NH) with patient‐controlled boluses of 20 µg each as a starting dose, regardless of parturient weight, and a 3 min lockout interval without basal infusion. The dose was increased by the attending anaesthesiologist every 15 to 20 min by 5 µg increments, on woman's request, to a maximum dose limit of 1500 µg/h. If any parturient had reached the maximum dose, a single bolus would have had 70 µg (0.93 µg/kg). Meperidine group (n = 45): Parturients in the control group received 75 mg of meperidine in 100 mL of normal saline over 30 min (approximately 1 mg/kg in a single bolus). In case of insufficient analgesia, another dose of 75 mg, followed by 50 mg when necessary, was administered, to a maximum dose of 200 mg of meperidine. | |
| Outcomes | The primary endpoint of the study was not explicitly stated but power analysis was performed for pain score. Continuous: ‐ overall satisfaction (NRS 1 to 4, within 24 h after delivery) ‐ pain intensity (VAS 0 to 100, at 0, 1 h and end of first stage of labour) ‐ umbilical cord pH (not specified) ‐ sedation score (observer, Ramsey sedation score, at 1 h and end oft 1st stage of labour) ‐ women: mean respiratory rate, mean SBP, mean HR (at 0, 1 h and end oft 1st stage of labour, respectively) Dichtomous: ‐ additional analgesia (epidural) ‐ rate of CS, rate of assisted birth (vacuum, forceps) ‐ feeding difficulties ‐ oxytocin use ‐ women: oxygen desaturation (< 95%), nausea + vomiting, pruritus ‐ newborns: Apgar score < 7 at 1 and 5 min, opioid‐induced loss of FHR, FHR reactive | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS pain scores, n = 88 in total) Concomitant medication: For inadequate analgesia, adverse effects due to opioids, or failure of the technique (VAS > 40), epidural analgesia was offered. The decision to cross‐over from systemic opioids to epidural analgesia was made by the parturient in corroboration with the anaesthesiologist and after an additional trial of increasing the dose of the analgesic and a repeat VAS score of > 40. A VAS of 40 was considered an indication for cross‐over to epidural analgesia. To avoid possible hypoxaemia, supplemental oxygen was administered to the parturients whenever SpO2 decreased to less than 95%. Funding: NA Intervention: Lockout time 3 min seems too long for adequate analgesia (borderline). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was based on computer‐generated codes […]” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] kept in sequentially numbered opaque envelopes until just before use.” Not specifically mentioned sealed envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “For blinding, PCIA remifentanil parturients were connected to a dummy IV saline bag, and IV meperidine parturients were connected to a dummy saline PCIA.”, “A senior anaesthesiologist, not involved in data recording, attended each parturient throughout labor.” Blinding was attempted to achieve by insertion of both an IV catheter and a PCA pump. However, we assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. The used method of blinding may only work for the outcome assessors (which was not explicitly mentioned in the published report for all outcomes). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “For blinding, PCIA remifentanil parturients were connected to a dummy IV saline bag, and IV meperidine parturients were connected to a dummy saline PCIA.”, “A senior anaesthesiologist, not involved in data recording, attended each parturient throughout labor.”, "Patient satisfaction [...] was assessed 24 h after delivery by an anaesthesiologist blinded to the mode of labor analgesia." The study did not address this issue for most of the relevant outcomes with exception of the assessment of woman's satisfaction. We do not know who was responsible for other outcome assessment and attending personnel and parturients may be able to uncover group allocation. The subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. Therefore, insufficient information exists to judge "yes" or "no". |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ Dropout rate: 0% ‐ Large amount (2%/22%) of outcome data (AE for women: SpO2) not reported and imbalanced between groups. No reasons declared. ‐ Rate of escape (epidural): 12%/38% (may influence data on AE, satisfaction and pain) ‐ Rate of cross‐over: NA ‐ Data‐analysis: Partial‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Single‐blinded. Randomisation after onset of labour (VAS pain score ≥ 30 mm). The purpose of this trial was to test the hypothesis whether labour can induce hyperthermia during epidural analgesia, and to assess the effects of analgesic doses of the IV opioid remifentanil or antipyretic doses of acetaminophen in the prevention of hyperthermia during labour. The study was conducted in Wolfson Medical Center affiliated to Tel‐Aviv University (period unknown). Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 213 Number receiving treatment: 201 (12 women which did not receive any analgesia were excluded) Number analysed: 192 (44/50/49/49), only women with at least 2 h of labour Inclusion criteria: Healthy women with singleton cephalic presentation at term, spontaneous active labour Exclusion criteria: Fever (oral temperature ≥ 38°C), signs of infection, ruptured membranes for more than 24 h, CS Baseline details: IV Remifentanil group (n = 44): Age (years, mean (SD)): 29 (7) Weight (kg, mean (SD)): 75 (11) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps (1), CS (4) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Number of birth (n)(1/2/3/≥ 4): 20/7/11/11 Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Epidural ropivacaine group (n = 50): Age (years, mean (SD)): 28 (5) Weight (kg, mean (SD)): 79 (14) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps (3), CS (5) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Number of birth (n) (1/2/3/≥ 4): 28/12/5/5 Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Epidural ropivacaine and IV Remifentanil group (n = 49): Age (years, mean (SD)): 27 (5) Weight (kg, mean (SD)): 78 (10) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps (3), CS (11) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Number of birth (n) (1/2/3/≥ 4): 25/9/4/7 Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Epidural ropivacaine and IV acetaminophen group (n = 49): Age (years, mean (SD)): 27 (4) Weight (kg, mean (SD)): 74 (14) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps (3), CS (3) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Number of birth (1/2/3/≥ 4): 29/13/4/3 Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 44): Parturients randomised to PCIA with remifentanil initially received a basal infusion of 0.025 µg/(kg*min) combined with 20 µg bolus doses with a lockout of 3 min. The dose was increased by 25% every 15 to 20 min as required. Acetaminophen was administered by continuous infusion 30 min after the initiation of epidural analgesia with the PCIA machine device at a rate of 0.47 mg/(kg*min) to a maximal dose of 2 g. Epidural ropivacaine group (n = 50): Epidural analgesia was administered after pre‐hydration with 500 mL Ringer’s lactate solution. A test dose of 3 mL lidocaine (2% without epinephrine) was followed by increments of 5 mL to 10 mL of 0.2% ropivacaine; maintenance was provided with the same solution via patient‐controlled epidural analgesia (PCEA) with a background infusion of 10 mg/h and a 10 mg patient‐activated bolus with 20 min lockout. The maximal dose of ropivacaine was 20 mL/h. The same ropivacaine dose was administered to women in all epidural groups. Epidural ropivacaine and IV Remifentanil group (n = 49): Epidural analgesia was administered after pre‐hydration with 500 mL Ringer’s lactate solution. A test dose of 3 mL lidocaine (2% without epinephrine) was followed by increments of 5 mL to 10 mL of 0.2% ropivacaine; maintenance was provided with the same solution via PCEA with a background infusion of 10 mg/h and a 10 mg patient‐activated bolus with 20 min lockout. The maximal dose of ropivacaine was 20 mL/h. Parturients initially received a basal infusion of 0.025 µg/(kg*min) combined with 20 µg bolus doses with a lockout of 3 min. The dose was increased by 25% every 15 to 20 min as required. Epidural ropivacaine and IV acetaminophen group (n = 49): Epidural analgesia was administered after pre‐hydration with 500 mL Ringer’s lactate solution. A test dose of 3 mL lidocaine (2% without epinephrine) was followed by increments of 5 mL to 10 mL of 0.2% ropivacaine; maintenance was provided with the same solution via PCEA with a background infusion of 10 mg/h and a 10 mg patient‐activated bolus with 20 min lockout. The maximal dose of ropivacaine was 20 mL/h. Acetaminophen was administered by continuous infusion 30 min after the initiation of epidural analgesia with the PCIA machine device at a rate of 0.47 mg/(kg*min) to a maximal dose of 2 g. | |
| Outcomes | The primary outcome was the incidence of hyperthermia. Continuous: ‐ pain intensity (VAS 0 to 100 mm, averaged over study period) Dichotomous: ‐ additional analgesia (conversion remifentanil (PCA) to epidural) ‐ rate of CS, rate of assisted birth (forceps) | |
| Notes | ‐ Power analysis not described Concomitant medication: Women with breakthrough pain (VAPS > 30 mm) were given rescue analgesia: women in the ropivacaine or ropivacaine and acetaminophen groups were given up to 4 additional boluses of 8 mL ropivacaine (0.2%), even if they had reached the maximum dose specified above; and women in either remifentanil group had their baseline infusion and bolus doses increased, as necessary, in 25% increments. If 4 increases proved insufficient, women assigned to IV remifentanil were switched to epidural analgesia. Oxytocin augmentation was applied when the rate of cervical dilatation was less than 1 cm/h. Funding: Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Mallinckrodt Anesthesiology Products, Inc (St. Louis, MO) donated the thermocouples. Exergen, Inc (Boston, MA) donated the infrared skin‐temperature thermometer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on computer‐generated codes […].” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was based on computer‐generated codes that were maintained in sequentially numbered opaque envelopes until just prior to use. The randomisation envelopes were opened and the designated treatment started when the visual analogue pain score (VAPS) reached 30 mm.” |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “The treatment regimen was blinded for the evaluator anaesthesiologist by using two patient‐controlled analgesia machine devices (PCIA and PCEA) for every patient. A “dummy” IV saline infusion (PCIA) was attached to parturients with patient‐controlled epidural analgesia (PCEA) and the other was a “dummy” epidural catheter attached superficially to the skin and connected to a PCEA syringe in the group with patient‐controlled IV analgesia (PCIA) with remifentanil.” The study seemed to be not blinded for parturients and personnel and we assume that at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The treatment regimen was blinded for the evaluator anaesthesiologist by using two patient‐controlled analgesia machine devices (PCIA and PCEA) for every patient. A “dummy” IV saline infusion (PCIA) was attached to parturients with patient‐controlled epidural analgesia (PCEA) and the other was a “dummy” epidural catheter attached superficially to the skin and connected to a PCEA syringe in the group with patient‐controlled IV analgesia (PCIA) with remifentanil.” An attempt was made and reported in the method section to blind the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ Dropout rate: 10% (overall) Reasons for missing outcome data were described (12 women delivered quickly without requirement for analgesia and nine women with labour < 2 h were excluded). Reasons may be unlikely to be related to true outcome. ‐ Rate of escape: NA ‐ Rate of cross‐over: 0% (remifentanil (PCA) to epidural) ‐ Data‐analysis: Per‐protocol (women without requirement for analgesia or with labour < 2 h were excluded) |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Multicentre randomised, controlled equivalence trial. No blinding. Randomisation before start of actual labour. The purpose of this trial was to determine women’s satisfaction with pain relief using PCA with remifentanil compared with epidural analgesia during labour. The study was conducted in three academic hospitals, 11 teaching hospitals, and one general hospital in the Netherlands from 30 May 2011 to 24 October 2012. Trial Identifier: NTR2551 | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 1414 (709/705) Number receiving treatment (of interest): 698 (402/296) Number analysed: 1358 (687/671) Inclusion criteria: Healthy women or those who have a mild systemic disease (ASA I or II), aged 18 or older, scheduled to deliver vaginally after 32 weeks Exclusion criteria: Contraindications for epidural analgesia or hypersensitivity to one of the drugs used Baseline details: Remifentanil group (n = 687): Age (years, mean (SD)): 31.5 (5.1) BMI (kg/m², median (IQR)): 23.7 (21.5‐26.9) ASA I/II (n/n): 491/196 Type of delivery (n): spontaneous (518), instrumental (63), CS (106) Week of gestation (median (IQR)): 37.8 (35.5 ‐ 39.2) Singleton, twin, multiple pregnancy (n): multiple pregnancy (24) Parity (n): 0 (323), ≥ 1 (364) Duration of labour: ‐ First stage of labour (min, mean (IQR)): 236 (128 ‐ 376) ‐ Second stage of labour (min, mean (IQR)): 20 (10 ‐ 46) Epidural group (n = 671): Age (years, mean (SD)): 31.7 (4.8) BMI (kg/m², median (IQR)): 23.8 (21.4 ‐ 27.6) ASA I/II (n/n): 461/210 Type of delivery (n): spontaneous (501), instrumental (70), CS (100) Week of gestation (median (IQR)): 37.1 (35.3 ‐ 39.0) Singleton, twin, multiple pregnancy (n): multiple pregnancy (30) Parity (n): 0 (329), ≥ 1 (342) Duration of labour: ‐ First stage of labour (min, mean (IQR)): 309 (181 ‐ 454) ‐ Second stage of labour (min, mean (IQR)): 24 (10 ‐ 53) | |
| Interventions | Remifentanil group (n = 687): The patient‐controlled device was programmed to deliver 30 µg remifentanil (solution 20 µg/mL) on request with a lockout time of 3 min. The dose could be increased to 40 µg in case of insufficient pain relief or decreased to 20 µg in case of excessive side effects. No background infusion was allowed. Women who were treated with patient‐controlled remifentanil were instructed on how to use the device and to maximise analgesia by pressing the device’s button in anticipation of the next contraction. Epidural group (n = 671): Women randomised to epidural analgesia received this when they requested pain relief, according to local protocol. | |
| Outcomes | The primary outcome measure was satisfaction with pain relief measured on a VAS ranging from 0 to 100 mm. Continuous: ‐ satisfaction with pain relief (VAS scale unclear, during active labour, after pain relief, at request, averaged), overall satisfaction (NRS 0 to 10, after birth) ‐ pain intensity (VAS scale unclear, during active labour, after pain relief, at request, averaged) Dichotomous: ‐ additional analgesia (escape analgesia) ‐ rate of CS, rate of assisted birth (instrumental) ‐ need for neonatal admission ‐ oxytocin use ‐ umbilical cord pH (artery), pH < 7.1 (twin 1) ‐ women: respiratory depression (< 8 breaths/min), oxygen desaturation (< 95%, < 92%), hypotension (< 90 mmHg), nausea, vomiting, pruritus, postpartum haemorrhage (≥ 1000 mL) ‐ newborns: Apgar score ≤ 7 at 5 min (twin 1) | |
| Notes | ‐ Power analysis performed (satisfaction with pain relief, n = 102 per group) Concomitant medication: If pain relief was inadequate, women could request epidural analgesia. They were advised to discontinue using the device during the second stage of labour to minimise the risk of neonatal side effects. If pain relief after epidural analgesia was judged inadequate by the woman, she could receive patient‐controlled remifentanil instead of epidural analgesia. No advice was given regarding continuing epidural analgesia during the second stage of labour. Funding: This study was funded by a grant from ZonMW (Dutch Organization for Health Care Research and Development) project No 80‐82310‐97‐11039. Intervention: Lockout time of 3 min seems too long for adequate analgesia (borderline). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was performed through a web based randomisation program. We randomised in fixed blocks of three, stratified for centre and parity.” |
| Allocation concealment (selection bias) | High risk | Quote (full‐text publication): “The allocation code appeared after a patient’s initials were entered into the randomisation program.” Quote (protocol: BMC Pregnancy and Childbirth 2012, 12: 63): "The consent form must be signed before performance of any study‐related activity. After obtaining informed consent women will be randomized and will be informed on the assigned method of pain relief before labour starts (as in usual care). They are only given pain relief during labour at their request or if a medical reason should arise." Allocation concealment was uncovered for participants and personnel before the start of treatment. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “Blinding was not possible because of the nature of the two interventions.” No blinding and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “Blinding was not possible because of the nature of the two interventions.” No blinding and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 3%/5% ‐ Large amount (5.5% to 41.5%) of data for maternal side effects and neonatal data were missing. No reasons reported. ‐ Randomisation occurred before onset of labour and only 65% and 52% received an analgesic intervention in the remifentanil and epidural group, respectively. Only 57% of women in the remifentanil group and only 42% in the epidural group received the allocated intervention. ‐ Rate of women with other pain relief (not escape, immediate use): 10%/15% ‐ Rate of cross‐over (insufficient pain relief): 13%/1% ‐ Rate of cross‐over (protocol violation): 9%/10% ‐ Data analysis: A partial ITT analysis with all women (including those without pain relief) was performed for rate of CS, assisted vaginal birth, postpartum haemorrhage, Apgar score < 7 at 5 min, and neonatal admission. A partial‐ITT with only women received pain relief (cross‐over participants as randomised) was performed for satisfaction, pain, cross‐over rate, and maternal side effects (SpO2, BP, respiratory depression, PONV, pruritus). ‐ Study design: equivalence study (per‐protocol analysis recommended) ‐ Multiple imputation was used to correct missing primary outcome data |
| Selective reporting (reporting bias) | High risk | A published protocol (BMC Pregnancy and Childbirth 2012, 12: 63) and a registered protocol (NTR2551) are available and there are several deviations. In the registered protocol cost‐effectiveness was defined as the primary outcome and pain relief, woman's satisfaction, pain scores, and maternal and neonatal side effects were defined as secondary outcomes. In the published report, however, the authors stated: “Our published protocol stated that both effectiveness and cost effectiveness were primary outcome measures. Satisfaction with pain relief was the primary outcome measure for effectiveness from the start of the study [which was not reported in the protocol]. We planned to perform a cost effectiveness analysis as well, taking into account the primary outcome for effectiveness. Because this was not made clear enough in the original protocol and registry it was changed in the last amended protocol. This last amended protocol was submitted before the last randomised woman delivered and as a result we did not have access to the data.” The first protocol (NTR2551) was prospectively registered: Study registration: 10/2010 First enrolment: 05/2011 The second protocol (BMC Pregnancy and Childbirth 2012, 12: 63) was retrospectively registered: Protocol received: 04/2012 First enrolment: 05/2011 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. No statement on blinding. Randomisation after onset of labour. The purpose of this trial was to assess if there is a difference in duration of labour, the mode of delivery, average VAS pain scores, maternal overall satisfaction with analgesia, side effects and neonatal outcomes in nulliparous women who received early labour analgesia with either epidural, PCIA with remifentanil or combined spinal–epidural (CSE) techniques. The study was conducted in TAIBA Hospital in Kuwait during the period from September 2009 to August 2011. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 1460 Number randomised: 1140 (380/380/380), 320 women were excluded due to cervical dilation of 4 cm or more Number receiving treatment: 1140 (380/380/380) Number analysed: 1140 (380/380/380) Inclusion criteria: Spontaneous labour (with at least two painful uterine contractions in 10 min and the cervix is at least 80 % effaced and up to 3 cm dilated) and requesting labour analgesia Exclusion criteria: Allergy to opioids, a history of the use of centrally‐acting drugs of any sort, chronic pain, psychiatric diseases records, participants younger than 18 years or older than 40 years, not willing to, or could not finish the whole study, alcohol‐ or opioid‐dependent women, non‐vertex presentation or scheduled induction of labour, diabetes mellitus and pregnancy‐induced hypertension, twin gestation and breech presentation, any contraindication to neuraxial or systemic opioid analgesia, cervical dilation of 4 cm or more, estimated fetal weight above 4000 g and abnormal FHR tracing on admission Baseline details: Remifentanil group (n = 380): Age (years, mean (SD)): 28.35 (5.54) Weight (kg, mean (SD)): 81 (13) ASA I/II (n/n): NA Type of delivery (n): spontaneous (250), instrumental (35), CS (95) Week of gestation (mean (SD)): 39.2 (1.1) Singleton, twin, multiple pregnancy: singleton Parity: NA Duration of labour: ‐ First stage of labour (h, mean (SD)): latent phase: 7.7 (0.8), active phase: 1.80 (0.6) ‐ Second stage of labour (h, mean (SD)): 0.95 (0.4) Epidural group (n = 380): Age (years, mean (SD)): 28.6 (5.49) Weight (kg, mean (SD)): 83 (15) ASA I/II (n/n): NA Type of delivery (n): spontaneous (249), instrumental (36), CS (95) Week of gestation (mean (SD)): 39.0 (1.3) Singleton, twin, multiple pregnancy: singleton Parity: NA Duration of labour: ‐ First stage of labour (h, mean (SD)): latent phase: 7.8 (0.9), active phase: 1.88 (0.7) ‐ Second stage of labour (h, mean (SD)): 1.0 (0.5) Spinal‐epidural group (n = 380): Age (years, mean (SD)): 28.8 (5.50) Weight (kg, mean (SD)): 82 (14) ASA I/II (n/n): NA Type of delivery (n): spontaneous (255), instrumental (38), CS (87) Week of gestation (mean (SD)): 39.1 (1.2) Singleton, twin, multiple pregnancy: singleton Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): latent phase: 6.6 (0.7), active phase: 1.55 (0.4) ‐ Second stage of labour (h, mean (SD)): 0.80 (0.3) | |
| Interventions | Remifentanil group (n = 380): The PCIA device was set to deliver 0.1 µg/kg of Ultiva (remifentanil hydrochloride, Glaxo Operations UK Ltd, Barnard Castle, Durham, UK), diluted with saline and given as a solution of 25 µg/mL as a bolus infused during a period of 1 min, with a lockout time of 1 min, into an IV catheter attached to a 1‐way line providing continuous infusion of saline at approximately 100 mL/h. During the study, the IV PCIA bolus was increased following a dose escalation scheme (0.1 – 0.2 – 0.3 – 0.5 – 0.7 – 0.9 µg/kg) after every second contraction until the parturient answered ‘no’ to the question whether she would like to get more efficient pain relief or until a maximum dose of 0.9 µg/kg was achieved. Epidural group (n = 380): Blocks were performed in the sitting position. The epidural space was located at the L3–L4 interspace using loss of resistance to air (an 18‐gauge Tuohy needle was used). A 3 mL epidural test dose of 2% lidocaine was given through the epidural catheter. After the test dose, an 8 mL dose of 0.125% levobupivacaine with 2 µg/mL fentanyl was administered through the epidural catheter. Then the catheter was connected to an electronic pump set to deliver a continuous infusion of 8 mL/h of 0.125% levobupivacaine and 2 µg/mL fentanyl. Further boluses of 5 mL to 10 mL of 0.125% levobupivacaine were given by the attending anaesthesiologist upon request. Spinal‐epidural group (n = 380): Blocks were performed in the sitting position. The epidural space was located at the L3–L4 interspace using loss of resistance to air (an 18‐gauge Tuohy needle was used). A 3 mL epidural test dose of 2% lidocaine was given through the epidural catheter. A needle‐through‐needle technique was performed with 2 mg levobupivacaine and 15 µg fentanyl (total volume of 2 mL) was injected intrathecally and the spinal needle was removed. Then the epidural catheter was inserted and connected to an electronic pump set to deliver the same previously mentioned mixture. | |
| Outcomes | The primary outcome was the rate of caesarean delivery. Continuous: ‐ overall satisfaction (VRS 1 to 4, 24 h after delivery) ‐ pain intensity (VAS 0 to 100, averaged) ‐ umbilical cord pH (artery, vein) ‐ women: mean respiratory rate, mean systolic and diastolic blood pressure, mean HR (averaged, respectively) Dichotomous: ‐ rate of CS, rate of assisted birth (instrumental) ‐ umbilical cord pH (artery), pH < 7.2 ‐ oxytocin use after analgesia ‐ women: nausea, vomiting, pruritus ‐ newborns: Apgar score < 7 at 1 and 5 min, non‐reassuring fetal status (indication for CS), need for naloxone | |
| Notes | ‐ Power analysis not performed Concomitant medication: NA Funding: NA Intervention: Maximum dose might be too high (might cause adverse effects). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “[…] the participants were randomized (in 3 blocks of 380 participants per block) through a computer‐generated, random‐number list […]” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The group assignment numbers were sealed in an envelope and kept by the study supervisor.” Not specifically mentioned sequentially numbered, opaque envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The study did not address this issue. However, we assume that blinding of outcome assessment did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. The study reported only significant positive results in the abstract. The non‐significant primary outcome was not there reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Single‐blinded. Randomisation after onset of labour. The purpose of this trial was to compare the efficacy and adverse maternal and neonatal effects of remifentanil given by bolus PCA versus continuous IV infusion for labour analgesia. The study was conducted at the Department of Anesthesiology of Ali Ebn‐e Abitaleb Hospital, Zahedan, Iran from January 2010 to March 2013. Trial Identifier: IRCT2012100811020N2 | |
| Participants | Participant flow: Number assessed for eligibility: NA Number randomised: 82 (41/41) Number receiving treatment: 82 (41/41) Number analysed: 82 (41/41) Inclusion criteria: Aged 18 to 35 years, gestational ages of 37 to 40 weeks Exclusion criteria: BMI > 30 or < 20 kg/m², pre‐eclampsia, using psychiatric drugs, opioid or alcohol consumption, occurrence of antenatal haemorrhage, fetal distress and requesting epidural analgesia Baseline details: Remifentanil infusion group (n = 41): Age (years, mean (SD)): 24.83 (4.67) BMI (kg/m², mean (SD)): 24.07 (2.21) ASA I/II (n/n): NA Type of delivery: NA Week of gestation (mean (SD)): 38.61 (1.16) Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): 165.3 (38.7) ‐ Second stage of labour (min, mean (SD)): 42.1 (12) Remifentanil bolus group (n = 41): Age (years, mean (SD)): 24.83 (4.67) BMI (kg/m², mean (SD)): 24.07 (2.21) ASA I/II (n/n): NA Type of delivery: NA Week of gestation (mean (SD)): 38.49 (1.23) Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): 153.2 (34.2) ‐ Second stage of labour (min, mean (SD)): 40 (10.3) | |
| Interventions | Remifentanil infusion group (n = 41): Remifentanil was incrementally infused with the starting dosage of 0.025 µg/(kg*min) and as required the infusion rate was increased to reach the doses of 0.05, 0.075 and 0.1 µg/(kg*min). Remifentanil bolus group (n = 41): Remifentanil was given by bolus PCA using an IVAC PCAM model P5000 pump, with the starting dosage of 0.25 µg/kg and as required increased to reach the dose of 0.4 µg/kg with a lockout time of 4 min. | |
| Outcomes | The primary endpoint of the study was reduction of labour pain. Continuous: ‐ pain intensity (VNRS 0 to 10, at baseline, averaged at stage 1 (every 15 min) and at stage 2 (every 15 min)) ‐ averaged oxytocin use ‐ sedation score (observer, MOAA/S 1 to 5, after remifentanil administration) Dichotomous: ‐ overall satisfaction (good to excellent, time point unclear) ‐ women: respiratory depression (< 8 breaths/min), oxygen desaturation (< 90 %), hypotension (< 90 mmHg), bradycardia (< 50 beats/min), nausea + vomiting ‐ newborns: Apgar score < 7 at 1 min, need for naloxone | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis not performed Concomitant medication: Remifentanil dosage was increased when VNRS was ≥ 7. Remifentanil was discontinued if any of the following criteria were detected: HR < 50 beats/min, SBP < 90 mmHg, SPO2 < 90% and respiratory rate (RR) < 8 breaths/min. Based on the standard protocols, oxytocin was infused in cases with inappropriate labour progress. Funding: Zahedan University of Medical Sciences Intervention: Lockout time 4 min (bolus group) seems too long for adequate analgesia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computerized random number generator was used for sequence generation. Simple randomisation with a 1:1 allocation ratio was used in this study.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “We used the consecutive opaque envelopes for the allocation concealment. The envelopes were opaque when held to the light and opened sequentially and only participant’s name and other details were written on the appropriate envelope.” |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “This randomized, single‐blind clinical trial…” The authors did not describe how they have blinded the parturients and personnel. We assume from the description of the intervention that blinding was not possible since a PCA pump was used only in the remifentanil PCA group. |
| Blinding of outcome assessment (detection bias) | High risk | No statement on blinding of outcome assessors. However, we assume from the description of the intervention that blinding was not possible since a PCA pump was used only in the remifentanil PCA group. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | The study protocol (IRCT2012100811020N2) is available and all pre‐specified primary and secondary outcomes have been reported in the final report. However, the outcomes remifentanil dose, maternal satisfaction, and maternal and neonatal side effects have not been pre‐specified in the protocol. The protocol was retrospectively registered: Protocol registration: 02/2013 First enrolment: 01/2012 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Double‐blinded. Randomisation after onset of labour. The purpose of this trial was to compare the efficacy of PCA remifentanil with IM pethidine for labour analgesia. There are no details where or when the study was conducted. The authors’ origin is China. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 69 Number randomised: 68 (34/34) Number receiving treatment: 68 (34/34) Number analysed: 68 (34/34) Inclusion criteria: Full term (36 to 40 weeks' gestation) parturients, ASA I and II, in the first stage of spontaneous labour, who requested parenteral opioid for labour analgesia Exclusion criteria: Complicated obstetric history (such as gestational diabetes, pregnancy induced hypertension or antepartum haemorrhage), multiple pregnancies, non‐cephalic presentation Baseline details: Remifentanil group (n = 34): Age (years, mean (SD)): 28 (5) Weight (kg, mean (SD)): 68.2 (11.1) ASA I/II (n/n): 24/10 Type of delivery: NA Week of gestation (median (IQR [range])): 39 (38 ‐ 40 [37 ‐ 41]) Singleton, twin, multiple pregnancy: singleton Parity (n): 0 (30), ≥ 1 (4) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Pethidine group (n = 34): Age (years, mean (SD)): 29 (5) Weight (kg, mean (SD)): 68.0 (8.9) ASA I/II (n/n): 27/7 Type of delivery: NA Week of gestation (median (IQR [range])): 39 (39 ‐ 40 [37 ‐ 41]) Singleton, twin, multiple pregnancy: singleton Parity (n): 0 (28), ≥1 (6) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 34): All parturients were provided with a PCA device (Omnifuse PCA, Smiths Medical, Kent, UK). The machine was loaded with a 50 mL syringe of study drug containing remifentanil 20 µg/mL. For each successful PCA demand, an IV bolus of 1.25 mL study drug (remifentanil 25 µg) was delivered to parturients weighing < 60 kg and 1.5 mL (remifentanil 30 µg) for those weighing ≥ 60 kg. The bolus was delivered at a rate of 20 mL/h. The effective lockout interval was 3.75 – 4.50 min with an hourly limit of 25 mL. A background infusion was not used. Parturients in the remifentanil group received an IM injection of 1.5 mL saline 0.9%. Parturients were then instructed to press the PCA demand button as soon as they felt the start of uterine contraction. Pethidine group (n = 34): All parturients were provided with a PCA device (Omnifuse PCA, Smiths Medical, Kent, UK). The machine was loaded with a 50 mL syringe of study drug containing 0.9% saline. For each successful PCA demand, an IV bolus of 1.25 mL study drug (saline) was delivered to parturients weighing < 60 kg and 1.5 mL (saline) for those weighing ≥ 60 kg. The bolus was delivered at a rate of 20 mL/h. The effective lockout interval was 3.75 to 4.50 min with an hourly limit of 25 mL. A background infusion was not used. Parturients were then instructed to press the PCA demand button as soon as they felt the start of uterine contraction. A single IM injection of pethidine 50 mg diluted to 1.5 mL with saline was given to parturients weighing < 60 kg and pethidine 75 mg in 1.5 mL to those weighing ≥ 60 kg. | |
| Outcomes | The primary endpoint of the study was VAS pain score during the entire duration of the study. Continuous: ‐ overall satisfaction (NRS 0 to 10, after delivery, median + IQR + range (symmetric)) ‐ pain intensity (VAS 0 to 100, at 0 h, every hour until 4 h, > 5 h, diagrammed) ‐ women: mean highest respiratory rate, oxygen saturation, SBP and pulse rate ‐ newborns: Apgar score at 1 and 5 min (median + IQR + range (symmetric)), mean FHR Dichotomous: ‐ additional analgesia (Entonox, pethidine IM) ‐ rate of CS, rate of assisted birth (ventouse) ‐ syntocinon use ‐ women: nausea, vomiting, pruritus (women reporting VAS ≥ 30 mm or requiring treatment, respectively), dizziness, sedation score = 1 = alert ‐ newborns: fetal distress with impaired CTG | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS pain, n = 34 per group) Concomitant medication: Rescue analgesia with IM pethidine and Entonox was offered to parturients with VAS > 50 mm or upon request. The time from the start of the study to the first request for rescue analgesia was recorded. Funding: NA Intervention: Bolus application time seems too slow (0.11 µg/s). Contact to the authors: We contacted Dr. Ng via e‐mail (23 June 2016) to inquire the number of women who reported 'pain intensity at 2 hours'. We received the missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “parturients were randomly assigned to receive either PCA remifentanil or intramuscular pethidine for labour analgesia according to a computer‐generated code […].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] code concealed in an opaque envelope.” Not specifically mentioned sequentially numbered, sealed envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “All parturients were provided with a PCA device (Omnifuse PCA, Smiths Medical, Kent, UK). The machine was loaded with a 50‐ml syringe of study drug containing either remifentanil 20 µg/ml or 0.9% saline.”, “Parturients in the remifentanil group received an intramuscular injection of 1.5 ml saline 0.9%.”, “Study drugs were prepared by an anaesthetist not otherwise involved in the study.”, “The parturient, the attending obstetricians, midwives and research staff responsible for data collection and outcome assessment were blinded to the group identity.” Blinding was attempted to achieve by application of both a PCA pump and an IM injection. However, we assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. The used method of blinding may only work for the outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “All parturients were provided with a PCA device (Omnifuse PCA, Smiths Medical, Kent, UK). The machine was loaded with a 50‐mL syringe of study drug containing either remifentanil 20 µg/mL or 0.9% saline.”, “The parturient, the attending obstetricians, midwives and research staff responsible for data collection and outcome assessment were blinded to the group identity.” An attempt was made and reported in the method section to blind the outcome assessor. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation ‐ Rate of escape (pethidine IM or Entonox): 50%/85% (may influence data on AE, satisfaction and pain) ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Double‐blinded. Randomisation after onset of labour. The purpose of this trial was to compare the maternal and neonatal effects of remifentanil given by PCA or continuous infusion for labour analgesia. The study was conducted in China from July 2008 and September 2009. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 60 Number randomised: 60 (30/30) Number receiving treatment: 60 (30/30) Number analysed: 53 (27/26) Inclusion criteria: ASA I to II, singleton term pregnancy, a cervical dilation of 1 to 3 cm, healthy fetus with a cephalic presentation, normal FHR pattern Exclusion criteria: Inability to understand PCA and VAS score, age less than 18 years or more than 45 years, morbid obesity, prior administration of regional or systemic analgesia, alcohol abuse, diabetes mellitus, pregnancy‐induced hypertension, pre‐eclampsia, severe disease of brain, heart, lung, liver or kidney Baseline details: Remifentanil bolus group (n = 27): Age (years, mean (SD)): NA Weight (kg, mean (SD)): NA ASA I/II (n/n): NA Type of delivery: NA Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Remifentanil infusion group (n = 26): Age (years, mean (SD)): NA Weight (kg, mean (SD)): NA ASA I/II (n/n): NA Type of delivery: NA Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity: NA Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil bolus group (n = 30): Two syringe pumps (Graseby 3300; Graseby Medical Ltd., Watford, UK) were connected, one set up for PCA and the other for continuous infusion. A nurse made up two labelled syringes, one with remifentanil and the other with 0.9% saline. Remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, Hubei, China) was diluted to 10 µg/mL with saline 0.9%. The initial PCA set‐up was with a bolus of 0.1 µg/kg given over 30 s with a 2‐min lockout interval. The parturients were advised to press the PCA button at the start of a uterine contraction. No additional training was given to parturients in respect of how to recognise the beginning of a contraction, and the decision of whether to press the PCA button depended on the parturient alone. The initial continuous infusion was with a dose of 0.05 µg/(kg*min). The PCA bolus dose was increased in increments of 0.1 µg/kg from 0.1 to 0.4 µg/kg. The continuous infusion pump was increased stepwise from 0.05 to 0.2 µg/(kg*min) with an increment of 0.05 µg/(kg*min). The two pumps were disconnected at the time of delivery, and the final dose of remifentanil was recorded. Remifentanil infusion group (n = 30): Two syringe pumps (Graseby 3300; Graseby Medical Ltd., Watford, UK) were connected, one set up for PCA and the other for continuous infusion. A nurse made up two labelled syringes, one with remifentanil and the other with 0.9% saline. The continuous infusion pump was increased stepwise from 0.05 to 0.2 µg/(kg*min) with an increment of 0.05 µg/(kg*min). The two pumps were disconnected at the time of delivery, and the final dose of remifentanil was recorded. | |
| Outcomes | The primary endpoint of the study was not explicitly stated but power analysis was performed for pain score. Continuous: ‐ overall satisfaction (NRS 0 to 10, 1 h after delivery, median + IQR + range (asymmetric)) ‐ pain intensity (VAS 0 to 10, 0, every 30 min until 120 min, delivery, diagrammed), median pain relief score (NRS 0 to 5, 0, every 30 min until 120 min, delivery, diagrammed) (median + IQR + range, respectively (symmetric)) ‐ sedation score (observer, Ramsey sedation score, at 0, 30, 60, 120 min and after delivery, median + IQR + range (symmetric)) Dichotomous: ‐ additional analgesia (epidural) ‐ need for neonatal resuscitation ‐ women: respiratory depression (< 8 breaths/min), oxygen desaturation (< 95%), hypotension, bradycardia, nausea, vomiting, pruritus ‐ newborns: non‐reassuring FHR/transient fetal bradycardia, need for naloxone, respiratory depression | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (pain score, n = 30 per group) Concomitant medication: During labour, if the parturient requested a higher dose of analgesia then both pumps were adjusted simultaneously. To avoid potential hypoxaemia, parturients received oxygen by nasal catheter with 2 L/min oxygen flow when there was an episode of desaturation, which was defined as SpO2 < 95%, and the dose of remifentanil was not increased. The bolus or the infusion was discontinued in the following circumstances: HR < 50 beats/min, respiratory rate < 8 breaths/min, SpO2 < 90%, mean arterial pressure > 25% decrease from baseline, FHR < 110 beats/min, or sedation score > 4. When these variables returned to a normal level, the remifentanil administration was restarted at a dose one step lower than that preceding the event. If any adverse reactions persisted, the trial was stopped, the parturient was excluded and the appropriate treatment was followed. For adverse reactions, unsatisfactory analgesia, or unwillingness to continue the trial, epidural analgesia was provided unless there was a contraindication. The decision to cross‐over to epidural analgesia was made by the parturient in collaboration with the anaesthesiologist. Adverse reactions were recorded throughout the labour. Funding: This study was supported by grant YKK08119 and YKK11058 from Medical Science and Technology Development Foundation, Nanjing Department of Health, Nanjing, Jiangsu, China, grant CSZ00838 from Wuxi Municipal Science and Technology Projects, Wuxi, Jiangsu, China and grant 08NMUM063 from the Science & Technology Development Foundation of Nanjing Medical University, Nanjing, Jiangsu, China. Intervention: Application time of remifentanil seems too long (0.003 µg/(kg*s). Contact to the authors: We contacted Dr. Shen via e‐mail (23 June 2016) to inquire the number of women who reported 'pain intensity at 2 hours'. We received the missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation code was generated by computer […].” |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] and then sealed in sequentially numbered, opaque envelopes before the beginning of the trial.”, “Only one investigator who selected the envelope knew the grouping.” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The anaesthesiologists who set up the analgesia and recorded the data, the nurses who prepared the medication, midwives, obstetricians and mothers were all blinded to the group allocation. Only one investigator who selected the envelope knew the grouping.”, “Two syringe pumps […] were connected to the cannula, one set up for PCA and the other for continuous infusion. A nurse made up two labelled syringes, one with remifentanil and the other with 0.9% saline. Remifentanil […] was diluted to 10 µg/ml with saline 0.9%. The investigator opened the randomisation envelope and then put the syringes into the appropriate pumps according to the group allocation. The syringe label was then covered with black paper.” Blinding for participants and personnel adequate. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The anaesthesiologists who set up the analgesia and recorded the data, the nurses who prepared the medication, midwives, obstetricians and mothers were all blinded to the group allocation. Only one investigator who selected the envelope knew the grouping.”, “Two syringe pumps […] were connected to the cannula, one set up for PCA and the other for continuous infusion. A nurse made up two labelled syringes, one with remifentanil and the other with 0.9% saline. Remifentanil … was diluted to 10 µg/ml with saline 0.9%. The investigator opened the randomisation envelope and then put the syringes into the appropriate pumps according to the group allocation. The syringe label was then covered with black paper.” Blinding for outcome assessment adequate. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 10%/13% Quote: “Two women in the PCA group and four subjects in the infusion group chose to cross over to epidural analgesia because of inadequate analgesia despite using the maximum dose, and there was one protocol violation in the PCA group. These mothers delivered their babies spontaneously, with Apgar scores > 9 at 1 min and 10 at 5 min, and no data were included from these subjects.” The protocol violation was not described. Reasons for missing outcome data likely to be related to true outcome. ‐ Rate of escape (epidural): 7%/13% ‐ Rate of cross‐over: NA ‐ Data‐analysis: Per‐protocol |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Not blinded. Randomisation after onset of labour. The purpose of this trial was to compare the analgesia efficacy and maternal satisfaction of remifentanil labour analgesia with standard treatment (epidural analgesia). The study was conducted in a Jerusalem tertiary hospital labour and delivery suite from February 2010 to August 2010. Trial Identifier: NCT00801047 | |
| Participants | Participant flow: Number assessed for eligibility: 144 Number randomised: 40 (20/20) Number receiving treatment: 39 (19/20) Number analysed: 39 (19/20) Inclusion criteria: Healthy, ASA I or II, age 18 to 40 years, body weight < 110 kg, gestational age > 36 completed weeks, with singleton pregnancy and vertex presentation Exclusion criteria: Contraindication to epidural analgesia, opioid administration in the previous 2 h, previous uterine surgery, pre‐eclampsia, inability to understand the consent form, nasal obstruction for any reason, medical indication for epidural analgesia (e.g. cardiac disease, suspected difficult airway), or non‐reassuring FHR tracing Baseline details: Remifentanil group (n = 19): Age (years, mean (SD)): 31 (5) Weight (kg, mean (SD)): 72 (10) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), vacuum delivery (2), CS (0) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (median (IQR) or n): 1 (1‐1), Nulliparous (4) Duration of labour: ‐ First stage of labour (min, mean (SD)): 329 (215) ‐ Second stage of labour (min, mean (SD)): 35 (41) Epidural group (n = 20): Age (years, mean (SD)): 30 (6) Weight (kg, mean (SD)): 73 (13) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), vacuum delivery (1), CS (4) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (median (IQR) or n): 1 (0‐1), Nulliparous (6) Duration of labour: ‐ First stage of labour (min, mean (SD)): 404 (259) ‐ Second stage of labour (min, mean (SD)): 69 (81) | |
| Interventions | Remifentanil group (n = 19): PCA: The bolus dose was titrated to effect from 20 µg up to a maximum of 60 µg as required; the lockout interval was initially set at 2 min, without a background infusion. The PCIA bolus/lockout interval was titrated to an endpoint of either woman's comfort or a maximal bolus dose of 60 µg/minimal lockout interval of 1 min by the recruiting anaesthesiologist at any time during labour. The PCIA pump tubing was “piggybacked” into the distal most port of the mainline IV fluid tubing. The mainline tubing contained an antireflux valve designed to prevent remifentanil inadvertently backing up in the IV line during administration. Epidural group (n = 20): A 17‐gauge Tuohy needle was inserted by the midline approach using loss of resistance to air at intervertebral space L3‐4 or L2‐3. An incremental initial loading dose of 15 mL of 0.1% bupivacaine with 50 µg fentanyl was administered followed by patient‐controlled epidural analgesia infusion of 0.1% bupivacaine with 2 µg/mL fentanyl: basal infusion of 5 mL/h, patient‐controlled bolus 10 mL, and lockout interval 20 min. Additional epidural bolus doses (either 0.1% bupivacaine 10 mL during the first stage of labour or 1% lidocaine 8 mL during the second stage of labour) were administered by the anaesthesiologist to treat breakthrough pain. If epidural analgesia failed, the epidural catheter was reinserted. | |
| Outcomes | The primary endpoint of the study was to demonstrate non‐inferiority of remifentanil labour analgesia compared with epidural analgesia in labouring women, measured by hourly assessment of NRS for pain throughout the duration of labour and maternal satisfaction. Continuous: ‐ satisfaction with pain relief (NRS 0 to 10, at 10 min and postpartum) ‐ pain intensity (NRS 0 to 10, at 0, 30 min, 1, 2, 3, 4, 5, 6 h) ‐ newborns: Apgar score at 1 and 5 min (median + IQR + range (symmetric), data for Apgar score at 5 min dichotomised: all newborn had an Apgar score of 10 at 5 min) Dichotomous: ‐ additional analgesia after 1 h (rescue), additional analgesia (cross‐over to the other treatment arm) ‐ rate of CS, rate for assisted birth (vacuum) ‐ need for neonatal resuscitation ‐ umbilical cord BE (artery), umbilical cord pH (artery) ‐ oxytocin use ‐ sedation score (observer, awake or easily arousable at 1 h) ‐ women: apnoea (> 20 s of zero respiratory rate), respiratory depression (< 8 breaths/min), oxygen desaturation (< 94%), nausea (at 1 h and postpartum), pruritus (at 1 h and postpartum) | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (NRS pain, n = 17 per group) Concomitant medication: Women were informed before study enrolment that conversion to the other treatment would be possible at any time during labour beginning 30 min after analgesia initiation with the study technique. All women received continuous supplementary oxygen (2 L/min) through an oral‐nasal cannula. Funding: Contact to the authors: We contacted Dr. Weiniger via e‐mail (16 March 2016) to inquire the number of women who reported 'pain intensity at 30 min, 1, and 2 hours'. We received the missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization and group allocation were determined: cards were divided into groups of 8 cards. Each group contained 4 allocation cards for remifentanil and 4 allocation cards for epidural analgesia (ratio 1:1), and 8 opaque envelopes numbered in groups from 1 – 8, 9 – 16, etc. were assigned to each group of cards. The cards were placed face down, manually shuffled, randomly selected, and then inserted into the numbered, opaque envelopes by a person not involved in the study. These envelopes were then sealed. Treatment assignment was revealed by breaking the seal of an envelope in consecutive order from number 1.” |
| Allocation concealment (selection bias) | Low risk | Quote: “…inserted into the numbered, opaque envelopes by a person not involved in the study. These envelopes were then sealed. Treatment assignment was revealed by breaking the seal of an envelope in consecutive order from number 1.” |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “This randomized nonblinded controlled noninferiority study […].” The study was not blinded due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “The investigator inquired whether opioid side effects (i.e., pruritus and/or nausea and vomiting) were present or absent.”, “After delivery, face‐to‐face follow‐up was performed on the first postpartum day for both mother and child by one of the investigators”, “Hence, the mathematician was not blinded to group allocation. However, she was blinded to our study hypothesis that remifentanil was noninferior to epidural analgesia and that remifentanil may have respiratory effects different from those seen with epidural analgesia.” The study did not adequately report on blinding of outcome assessors. Therefore, we assume that the study was not blinded due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ Dropout rate: 5%/0% One woman was excluded after enrolment but before analgesia due to obstetrician request. Reasons for missing outcome data unlikely to be related to true outcome. ‐ Rate of escape (other pain relief after 1 h): 0%/17% (may influence data on AE, satisfaction and pain) ‐ Rate of cross‐over: 16%/5% ‐ Data‐analysis: Partial‐ITT ‐ Study design: non‐inferiority study (per‐protocol analysis recommended) |
| Selective reporting (reporting bias) | High risk | A registered protocol (NCT00801047) is available and there are several deviations. The VAS pain score was defined as primary outcome and no other primary or secondary outcomes were defined in the protocol. In the published report, however, the authors defined maternal satisfaction as another primary endpoint, and incidence of apnoea was defined as secondary outcome. The primary outcome pain score was reported on a 11‐point NRS scale in the final report. The authors further reported on SpO2, sedation, pruritus, nausea, and adverse effects on the newborn. The protocol was prospectively registered: Protocol registration: 12/2008 First enrolment: 02/2010 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. No statement on blinding. Randomisation after onset of labour. The purpose of this trial was to compare the analgesic efficacy of parturient‐controlled IV remifentanil administration with epidural analgesia during first stage of labour with regard to maternal and early neonatal side effects. The study was conducted in Czech Republic from March 2010 to May 2010. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 81 Number randomised: 28 (13/15) Number receiving treatment: 28 (13/15) Number analysed: 24 (12/12) Inclusion criteria: No comorbidity (ASA I), singleton head‐down full term pregnancy, spontaneous or induced labour, request for labour analgesia Exclusion criteria: NA Baseline details: Remifentanil group (n = 12): Age (years, mean (SD)): 27.9 (2.95) Weight (kg, mean (SD)): 84 (16.75) ASA I/II (n/n): NA Type of delivery (n): spontaneous (2), induced (10) Week of gestation (weeks + days, mean (SD)): 39 + 3 (1 + 3) Singleton, twin, multiple pregnancy: singleton Parity (n): 1 (10), 2 (2), 3 (0) Duration of labour: ‐ First stage of labour (min, mean (SD)): 260.8 (65.5) ‐ Second stage of labour (min, mean (SD)): 11.25 (10.01) Epidural group (n = 12): Age (years, mean (SD)): 29.4 (2.05) Weight (kg, mean (SD)): 85.83 (16.75) ASA I/II (n/n): NA Type of delivery (n): spontaneous (4), induced (8) Week of gestation (weeks + days, mean (SD)): 40 + 0 (1 + 0) Singleton, twin, multiple pregnancy: singleton Parity (n): 1 (8), 2 (3), 3 (1) Duration of labour: ‐ First stage of labour (min, mean (SD)): 246.7 (76.3) ‐ Second stage of labour (min, mean (SD)): 13.75 (13.51) | |
| Interventions | Remifentanil group (n = 13): After the parturient's consent, her peripheral vein was cannulated and infusion of normal saline up to 2 mL/(kg*h) was started. The PCA device was connected to the same cannula. Remifentanil (Ultiva, Glaxo‐Smith‐Kline, Great Britain) was then administered via the PCA device (Technic 1, AMV Technics, Czech Republic) from a 50 mL syringe in a concentration of 20 µg/mL. On demand, the parturient received an IV bolus of 20 µg (1 mL) of remifentanil. Lockout interval was set to 3 min. The significant analgesic effect was set to a minimal VAS score decrease of 2, based on existing evidence. In case of inadequate analgesic VAS decrease (< 2), it was possible for the anaesthesiologist to increase the dose in 10 µg steps. Epidural group (n = 15): The parturients undergoing EA had an epidural catheter inserted into the epidural space by an anaesthesiologist. The dosage regimen of bupivacaine and sufentanil in the EA group was rigidly set to induction dose of 12.5 mg of bupivacaine and 5 µg of sufentanil in 10 mL of normal saline; top‐up boluses of half‐size dose were repeated in 60‐min to 90‐min intervals. The epidural catheter was extracted after the delivery, at least 2 hours before the parturient's transport to post‐natal care ward. | |
| Outcomes | The primary endpoint of the study was not defined. Continuous: ‐ overall satisfaction (mean % score + range, at 2 ‐ 24 h after delivery) ‐ pain intensity (VAS 0 to 10, 0, 30 min, until delivery (4 h), median + IQR (symmetric)) ‐ umbilical cord pH (not specified) ‐ newborns: Apgar score at 1, 5 and 10 min Dichotomous: ‐ rate of CS ‐ women: hypotension (> 25% decrease from baseline), bradycardia (< 50 beats/min), nausea + vomiting, drowsiness + dizziness ‐ newborns: pathological CTG (conversion to CS) | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis not performed Concomitant medication: If any signs of sedation (drowsiness), dizziness, muscle rigidity or bradypnoea (< 10 breaths/min) were observed, the lockout interval could be extended by 1 min. Funding: NA Contact to the authors: We contacted Dr. Stourac via e‐mail (29 June 2016) to inquire details on the method used for randomisation. We received the missing information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women who had asked for obstetric analgesia and met the inclusion criteria were offered the PCA using remifentanil (randomisation by the parturient)." On request the trial author offered the following information on the randomisation method: "Then she rolled the dice and based on the result she was assigned into remi (even) or epi (odd) groups". |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because of the method used for randomisation (throwing dice). |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The study did not address this issue. However, we assume that blinding of outcome assessment did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 8%/20% Quote: “The data of the parturients whose pregnancies were terminated by Caesarean Section were excluded from statistical evaluation. Four pregnancies were terminated by Caesarean Section (2 of them due to labour progress stagnancy, 2 because of a pathological cardiotocography (CTG) record (1 in each branch)).” Reasons for missing outcome data likely to be related to true outcome. ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: Per‐protocol |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Unclear risk | Early stopping. Quote: “28 parturients met the requirements to take part in this prospective randomised trial. This low count was caused by high parturient refusal to participate in the study (N=53) despite agreement with labour analgesia. We are aware of the decreased power with the lower sample size; nevertheless the 95% confidence interval for endpoint estimate in the groups showed clinically non‐significant results within its whole range which supports our findings from the statistical testing. Therefore we decide to terminate the enrolment.” |
| Methods | Randomised, controlled trial. No statement on blinding. No statement on time of randomisation. The purpose of this trial was to compare the analgesic effect of remifentanil given as PCA with IM meperidine during labour. There are no details where or when the study was conducted. The authors’ origin is the UK. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 36 Number randomised: 36 (18/18) Number receiving treatment: 36 (18/18) Number analysed: 36 (18/18) Inclusion criteria: Aged between 18 and 40 years, between 38 to 42 weeks of gestation in early labour Exclusion criteria: < 50 kg or >100 kg Baseline details: Remifentanil group (n = 18): Age (years, median (IQR)): 28 (22 ‐ 32) Weight (kg, median (IQR)): 66.5 (58 ‐ 78) ASA I/II (n/n): NA Type of delivery (n): spontaneous (11), ventouse (4), CS (3) Week of gestation (median (IQR)): 40.1 (39.25 ‐ 41) Singleton, twin, multiple pregnancy: NA Parity (n): 1 (13), 2 (0), 3 (3), 4 + (2) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Meperidine group (n = 18): Age (years, median (IQR)): 29 (25 ‐ 30) Weight (kg, median (IQR)): 64 (58 ‐ 76) ASA I/II (n/n): NA Type of delivery (n/n): spontaneous (16/17), ventouse (1/17), CS (0/17) Week of gestation (median (IQR)): 39.6 (39 ‐ 40) Singleton, twin, multiple pregnancy: NA Parity (n): 1 (13), 2 (0), 3 (2), 4 + (0) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 18): PCA: starting with a 5 µg bolus, an increasing dose of remifentanil was given at the beginning of each painful contraction until the contraction was pain‐free, and the next painful contraction was noted. Meperidine group (n = 18): IM Meperidine 100 mg | |
| Outcomes | The primary endpoint of the study was not explicitly defined but power analysis was performed for pain score. Continuous: ‐ midwives' and mother's assessment of overall effective analgesia (VRS 1 to 5, within 2 h after delivery, individual patient data) ‐ pain intensity (VAS 0 to 100, at 0 and 1 h), max. pain score over 2 h (VAS 0 to 100) (median + IQR, respectively (asymmetric)) ‐ sedation score (unclear assessor, VAS 0 to 100, at baseline, median + IQR) Dichotomous: ‐ additional analgesia (Entonox, epidural) ‐ rate of CS, rate of assisted birth (ventouse) ‐ syntocinon use ‐ women: respiratory depression (< 8 breaths/min), oxygen desaturation (< 94%), nausea + vomiting | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (pain score, n = 30 in total) ‐ Abstract: fewer number of women ‐ The exact intervention in the remifentanil group remains unclear (“20 µg bolus over 20 s, 3 min lockout and no background infusion”) Concomitant medication: If the assigned analgesia was inadequate for the woman at any time, an alternative was offered (epidural analgesia) and further study recordings were discontinued. All women had access to Entonox at all times. Remifentanil group: No antiemetic was given unless indicated clinically. Meperidine group: antiemetic was given (promethazine 25 mg or prochlorperazine 12.5 mg) Funding: NA Intervention: Lockout time of 3 min seems too long for adequate analgesia (borderline). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “[…] women were assigned randomly to one of two groups by sequentially numbered, sealed opaque envelopes prepared by an independent practitioner.” The method used for randomisation was not described. |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] sequentially numbered, sealed opaque envelopes…” |
| Blinding of participants and personnel (performance bias) | High risk | The study did not address this issue. However, we assume that blinding of parturients and personnel did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The study did not address this issue. However, we assume that blinding of outcome assessment did not occur due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ Dropout rate: 0% ‐ There were no withdrawals. However, there are some outcome data (additional analgesia, mode of delivery) incomplete (missing from notes) without reasons. ‐ Rate of escape (Entonox): 55%/81% (may influence data on AE, satisfaction and pain) ‐ Rate of escape (epidural): 39%/17% ‐ Rate of cross‐over: NA ‐ Data‐analysis: Partial‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Not blinded. Randomisation after onset of labour. The purpose of this trial was to compare the analgesic efficacy and side effects of remifentanil (PCA) with walking epidural analgesia during labour, with regard to maternal and early neonatal side effects. There are no details where or when the study was conducted. The authors’ origin is Norway. Trial Identifier: NCT00202722 | |
| Participants | Participant flow: Number assessed for eligibility: 62 Number randomised: 39 (19/20) Number receiving treatment: 39 (19/20) Number analysed: 37 (17/20) Inclusion criteria: Mixed parity, ASA I or II with normal singleton pregnancies, regular uterine contractions, cervical dilatation more than 2 cm, anticipated vaginal delivery, fetus without suspected abnormality, normal fetal cardiotocographic pattern, no complications during pregnancy, gestation age 37 to 40 weeks Exclusion criteria: Request for an epidural, had received pethidine less than 8 h before the study period, contraindications to remifentanil Baseline details: Remifentanil group (n = 17): Age (years, mean (range)): 26 (20 ‐ 33) Weight (kg, mean (SD)): 79 (13.7) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps/ventouse (2), CS (1) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): Primiparous (12), Multiparous (5) Duration of labour: ‐ First stage of labour (min, mean (SD)): 360 (185.9) ‐ Second stage of labour (min, mean (SD)): 51 (33.5) Epidural group (n = 20): Age (years, mean (range)): 27 (20 ‐ 37) Weight (kg, mean (SD)): 80 (8.7) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), forceps/ventouse (3), CS (3) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): Primiparous (11), Multiparous (9) Duration of labour: ‐ First stage of labour (min, mean (SD)): 359 (165.5) ‐ Second stage of labour (min, mean (SD)): 42 (32.2) | |
| Interventions | Remifentanil group (n = 17): Those randomised to the RA group received remifentanil hydrochloride (Ultiva, GlaxoSmithKline, Oslo, Norway) diluted in physiological saline to a concentration of 50 µg/mL, given as stepwise bolus doses with no background infusion. The starting bolus dose was 0.15 µg/kg, with increasing dose steps of 0.15 µg/kg and no maximum limit. The dose was allowed to be increased or decreased every 15 min according to the woman’s request for dose adjustment, VAS pain score and side effects. The lockout period was 2 min. Remifentanil was administered using a PCA pump (Baxter 6060 Multi‐Therapy infusion pump, Baxter Healthcare Corporation, Kista, Sweden) with a bolus infusion speed of 2 mL/min (100 µg/min). Calculation of PCA doses was based on estimated bodyweight using the following formula: body height in centimetres ‐ 100 = estimated weight (kg). Epidural group (n = 20): Parturient women randomised to the epidural group had an epidural catheter inserted in the midline at L2–3/L3–4 by the investigator. They received a continuous epidural infusion of ropivacaine 1 mg/mL and fentanyl 2 µg/mL (‘walking epidural’). An initial bolus dose of 10 mL, followed by a 5 mL top‐up after 5 min (total 15 mL) was given before the start of infusion (10 mL/h). Thereafter, the midwife was allowed to adjust the infusion dose (5 mL/h to 15 mL/h) and give rescue doses (5 mL) if needed. If pain relief was inadequate, the position of the epidural catheter was adjusted or a new catheter was placed if necessary. | |
| Outcomes | The primary endpoint of the study was not defined but power analysis was performed for VAS pain reduction. Continuous: ‐ pain intensity (VAS 0 to 100, at 0, 30 min, until 240 min) ‐ sense of control in labour (VRS 1 to 5, within 24 h after delivery, median + range) ‐ umbilical cord BE (artery, vein), umbilical cord pH (artery, vein) ‐ newborns: Apgar score at 1, 5 and 10 min (median + range) Dichotomous: ‐ satisfaction with pain relief (very satisfied, recommend analgesia, same analgesia next time) ‐ additional analgesia (rescue, epidural group: additional bolus), additional analgesia (conversion to EA) ‐ rate of CS, rate of assisted birth (ventouse, forceps) ‐ neonatal abnormalities ‐ oxytocin use ‐ sedation score (parturient, sedated or very sedated, discomfort by sedation, amnesia from labour) ‐ women: respiratory depression (< eight breaths/min), oxygen desaturation (< 92%, supplemental oxygen), nausea, vomiting, pruritus, drowsiness ‐ newborns: Apgar score < 8 at 1 min, pathological FHR changes | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS pain reduction, n = 26 per group) ‐ If delivery took place within 30 min of analgesia, the woman was excluded. Concomitant medication: Oxytocin, metoclopramide, ephedrine and IV fluids were available if needed. Supplemental oxygen (4 L/min) was given immediately via nasal cannula if SaO2 was less than 92%. Remifentanil analgesia was temporarily stopped if SaO2 less than 92% persisted or respiratory frequency was less than nine breaths/min, SBP was less than 90 mmHg or HR was less than 50 beats/min. When physiological variables returned to normal, pain therapy was continued on a dose 1 step lower. Funding: The work was funded by Sørlandet Hospital HF, Sørlandets Kompetansefond and Helse Sør‐Øst, Norway. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was based on a computer‐generated list [...].” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] codes were kept in sealed envelopes until recruitment..” Not specifically mentioned sequentially numbered, opaque envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “Our study has some limitations; it was not blinded, […].” The study was not blinded due to technical reasons and at least the subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “[…] for observer sedation score […] the anaesthesiologist and attending midwife scored independently.”, “After the study, two additional obstetricians, blinded to the analgesia method and neonatal outcome, independently evaluated FHR recordings.” The study was not blinded due to technical reasons and at least all other subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. However, the authors attempted to minimise bias by duplicate outcome assessment for the outcomes observer sedation score and FHR. |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 10%/0% Two women (10%) were excluded (per‐protocol analysis) from the remifentanil group due to cross‐over to the epidural group (reasons: suspicious FHR changes and inadequate analgesia). However, there are five women in the epidural group who received an extra bolus dose (rescue medication) due to unsatisfactory analgesia and were not excluded. ‐ There were missing outcome data (15% to 70%) regarding neonatal outcomes without reasons. ‐ Rate of escape (additional epidural bolus): NA/25% ‐ Rate of cross‐over: 10%/NA, ‐ Data‐analysis: Per‐protocol |
| Selective reporting (reporting bias) | High risk | A registered protocol (NCT00202722) is available. However, there are large discrepancies between the protocol and the published study report. The protocol deals only with a single group assignment (remifentanil PCA) and 41 enrolled women (39 within the published report), whereupon we contacted the study author. The author confirmed on request by mail that this protocol matches the published study. The protocol was retrospectively registered: Protocol registration: 09/2005 First enrolment: 01/2004 |
| Other bias | Unclear risk | Early stopping. Quote: “We had a technical problem with our infusion pumps after inclusion of 39 patients, and were not able to find new pumps with exactly the same technical specifications. Therefore, we closed the study at this point, leaving us with a number of participants close to the estimation from the power calculation.” |
| Methods | Randomised, controlled trial. Double‐blinded. Randomisation after onset of labour. The purpose of this trial was to compare the efficacy of analgesia and side effects of a remifentanil PCA and pethidine PCA, using a VAS scoring system, throughout the first and second stages of labour. There are no details where or when the study was conducted. The authors’ origin is the UK. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 17 Number randomised: 17 (9/8) Number receiving treatment: 17 (9/8) Number analysed: 17 (9/8) Inclusion criteria: Healthy women at 36 to 40 weeks’ gestation, ASA I or II, with no known obstetric complications, and requesting pethidine analgesia Exclusion criteria: Any contraindication to pethidine or remifentanil and a request for early epidural analgesia in labour Baseline details: Remifentanil group (n = 9): Age (years, mean (SD)): 28.6 (4.7) Weight (kg, mean (SD)): 81.1 (24.1) ASA I/II (n/n): NA Type of delivery (n): spontaneous (5), forceps/ventouse (2), CS (2) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): primiparous (5), multiparous (4) Duration of labour (min, mean (SD)): 362 (300) ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Pethidine group (n = 8): Age (years, mean (SD)): 28.9 (4.6) Weight (kg, mean (SD)): 67.2 (14.3) ASA I/II (n/n): NA Type of delivery (n): spontaneous (5), forceps/ventouse (2), CS (1) Week of gestation: NA Singleton, twin, multiple pregnancy: NA Parity (n): primiparous (4), multiparous (4) Duration of labour (min, mean (SD)): 348 (283) ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 9): PCA (IVAC PCAM model P5000 pump (ALARIS Medical Systems, UK)) with a remifentanil bolus of 0.5 µg/kg, a lockout period of 2 min and no hourly maximum limit. The remifentanil bolus was calculated using the maternal weight recorded at the antenatal booking clinic. Pethidine group (n = 8): PCA (IVAC PCAM model P5000 pump (ALARIS Medical Systems, UK)) with a pethidine bolus 10 mg, a lockout period of 5 min and a maximum limit of 100 mg per h. | |
| Outcomes | The primary endpoint of the study was not defined but power analysis was performed for VAS pain. Continuous: ‐ pain intensity (VAS 0 to 100, at 0 h, every hour until 5 h, diagrammed) ‐ women: mean nausea and pruritus (VAS 0 to 100, initial and mean hourly) ‐ newborns: Apgar score at 1 and 5 min (median + range) Dichotomous: ‐ additional analgesia (Entonox and epidural) ‐ rate of CS, rate of assisted birth (forceps/ventouse) ‐ need for neonatal admission ‐ syntocinon use before and after PCA ‐ women: respiratory depression (< 12 breaths/min), hypotension, bradycardia ‐ newborns: Apgar score < 7 at 5 min, need for naloxone | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (VAS pain, n = 17 in total) Concomitant medication: All the women in both groups were given metoclopramide 10 mg IV every 8 h. They received IV ondansetron 8 mg for persisting nausea and vomiting. The use of Entonox for any period during labour was noted. The PCA was stopped if epidural analgesia was requested but the VAS scores to that point were included in the analysis. Funding: NA Intervention: high bolus starting dose (0.5 mg/kg) Contact to the authors: We contacted Dr. Volikas via e‐mail (23 June 2016) to inquire the number of women who reported 'pain intensity at 2 hours'. We did not receive any answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The women were randomly allocated to one of two groups by selecting the next in a series of sealed envelopes prepared by our pharmacy department.” It is not specifically mentioned that the envelopes were opaque. Therefore, it might be possible that sequence generation is influenced by specifically selecting the preferred group. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…in a series of sealed envelopes prepared by our pharmacy department.” Not specifically mentioned sequentially numbered, opaque envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “The women and observer were blind to the treatment. This was achieved by having two investigators. One investigator selected the envelope and prepared the PCA pump with the appropriate drug. The pump was covered so that the other investigator, the observer, was unable to see which drug the woman was receiving.” We assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. The used method of blinding may only work for the outcome assessors. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “The women and observer were blind to the treatment. This was achieved by having two investigators. One investigator selected the envelope and prepared the PCA pump with the appropriate drug. The pump was covered so that the other investigator, the observer, was unable to see which drug the woman was receiving. The observer, who was an anaesthetist, stayed on the delivery suite at all times to record the visual analogue scale (VAS) scores and provide continuous monitoring of the individual under study. The observer did not have any other commitments on the labour ward.” An attempt was made to blind the outcome assessor. However, we assume, that the outcome assessor who stayed on the delivery suite all the time might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. |
| Incomplete outcome data (attrition bias) | Low risk | ‐ No missing outcome data after randomisation. ‐ Rate of escape (Entonox): 44%/63% (may influence data on AE, satisfaction and pain) ‐ Rate of escape (epidural): 11%/13% ‐ Rate of cross‐over: NA ‐ Data‐analysis: Full‐ITT |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, controlled trial. Double‐blinded. Randomisation after onset of labour. The purpose of this trial was to evaluate if IV PCA with remifentanil could provide as satisfactory pain relief for labour as epidural analgesia. There are no details where or when the study was conducted. The authors’ origin is Finland. Trial Identifier: NA | |
| Participants | Participant flow: Number assessed for eligibility: 52 Number randomised: 52 (27/25) Number receiving treatment: 51 (27/24) Number analysed: 45 (24/21) Inclusion criteria: Healthy term parturients with uncomplicated singleton pregnancies, normal cephalic presentation, no prior administration of opioid analgesia for at least 4 h or regional analgesia Exclusion criteria: NA Baseline details: Remifentanil group (n = 24): Age (years, median (IQR)): 27 (24 ‐ 32) Weight (kg, median (IQR)): 80 (73 ‐ 86) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), vacuum extraction (4), CS (1) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Parity (n): primiparous (17), multiparous (7) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA Epidural group (n = 21): Age (years, median (IQR)): 28 (27 ‐ 31) Weight (kg, median (IQR)): 79 (75 ‐ 87) ASA I/II (n/n): NA Type of delivery (n): spontaneous (NA), vacuum extraction (1), CS (1) Week of gestation: NA Singleton, twin, multiple pregnancy: singleton Parity (n): primiparous (16), multiparous (5) Duration of labour: ‐ First stage of labour (min, mean (SD)): NA ‐ Second stage of labour (min, mean (SD)): NA | |
| Interventions | Remifentanil group (n = 24): The PCA (Graseby 3300, Graseby Medical Ltd, Watford, UK) device was set to deliver 0.1 mg/kg of Ultiva (remifentanil hydrochloride, Glaxo Operations UK Ltd, Barnard Castle, Durham, UK), diluted with saline and given as a solution of 25 µg/mL as a bolus infused during a period of 1 min, with a lockout time of 1 min, into an IV catheter attached to a 1‐way line providing continuous infusion of saline at approximately 100 mL/h. In order to mimic a normal clinical situation, the subjective signs of anticipating the next uterine contraction were not specified, and no attempts were made to train the parturient in early recognition of the onset of contractions. The decision as to whether to start the PCA dose was left solely to the woman. Epidural saline was used for blinding during remifentanil administration. The first epidural bolus was given simultaneously with the first PCA bolus. During the study, the IV PCA bolus was increased following a dose escalation scheme (0.1 – 0.2 – 0.33 – 0.5 – 0.7 – 0.9 µg/kg) after every second contraction until the parturient answered ‘no’ to the question whether she would like to get more efficient pain relief or until a maximum dose of 0.9 mg/kg was achieved. If she answered ‘no’ and her pain score was higher than what she had estimated before as acceptable for herself, she was asked why she did not want to get more efficient pain relief. The study period was terminated if the parturient answered ‘yes’ when she was asked whether she wanted more efficient pain relief although the 0.9 µg/kg bolus had been reached, if the parturient wished to stop participation for any reason, or if the midwife noted that the parturient had reached full cervical opening. Epidural group (n = 21): An epidural catheter was sited at the L2/3 lumbar interspace. The parturient received epidurally 20 mL of levobupivacaine (0.625 mg/mL) and fentanyl 2 µg/mL in saline divided into 2 10 mL boluses given by the researcher manually with a 5‐min interval. IV saline was used for blinding in the epidural group. The first epidural bolus was given simultaneously with the first PCA bolus. After the study period was over, an epidural bolus was given when the parturient requested more efficient pain relief. | |
| Outcomes | The primary endpoint of the study was not defined but power analysis was performed for pain score. Continuous: ‐ pain relief score (NRS 0 to 4, every 10 min until 60 min), median pain relief score (NRS 0 to 4, averaged, median + IQR (symmetric)) ‐ pain intensity (NRS 0 to 10, every 10 min until 60 min, median + IQR (asymmetric)) ‐ umbilical cord pH (artery, median + IQR (asymmetric)) ‐ sedation score (VRS 0 to 3, average over 60 min every 10 min) ‐ women: mean blood pressure, mean HR ‐ newborns: Apgar score at 1 min (median + IQR (asymmetric)) Dichotomous: ‐ rate of CS, rate of assisted birth (vacuum) ‐ oxytocin use (started or increased during the study) ‐ women: oxygen desaturation (< 95%, supplemental oxygen), nausea (before and during the study) ‐ newborns: abnormal FHR (during and 30 min after the study period) | |
| Notes | ‐ Small trial sample size (< 200 participants) ‐ Power analysis performed (Pain score, n = 20 per group) ‐ The parturients were requested to use sunglasses in order to hide the miosis peculiar to systemic opioid treatment. ‐ The women used the study medicines during a 60‐min study period, which was thought to be long enough for a complete dose escalation for the IV PCA medication. Concomitant medication: SaO2 < 95% was treated with supplemental oxygen at a rate of 2 L/min via nasal cannula. Funding: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly allocated to two groups using sealed envelopes numbered according to a computer‐generated list that was stratified according to parity.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] using sealed envelopes numbered according to a computer‐generated list.” Not specifically mentioned opaque envelopes (SNOSE). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “[…] epidural saline was used for blinding during remifentanil administration. IV saline was used for blinding in the epidural group.”, “Midwives and nurses not involved in the study prepared the medications and placebo syringes. Both the parturient and all the personnel present during the study were blinded as to which medication was used during the study. The parturients were requested to use sunglasses in order to hide the miosis peculiar to systemic opioid treatment.” Blinding was attempted to achieve by insertion of both an epidural catheter and a PCA pump. However, we assume that participants and attending personnel might be able to uncover group allocation due to the different pharmacokinetics of the two interventions. The used method of blinding may only work for the outcome assessors (which was not mentioned in the published report). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “The FHR tracings were analysed afterwards by an obstetrician who was blinded to the method of analgesia and the outcome of the newborn.” The study did not address this issue for most of the relevant outcomes with exception of the assessment of FHR patterns. We do not know who was responsible for outcome assessment and attending personnel may be able to uncover group allocation. The subjective1 outcomes or outcome measurements are likely to be influenced by lack of blinding. Therefore, insufficient information exists to judge "yes" or "no". |
| Incomplete outcome data (attrition bias) | High risk | ‐ Dropout rate: 11%/16% It is unclear from the description why and how many women in each group were excluded: two vs. four (flow diagram: three vs. three) women discontinued the intervention due to insufficient pain relief (flow diagram: due to entering second stage of labour); one woman (epidural) did not receive allocated intervention (dural tap). Exclusion of parturients due to insufficient pain relief is likely to be related to true outcome. ‐ Rate of escape: NA ‐ Rate of cross‐over: NA ‐ Data‐analysis: Per‐protocol |
| Selective reporting (reporting bias) | Unclear risk | There is no reference to a trial registry and no published study protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
1Subjective outcomes: satisfaction with pain relief, adverse events for women, adverse events for the newborn, pain intensity, additional analgesia required, rate of caesarean delivery, rate of assisted vaginal birth, satisfaction with childbirth experience, sense of control in labour, effect (negative) on mother/baby interaction, breastfeeding initiation, need for neonatal resuscitation, long‐term childhood development, cost.
Objective outcomes: umbilical cord base excess (arterial and venous), umbilical cord pH (arterial and venous), vomiting, postpartum haemorrhage
Abbreviations:
AE: adverse events; ASA: American Society of Anesthesiologists; BE : base excess ; BMI: Body‐Mass‐Index; CS: caesarean section; CSE : combined spinal‐epidural analgesia ; CTG: cardiotocography; EA: epidural analgesia ; FHR: fetal heart rate; HR: heart rate;IM: intramuscular; IQR: interquartile range; ITT: intention‐to‐treat; IV: intravenous; MOAAS: Modified Observer's Assessment of Alertness/Sedation ; NA: not applicable; NACS: neurologic and adaptive capacity score ; NRS: numerical rating scale; PCA: patient‐controlled analgesia; PCIA: patient‐controlled IV analgesia ; PONV: p ostoperative nausea and vomiting ; RPCA: remifentanil patient‐controlled analgesia ; SBP: systolic blood pressure; SD: stand ard deviation; VAS: visual analogue scale; VNRS: verbal numerical rating scale ; VRS: verbal rating scale .
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong intervention (PCA versus PCA) | |
| Cross‐over trial | |
| No patient‐controlled anaesthesia | |
| No randomisation | |
| Cross‐over trial | |
| Cross‐over trial | |
| Cross‐over trial | |
| Cross‐over trial | |
| Cross‐over trial |
PCA: patient‐controlled analgesia
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, controlled trial, double‐blinded. The purpose of this trial is to assess whether the combination of dexmedetomidine (DMET) with remifentanil produces a synergistic effect that results in lower analgesic requirements. The study was conducted in Ain Shams University Hospital, Cairo, Egypt. There are no details when the study was conducted. Trial identifier: NA |
| Participants | Inclusion criteria: pregnant women, ASA I‐II, full term (37‐40 weeks), singleton fetus with cephalic presentation, first stage of spontaneous labour Exclusion criteria: known relevant drug allergy, significant respiratory depression from previous exposure to opioids, obstetric complications |
| Interventions | Remifentanil PCA + DMET versus remifentanil PCA + placebo |
| Outcomes | VAS pain scores, maternal and fetal complications, patients' satisfaction |
| Notes | This study is expected to be excluded due to wrong intervention (PCA versus PCA, same regimen). |
| Methods | Case report: "Labour analgesia challenge ‐ when epidural is not possible, what can we do?" The author's origin is Portugal. |
| Participants | Pregnant 31 year‐old female, 86 kg, 1.63 m, with severe scoliosis |
| Interventions | Remifentanil PCA |
| Outcomes | Pain score, occurrence of vomiting, neonatal outcome (Apgar score) |
| Notes | This case report is expected to be excluded (not RCT). |
| Methods | Randomised, controlled trial. The purpose of this study is to compare two different remifentanil PCA protocols (bolus and bolus + infusion) with intramuscular meperidine for labour analgesia. The study was conducted in Cukurova University Medical Faculty, Turkey. There are no details when the study was conducted. Trial identifier: NA |
| Participants | Inclusion criteria: pregnant women, ASA I‐II, mean gestational age of 270 ± 10 days, planned for vaginal delivery Exclusion criteria: gestational pathology, obesity (BMI > 40 kg/m2), high risk cases (pre‐eclampsia, severe asthma, insulin‐dependent diabetes, hepatorenal disease), history of opioid allergy, long‐term opioid use or chronic pain |
| Interventions | remifentanil PCA bolus versus remifentanil PCA bolus + infusion versus meperidine IM |
| Outcomes | pain‐comfort and sedation scores, remifentanil consumption, side effects, Apgar scores |
| Notes | NA |
| Methods | Insufficient information about study design. |
| Participants | Inclusion criteria: pregnant women, spontaneous labour, ASA I |
| Interventions | Continuous epidural infusion versus remifentanil PCA |
| Outcomes | The primary outcome is maternal and neonatal safety. The secondary outcome is efficacy evaluated through hourly pain and satisfaction scores. |
| Notes | There is no full text available (abstract only). |
| Methods | Parallel randomised trial investigating mosaprid in patients receiving remifentanil for labour analgesia. The study is not yet recruiting. The author's origin is Japan. Trial identifier: JPRN‐UMIN000021322 |
| Participants | Inclusion criteria: pregnant women with the wish for labour pain relief, age > 20 years. Exclusion criteria: patients who can not consent, < 22 weeks of pregnancy, history of a high degree of hypersensitivity reactions to other drugs, impaired consciousness. |
| Interventions | Mosaprid administration versus mosaprid 5 mg every 4 h during the first labour phase versus no treatment |
| Outcomes | The primary outcome is pain control and the presence or absence of maternal respiratory depression. Secondary outcomes are drug administration time, drug bolus administration number of times, the presence or absence of labour induction, patient satisfaction, final delivery mode, Apgar score, the presence or absence of umbilical cord arterial blood pH, birthweight, neonatal complications. |
| Notes | This study is expected to be excluded (wrong intervention). |
| Methods | Safety study with single‐group assignment investigating vital‐sign patient‐assisted intravenous analgesia with remifentanil. The study is not yet recruiting. The author's origin is Singapore. Trial identifier: NCT02733835 |
| Participants | Inclusion criteria: patients who choose to use parenteral opioid for pain relief, written informed consent, refuse labour epidural analgesia, contraindication to epidural analgesia, gestational age ≥ 36 weeks. Exclusion criteria: inability to understand instructions or to self‐administer PCA boluses, language differences, hypersensitivity to remifentanil or any component of its formulation or to other fentanyl analogue, severe respiratory disease, history of drug dependence or recreational drug abuse, unmanaged fetal bradycardia. |
| Interventions | patient‐assisted IV remifentanil |
| Outcomes | The primary outcome is maternal desaturation. The secondary outcomes are apnoea/hypopnoea and maternal bradycardia. |
| Notes | This study is expected to be excluded (no control intervention). |
| Methods | Randomised, controlled equivalence trial. Not blinded. The purpose of this study is to compare pain appreciation during labour between RPCA and EA. The study was conducted in the Academic Medical Center, Amsterdam, NL from September 2012 to May 2013. Trial identifier: NTR3687 |
| Participants | Inclusion criteria: age > 18 years, ASA I or II, low‐risk pregnant women Exclusion criteria: drug allergy: history of hypersensitivity to opioid or local anaesthetic, substances, labour before 32 weeks or after 42 weeks of gestation, initial maternal SpO2 of less than 95%, initial maternal temperature of 38°C or higher, prior administration of regional of opioid analgesia (during this delivery) |
| Interventions | Epidural anaesthesia versus remifentanil PCA |
| Outcomes | The primary endpoint of this study is pain appreciation, expressed by women's satisfaction with pain on a VAS scale, measured hourly from the onset of active labour. Secondary outcomes are overall satisfaction with pain during delivery judged 2 h and 6 weeks after delivery, pain scores during labour and maternal and neonatal side effects. |
| Notes | This publication belongs to the ongoing study abstract Logtenberg 2014. |
| Methods | Case report: "General anesthesia with target controlled infusion of propofol and remifentanil for planned caesarean section in a parturient with unrupted intracranial aneurysm" The authors' origin is Portugal. |
| Participants | NA. |
| Interventions | Remifentanil target‐controlled intravenous infusion and propofol for general anaesthesia. |
| Outcomes | Mean arterial blood pressure, neonatal outcomes, time of assisted mask ventilation. |
| Notes | This case report is expected to be excluded (not RCT). |
| Methods | Randomised parallel trial investigating the effects of different opioids on emergence from general anaesthesia for short gynaecological surgery. The study recruitment is completed. The author's origin is Turkey. Trial identifier: ISRCTN23443592 |
| Participants | Inclusion criteria: female patients, aged 18 ‐ 60, ASA I ‐ II, have undergone dilatation curettage and/or endometrial biopsy procedures. Exclusion criteria: psychiatric disorder, opioid drug abuse. |
| Interventions | IV remifentanil versus IV fentanyl for general anaesthesia |
| Outcomes | The primary outcomes are emergence time from general anaesthesia and discharge time from post‐anaesthesia care unit. Secondary outcomes are pain scores, additional analgesia requirement, patient's satisfaction and intra‐operative dreaming. |
| Notes | This study is expected to be excluded (wrong intervention). |
| Methods | Observational study investigating remifentanil and neuraxial anaesthesia for labour in multiparous women. The study is not yet recruiting. The author's origin is Slovenia. Trial identifier: NCT02963337 |
| Participants | Inclusion criteria: patient's request for pain relief, ASA I ‐ III, aged 18 ‐ 55 years, uncomplicated singleton pregnancy with cephalic presentation, gestation age > 37 weeks, regular uterine contractions, cervical dilation 2 cm to 6 cm, anticipated vaginal delivery, fetus without suspected abnormality and normal CTG. Exclusion criteria: contraindications for remifentanil use or for CSE. |
| Interventions | Remifentanil PCA versus CSE. |
| Outcomes | The primary outcome is pain relief. The secondary outcomes are duration of the first and second stage of labour and patient's satisfaction with pain relief. |
| Notes | This study is expected to be excluded (observational study). |
| Methods | Safety analysis of a prospective IRB‐approved study of healthy women receiving IV patient‐controlled boluses of remifentanil. The purpose was to detect respiratory depression in labouring women receiving remifentanil. The authors' origin is Israel. |
| Participants | Healthy women in labour |
| Interventions | Remifentanil PCA |
| Outcomes | Number of apneic episodes |
| Notes | This safety analysis is expected to be excluded (not RCT). |
ASA: American Society of Anesthesiologists; CSE: combined spinal‐epidural analgesia; CTG: cardiotocography; EA: epidural analgesia; IRB: institutional review board; IV: intravenous; NA: not applicable; PCA: patient‐controlled analgesia; RCT: randomised controlled trial; RPCA: remifentanil patient‐controlled analgesia; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A comparison of pethidine/meperidine intramuscularly and remifentanil patient‐controlled analgesia during labour in Westfriesgasthuis ‐ PCA remifentanil during labour |
| Methods | Randomised, controlled trial, not blinded. The purpose of this trial is to compare woman's satisfaction using pethidine/meperidine IM and PCA remifentanil for pain relief during labour and to assess the safety of remifentanil for parturient and fetus. There are no details where the study is conducted. The authors' origin is the Netherlands. Trial identifier: EUCTR2007‐000736‐10‐NL |
| Participants | Inclusion criteria: pregnant women in labour aged > 18 years in Westfriesgasthuis, ASA I, planned vaginal delivery, informed consent, term pregnancy (37 + 0 to 42 + 0 weeks), head‐down position, no congenital abnormalities Exclusion criteria: requesting or undergoing epidural analgesia, known allergy for remifentanil, other fetal positions than head down, parturient who feels she does not have right amount of time to consider enrolling in this study, fetal congenital abnormalities |
| Interventions | Pethidine/meperidine IM and remifentanil PCA |
| Outcomes | The primary endpoints of this study are woman's satisfaction measured by the women's view of birth labour satisfaction questionnaire, pain relief measured by VAS scores, parturient and fetal safety. The secondary objective is the question if there is a difference in the pain perception of primipara and multipara. |
| Starting date | Date of registration: 11/09/2008 Date of first enrolment: 05/11/2007 |
| Contact information | NA |
| Notes | We did not contact the authors due to lack of contact information. |
| Trial name or title | Epidural analgesia versus remifentanil PCA during labour ‐ OER‐study |
| Methods | Randomised, controlled trial, not blinded. The purpose of this trial is to compare remifentanil PCA with epidural anaesthesia among healthy nulligravida during labour. There are no details where the study is conducted. The authors' origin is the Netherlands. Trial identifier: EUCTR2007‐005424‐33‐NL |
| Participants | Inclusion criteria: nulligravida, without serious systemic disease, in partu, less than 6 cm dilatation, in labour, aged > 18 years Exclusion criteria: ASA > II, (pre)eclampsia, HELLP‐syndrome, serious diabetic gravidarum, infection, placenta praevia, psychiatric disorder |
| Interventions | Epidural analgesia versus remifentanil PCA |
| Outcomes | The primary endpoint of this study is woman's satisfaction. The secondary objective is the outcome of the infant (Apgar score, vacuum or forceps deliveries). |
| Starting date | Date of registration: 10/10/2007 Date of first enrolment: 06/02/2008 |
| Contact information | NA |
| Notes | We did not contact the authors due to lack of contact information. |
| Trial name or title | Remifentanil patient‐controlled analgesia (RPCA) versus epidural analgesia (EA) during labour. A randomised multicenter trial |
| Methods | Randomised, controlled trial. Not blinded. The purpose of this study is to compare pain appreciation during labour between RPCA and EA. The study was conducted in the Academic Medical Center, Amsterdam, the Netherlands from September 2012 to May 2013 (abstract). The study has been completed according to the authors. Trial identifier: NTR3687 |
| Participants | Inclusion criteria: age > 18 years, ASA I or II, low‐risk pregnant women Exclusion criteria: drug allergy: history of hypersensitivity to opioid or local anaesthetic, substances, labour before 32 weeks or after 42 weeks of gestation, initial maternal SpO2 of less than 95%, initial maternal temperature of 38°C or higher, prior administration of regional of opioid analgesia (during this delivery) |
| Interventions | Epidural anaesthesia versus remifentanil PCA |
| Outcomes | The primary endpoint of this study is pain appreciation, expressed by women's satisfaction with pain on a VAS scale, measured hourly from the onset of active labour. Secondary outcomes are overall satisfaction with pain during delivery judged 2 h and 6 weeks after delivery, pain scores during labour and maternal and neonatal side effects. |
| Starting date | Date of protocol registration: 05/11/2012 Date of first enrolment (protocol): 10/10/2012 |
| Contact information | Sabine Logtenberg: [email protected]/[email protected], B.W. Mol: [email protected] |
| Notes | A published abstract is available Logtenberg 2014. We contacted the authors but no additional data could be provided yet. |
| Trial name or title | Intravenous remifentanil for labour analgesia (IRELAN) |
| Methods | Randomised, controlled trial. Double‐blinded. The purpose of this trial is to assess the safety and efficacy of IV remifentanil with patient‐controlled technique for labour analgesia. The study was conducted in Nanjing Maternal and Child Health Care Hospital in Nanjing, Jiangsu, China from July 2008 to September 2009. The study has been completed. Trial identifier: NCT00710086 |
| Participants | Inclusion criteria: nulliparous women, > 18 years and < 45 years, spontaneous labour, analgesia request, epidural puncture contraindications, tendency to bleeding Exclusion criteria: allergy to opioids, a history of the use of centrally‐acting drugs of any sort, chronic pain and psychiatric diseases records, those who were not willing to or could not finish the whole study at any time, using or used in the past 14 days of the monoamine oxidase inhibitors, alcohol‐addictive or narcotic‐dependent women were excluded for their influence on the analgesic efficacy of the epidural analgesics, participants with a non‐vertex presentation or scheduled induction of labour, cervical dilation was 5 cm or greater before performing epidural puncture and catheterisation, diagnosed diabetes mellitus and pregnancy‐induced hypertension, twin gestation and breech presentation |
| Interventions | Hydromorphone intravenous (1 mg at the woman's request if they felt uterine contraction pain) versus remifentanil PCA (0.2 µg/kg, lockout time 2 min, continuous infusion rate 0.2 ‐ 0.8 µg/(kg*min) |
| Outcomes | The primary endpoint of this study was the maternal VAS rating of pain. Secondary outcome measures: rate and indications of caesarean delivery, rate of instrument‐assisted delivery, duration of analgesia, maternal satisfaction with analgesia, maternal oral temperature, use of oxytocin after analgesia, maximal oxytocin dose, breastfeeding success at six weeks after vaginal delivery, neonatal Apgar score at 1 and 5 min, umbilical cord gas analysis, neonatal sepsis evaluation, neonatal antibiotic treatment, incidence of maternal side effects |
| Starting date | Date of registration: 02/07/2008 Date of first enrolment: July 2008 |
| Contact information | XiaoFeng Shen, Nanjing Medical University |
| Notes | We contacted the authors for further information without any response. |
| Trial name or title | Patient‐controlled intravenous analgesia with remifentanil infusion for labour |
| Methods | Randomised, controlled trial. Double‐blinded. The purpose of this trial is to assess the effectiveness of two methods remifentanil administration in the form of either an infusion or PCA demand bolus. The study was conducted in Mount Sinai Hospital, Toronto, Ontario, Kanada from February 2012 to December 2014. It has been terminated due to difficult recruitment. Trial identifier: NCT01563939 |
| Participants | Inclusion criteria: age 18 ‐ 50 years, written informed consent, term pregnancy in labour with singleton fetus in cephalic presentation, women requesting systemic analgesia, women with contraindication for regional anaesthesia without fetal compromise (coagulopathy, thrombocytopenia, refusal, etc.) Exclusion criteria: refusal to sign written informed consent, inability to communicate in English, opioid dependence or addiction, women on methadone, allergy or hypersensitivity to remifentanil, fetal heart rate abnormalities, fetal congenital anomalies |
| Interventions | Continuous remifentanil IV infusion (stepwise increase in infusion rates and placebo demand bolus of normal saline) versus demand bolus of remifentanil (stepwise increase in bolus dose and placebo continuous infusion of normal saline) |
| Outcomes | The primary endpoint of the study was the pain score (VNRS from 0 to 10). Secondary outcome measures: maternal satisfaction, consumption of remifentanil, cross‐over to epidural, side effects, fetal and neonatal outcomes (non‐reassuring fetal heart rate as determined by obstetrician, neonatal weight, Apgar scores, naloxone administration, need for resuscitation, NICU admission) |
| Starting date | Date of registration: 23/03/2012 Date of first enrolment: February 2012 |
| Contact information | Mrinalini Balki, Mount Sinai Hospital, New York; Samuel Lunenfeld Research Institute, Mount Sinai Hospital |
| Notes | We contacted the authors for further information without any response. |
| Trial name or title | A randomised controlled trial of remifentanil intravenous patient‐controlled analgesia (PCA) versus intramuscular pethidine for pain relief in labour (RESPITE) |
| Methods | Randomised, controlled trial. Not blinded. The purpose of this study is to conduct a RCT to determine if remifentanil (PCA) reduces the proportion of women who subsequently require an epidural for pain relief in comparison to intermittent pethidine IM. The study was conducted in Birmingham, United Kingdom. The study has been completed according to the authors. Trial identifier: EUCTR2012‐005257‐22‐GB and NCT02179294 |
| Participants | Inclusion criteria: requesting systemic opioid analgesia, aged > 16 years, beyond 37 weeks' gestation, in established labour with vaginal birth intended, able to understand all information (written and oral) presented (using an interpreter if necessary), not participating in any other clinical trial of a medical product, live singleton pregnancy with cephalic presentation Exclusion criteria: contraindication to epidural analgesia, contraindication to intramuscular injection, history of drug sensitivity to pethidine or remifentanil, women taking long‐term opioid therapy including methadone, systemic pain relief opioid in the last 4 hours |
| Interventions | Pethidine 100 mg IM (up to 4 hourly in frequency, maximum of 4 doses, maximum dose being 400 mg in 24 h) versus remifentanil PCA (bolus 40 µg, lockout 2 min) |
| Outcomes | The primary endpoint is the proportion of women who receive epidural analgesia for pain relief in labour, in each group, after randomisation. Secondary outcome measures: effectiveness of pain relief, incidence of maternal side effects (excessive sedation score, oxygen saturation < 94% whilst breathing room air, nausea requiring anti‐emetic administration, requirement for supplemental oxygen, respiratory depression (< 8 breaths/min)), delivery mode, incidence of fetal distress requiring delivery, neonatal status at delivery (Apgar score at 5 min, incidence of fetal acidosis determined by umbilical cord gas analysis, requirement for neonatal resuscitation, incidence of admission to Special Care Baby Unit), rate of initiation of breast feeding within the first hour of birth, maternal satisfaction with childbirth experience determined by postpartum questionnaire prior to discharge from the delivery ward, resources used intra‐ and postoperatively, including PCA consumables, anaesthetist attendance, costs of staff training, service procurement, provision of care |
| Starting date | Date of registration: 21/06/2013 Date of first enrolment: 12/07/2013 |
| Contact information | Leanne Homer, [email protected] |
| Notes | We contacted the authors but no additional data could be provided yet. |
| Trial name or title | Remifentanil by patient‐controlled analgesia compared with epidural analgesia for pain relief in labour |
| Methods | Randomised, controlled trial. No statement on blinding. The purpose of this trial is to compare the use of PCA remifentanil to epidural analgesia in labour. There is no statement when or where the study is conducted. The authors' origin is Riyadh, Kingdom of Saudi Arabia. Trial identifier: NA |
| Participants | Inclusion criteria: healthy pregnant women, ASA I or II, no obstetric complications or contraindication to remifentanil or epidural analgesia Exclusion criteria: NA |
| Interventions | Epidural infusion (bupivacaine 1% plus 2 µg/mL fentanyl) versus remifentanil PCA (bolus of 0.4 µg/kg over 20 s, lockout 1 min) |
| Outcomes | There is no primary outcome defined. General outcomes: pain relief, safety of the mother and the fetus, side effects (bradycardia, hypotension, desaturation, nausea, fetal heart rate changes, Apgar scores at 1 min and 5 min, umbilical cord gas analysis and lactate levels), overall parturient's satisfaction, sedation scores |
| Starting date | Date of registration: NA Date of first enrolment: NA |
| Contact information | M.E. Rabie, H. H. Negmi, A. M. Moustafa, H. Al Oufi, Anesthesia Department, King Faisal Specialist Hospital & Research Centerm Riyadh, KSA; [email protected] |
| Notes | A published abstract is available Rabie 2006. We contacted the authors for further information without any response. |
ASA: American Society of Anesthesiologists; HELLP: Haemolysis, ElevatedLiver enzymes, and Low Platelet count; IM: intramuscular; IV: intravenous; NICU: neonatal intensive care unit; OER: Open Educational Resources; PCA: patient‐controlled analgesia; VAS: visual analogue scale; VNRS: verbal numerical rating scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 4 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 2.11 [0.72, 3.49] |
| Analysis 1.1  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 1 Satisfaction with pain relief. | ||||
| 2 Respiratory depression (< 8 breaths/min) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 2 Respiratory depression (< 8 breaths/min). | ||||
| 3 Oxygen desaturation (SpO2 < 95%) Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.00, 47.37] |
| Analysis 1.3  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 3 Oxygen desaturation (SpO2 < 95%). | ||||
| 4 Nausea (and vomiting) Show forest plot | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 0.99] |
| Analysis 1.4  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 4 Nausea (and vomiting). | ||||
| 5 Vomiting Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 5 Vomiting. | ||||
| 6 Pruritus Show forest plot | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.6  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 6 Pruritus. | ||||
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 7 Sedation (1 h). | ||||
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 8 Apgar score < 7 at 5 min. | ||||
| 9 Apgar score at 5 min Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 9 Apgar score at 5 min. | ||||
| 10 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.90] |
| Analysis 1.10  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 10 FHR/CTG abnormalities, non‐reassuring fetal status. | ||||
| 11 Pain intensity 'early' (1 h) Show forest plot | 3 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.69, ‐0.48] |
| Analysis 1.11  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 11 Pain intensity 'early' (1 h). | ||||
| 12 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 12 Pain intensity 'late' (2 h). | ||||
| 13 Additional analgesia required (escape analgesia) Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| Analysis 1.13  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 13 Additional analgesia required (escape analgesia). | ||||
| 14 Rate of caesarean delivery Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.34, 1.41] |
| Analysis 1.14  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 14 Rate of caesarean delivery. | ||||
| 15 Rate of assisted birth Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.09] |
| Analysis 1.15  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 15 Rate of assisted birth. | ||||
| 16 Augmented labour Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.29] |
| Analysis 1.16  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 16 Augmented labour. | ||||
| 17 Breastfeeding initiation (feeding difficulties) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.17  Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 17 Breastfeeding initiation (feeding difficulties). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 1 Satisfaction with pain relief. | ||||
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.49, 3.30] |
| Analysis 2.2  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 2 Oxygen desaturation (SpO2 < 95%). | ||||
| 3 Hypotension Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 3 Hypotension. | ||||
| 4 Bradycardia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 4 Bradycardia. | ||||
| 5 Nausea (and vomiting) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 5 Nausea (and vomiting). | ||||
| 6 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 6 Pruritus. | ||||
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 7 Sedation (1 h). | ||||
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 8 Apgar score < 7 at 5 min. | ||||
| 9 Apgar score at 5 min Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.9  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 9 Apgar score at 5 min. | ||||
| 10 Need for naloxone Show forest plot | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.47] |
| Analysis 2.10  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 10 Need for naloxone. | ||||
| 11 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 11 FHR/CTG abnormalities, non‐reassuring fetal status. | ||||
| 12 NACS at 15/30 min Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐0.65, 2.87] |
| Analysis 2.12  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 12 NACS at 15/30 min. | ||||
| 13 Pain intensity 'early' (30 min/1 h) Show forest plot | 3 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.01, ‐0.00] |
| Analysis 2.13  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 13 Pain intensity 'early' (30 min/1 h). | ||||
| 14 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.14  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 14 Pain intensity 'late' (2 h). | ||||
| 15 Additional analgesia required (escape analgesia) Show forest plot | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.28] |
| Analysis 2.15  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 15 Additional analgesia required (escape analgesia). | ||||
| 16 Rate of caesarean delivery Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.99, 7.82] |
| Analysis 2.16  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 16 Rate of caesarean delivery. | ||||
| 17 Rate of assisted birth Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.37] |
| Analysis 2.17  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 17 Rate of assisted birth. | ||||
| 18 Augmented labour Show forest plot | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.59, 3.15] |
| Analysis 2.18  Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 18 Augmented labour. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 7 | 2135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.04] |
| Analysis 3.1  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 1 Satisfaction with pain relief. | ||||
| 2 Apnoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 2 Apnoea. | ||||
| 3 Respiratory depression (< 9, < 8 breaths/min) Show forest plot | 3 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.23, 9.90] |
| Analysis 3.3  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 3 Respiratory depression (< 9, < 8 breaths/min). | ||||
| 4 Oxygen desaturation (SpO2 < 92%) Show forest plot | 3 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.66, 6.32] |
| Analysis 3.4  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 4 Oxygen desaturation (SpO2 < 92%). | ||||
| 5 Oxygen desaturation (SpO2 < 95%) Show forest plot | 3 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [2.32, 4.61] |
| Analysis 3.5  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 5 Oxygen desaturation (SpO2 < 95%). | ||||
| 6 Hypotension Show forest plot | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.22, 1.49] |
| Analysis 3.6  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 6 Hypotension. | ||||
| 7 Bradycardia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.7  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 7 Bradycardia. | ||||
| 8 Nausea Show forest plot | 8 | 1909 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.19, 1.86] |
| Analysis 3.8  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 8 Nausea. | ||||
| 9 Vomiting Show forest plot | 6 | 1840 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.25, 2.13] |
| Analysis 3.9  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 9 Vomiting. | ||||
| 10 Pruritus Show forest plot | 7 | 1852 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.18] |
| Analysis 3.10  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 10 Pruritus. | ||||
| 11 Sedation (1 h) Show forest plot | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.03, 1.39] |
| Analysis 3.11  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 11 Sedation (1 h). | ||||
| 12 Apgar score ≤ 7 (< 7) at 5 min Show forest plot | 5 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.65, 2.51] |
| Analysis 3.12  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 12 Apgar score ≤ 7 (< 7) at 5 min. | ||||
| 13 Apgar score at 5 min Show forest plot | 3 | 137 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.27, 0.39] |
| Analysis 3.13  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 13 Apgar score at 5 min. | ||||
| 14 Need for naloxone Show forest plot | 2 | 1170 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.85] |
| Analysis 3.14  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 14 Need for naloxone. | ||||
| 15 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 5 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.49, 4.92] |
| Analysis 3.15  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 15 FHR/CTG abnormalities, non‐reassuring fetal status. | ||||
| 16 Pain intensity 'early' (1 h) Show forest plot | 6 | 235 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [0.31, 0.84] |
| Analysis 3.16  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 16 Pain intensity 'early' (1 h). | ||||
| 17 Pain intensity 'late' (2 h) Show forest plot | 4 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [0.66, 2.26] |
| Analysis 3.17  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 17 Pain intensity 'late' (2 h). | ||||
| 18 Additional analgesia required Show forest plot | 6 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 8.10 [3.50, 18.75] |
| Analysis 3.18  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 18 Additional analgesia required. | ||||
| 19 Rate of caesarean delivery Show forest plot | 9 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.81, 1.21] |
| Analysis 3.19  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 19 Rate of caesarean delivery. | ||||
| 20 Rate of assisted birth Show forest plot | 8 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.26] |
| Analysis 3.20  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 20 Rate of assisted birth. | ||||
| 21 Augmented labour Show forest plot | 6 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| Analysis 3.21  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 21 Augmented labour. | ||||
| 22 Umbilical cord base excess (artery) Show forest plot | 3 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.65, 0.72] |
| Analysis 3.22  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 22 Umbilical cord base excess (artery). | ||||
| 23 Umbilical cord base excess (venous) Show forest plot | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.39, 2.30] |
| Analysis 3.23  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 23 Umbilical cord base excess (venous). | ||||
| 24 Umbilical cord pH (artery) Show forest plot | 5 | 1245 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| Analysis 3.24  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 24 Umbilical cord pH (artery). | ||||
| 25 Umbilical cord pH (venous) Show forest plot | 4 | 1299 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| Analysis 3.25  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 25 Umbilical cord pH (venous). | ||||
| 26 Neonatal resuscitation Show forest plot | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.04, 25.09] |
| Analysis 3.26  Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 26 Neonatal resuscitation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Respiratory depression (< 8 breaths/min) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.1  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 1 Respiratory depression (< 8 breaths/min). | ||||
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 2 Oxygen desaturation (SpO2 < 95%). | ||||
| 3 Hypotension Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.3  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 3 Hypotension. | ||||
| 4 Bradycardia Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.4  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 4 Bradycardia. | ||||
| 5 Nausea (and vomiting) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.28, 2.54] |
| Analysis 4.5  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 5 Nausea (and vomiting). | ||||
| 6 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 6 Pruritus. | ||||
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.7  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 7 Sedation (1 h). | ||||
| 8 Need for naloxone Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.8  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 8 Need for naloxone. | ||||
| 9 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.9  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 9 FHR/CTG abnormalities, non‐reassuring fetal status. | ||||
| 10 Pain intensity 'early' (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.10  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 10 Pain intensity 'early' (1 h). | ||||
| 11 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.11  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 11 Pain intensity 'late' (2 h). | ||||
| 12 Additional analgesia required (escape analgesia) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.12  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 12 Additional analgesia required (escape analgesia). | ||||
| 13 Neonatal resuscitation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.13  Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 13 Neonatal resuscitation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 1 Satisfaction with pain relief. | ||||
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 2 Oxygen desaturation (SpO2 < 95%). | ||||
| 3 Hypotension Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 3 Hypotension. | ||||
| 4 Bradycardia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 4 Bradycardia. | ||||
| 5 Nausea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 5 Nausea. | ||||
| 6 Vomiting Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 6 Vomiting. | ||||
| 7 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 7 Pruritus. | ||||
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.8  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 8 Apgar score < 7 at 5 min. | ||||
| 9 Need for naloxone Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.9  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 9 Need for naloxone. | ||||
| 10 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.10  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 10 FHR/CTG abnormalities, non‐reassuring fetal status. | ||||
| 11 Additional analgesia required (escape analgesia) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.11  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 11 Additional analgesia required (escape analgesia). | ||||
| 12 Rate of caesarean delivery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.12  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 12 Rate of caesarean delivery. | ||||
| 13 Augmented labour Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.13  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 13 Augmented labour. | ||||
| 14 Umbilical cord base excess (artery) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.14  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 14 Umbilical cord base excess (artery). | ||||
| 15 Umbilical cord base excess (venous) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.15  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 15 Umbilical cord base excess (venous). | ||||
| 16 Umbilical cord pH (artery) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.16  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 16 Umbilical cord pH (artery). | ||||
| 17 Umbilical cord pH (venous) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.17  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 17 Umbilical cord pH (venous). | ||||
| 18 Neonatal resuscitation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.18  Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 18 Neonatal resuscitation. | ||||

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Abbreviations:
IV: intravenous; IM: intramuscular; PCA: patient‐controlled analgesia; CTG: cardiotocography; FHR: fetal heart rate; NACS: neonatal neurologic and adaptive capacity score; BE: base excess.
Direction of estimated effects (results of meta‐analyses) for all primary and secondary outcomes with two or more studies and level of evidence (GRADE) for all GRADE‐relevant, pre‐defined outcomes:
The direction of the estimated effects were labelled as green (favours remifentanil (PCA)), red (favours control), yellow (neither favour of remifentanil (PCA) nor control), (1) (only one RCT, no meta‐analysis performed), ∅ (no RCTs available).
The GRADE levels of the evidence were expressed as VERY LOW, LOW, MODERATE, and HIGH for all GRADE‐relevant outcomes (dark grey, bold). For details on GRADE levels of evidence see the summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5).
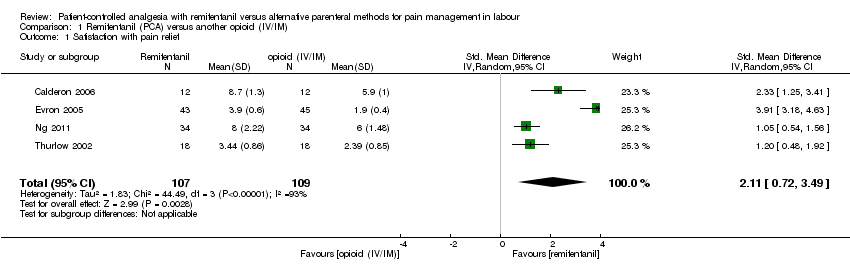
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 1 Satisfaction with pain relief.

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 2 Respiratory depression (< 8 breaths/min).
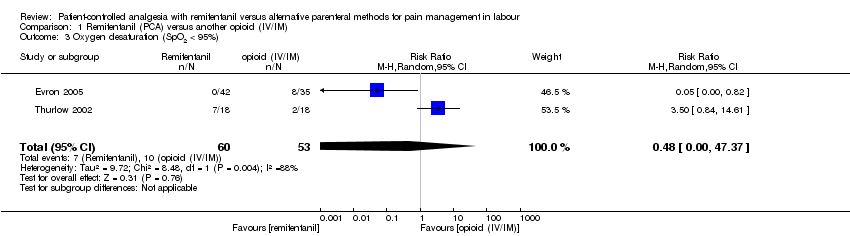
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 3 Oxygen desaturation (SpO2 < 95%).
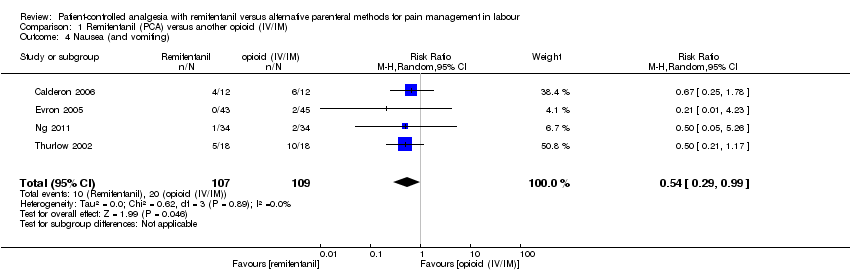
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 4 Nausea (and vomiting).

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 5 Vomiting.

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 6 Pruritus.

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 7 Sedation (1 h).

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 8 Apgar score < 7 at 5 min.

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 9 Apgar score at 5 min.
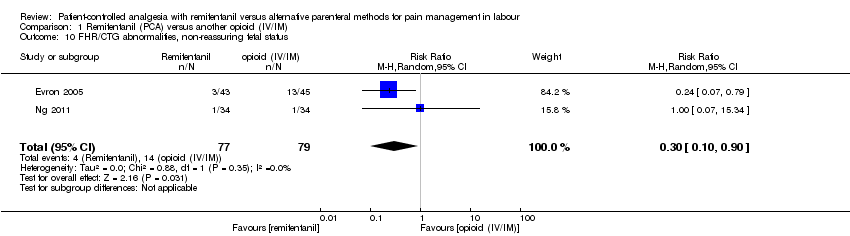
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 10 FHR/CTG abnormalities, non‐reassuring fetal status.
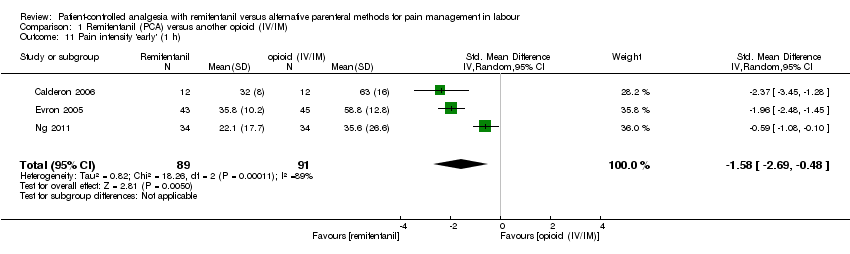
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 11 Pain intensity 'early' (1 h).

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 12 Pain intensity 'late' (2 h).

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 13 Additional analgesia required (escape analgesia).

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 14 Rate of caesarean delivery.
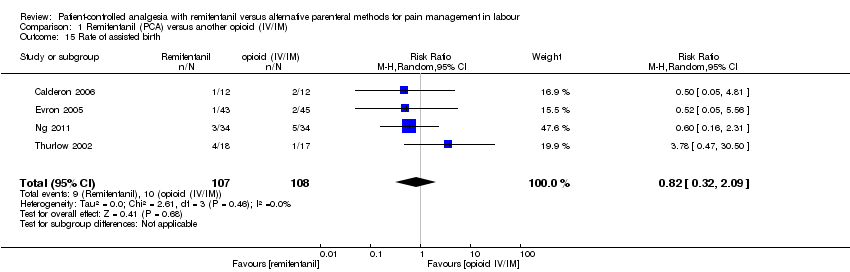
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 15 Rate of assisted birth.
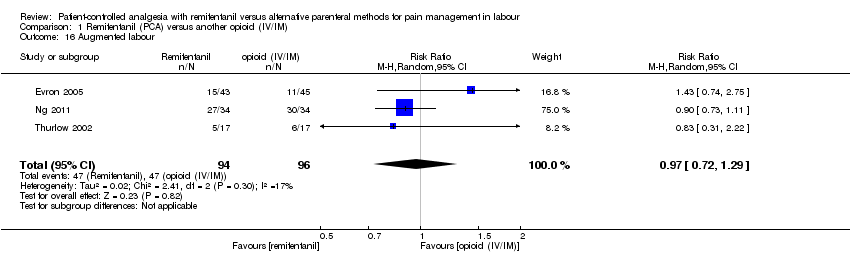
Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 16 Augmented labour.

Comparison 1 Remifentanil (PCA) versus another opioid (IV/IM), Outcome 17 Breastfeeding initiation (feeding difficulties).

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 1 Satisfaction with pain relief.
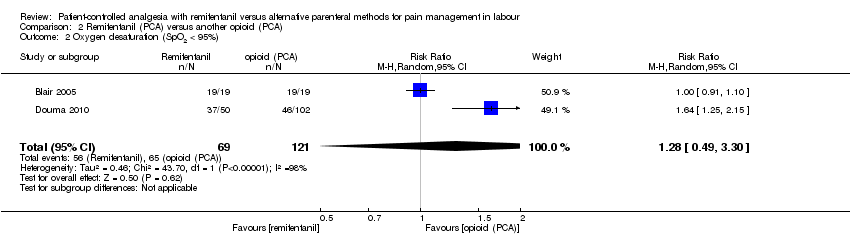
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 2 Oxygen desaturation (SpO2 < 95%).
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 3 Hypotension.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 4 Bradycardia.
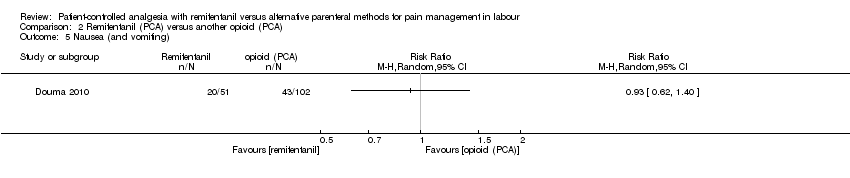
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 5 Nausea (and vomiting).

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 6 Pruritus.
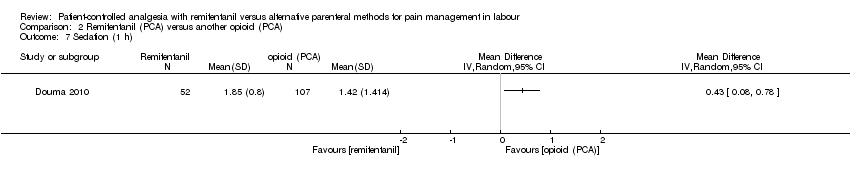
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 7 Sedation (1 h).

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 8 Apgar score < 7 at 5 min.
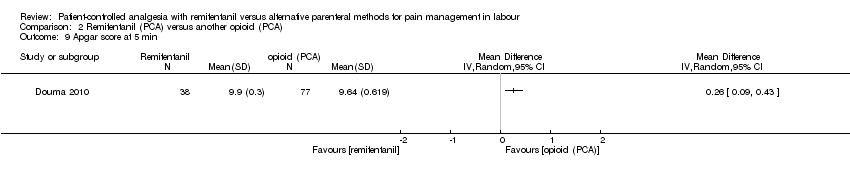
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 9 Apgar score at 5 min.
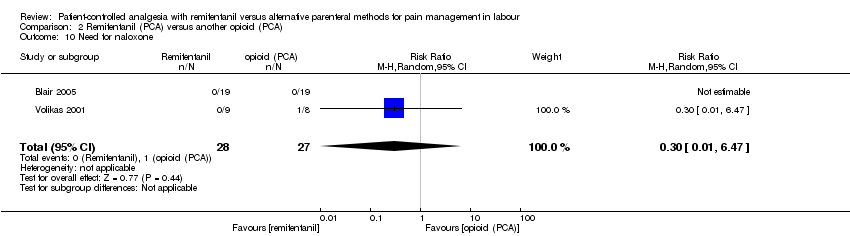
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 10 Need for naloxone.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 11 FHR/CTG abnormalities, non‐reassuring fetal status.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 12 NACS at 15/30 min.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 13 Pain intensity 'early' (30 min/1 h).
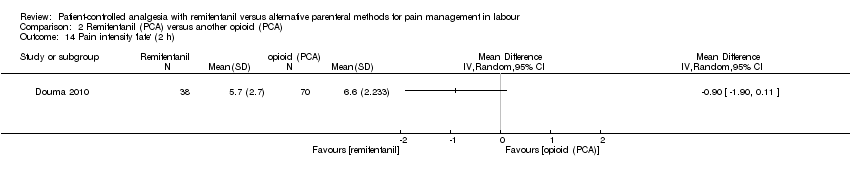
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 14 Pain intensity 'late' (2 h).
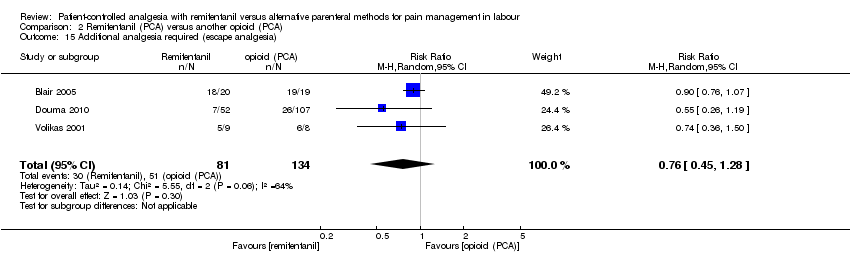
Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 15 Additional analgesia required (escape analgesia).

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 16 Rate of caesarean delivery.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 17 Rate of assisted birth.

Comparison 2 Remifentanil (PCA) versus another opioid (PCA), Outcome 18 Augmented labour.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 1 Satisfaction with pain relief.
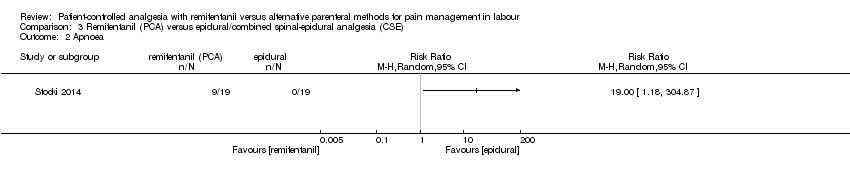
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 2 Apnoea.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 3 Respiratory depression (< 9, < 8 breaths/min).
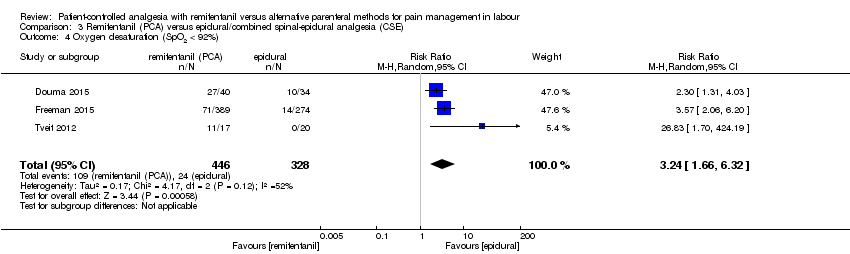
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 4 Oxygen desaturation (SpO2 < 92%).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 5 Oxygen desaturation (SpO2 < 95%).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 6 Hypotension.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 7 Bradycardia.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 8 Nausea.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 9 Vomiting.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 10 Pruritus.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 11 Sedation (1 h).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 12 Apgar score ≤ 7 (< 7) at 5 min.
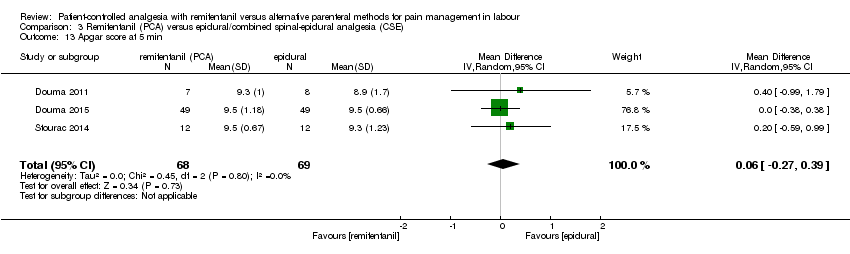
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 13 Apgar score at 5 min.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 14 Need for naloxone.
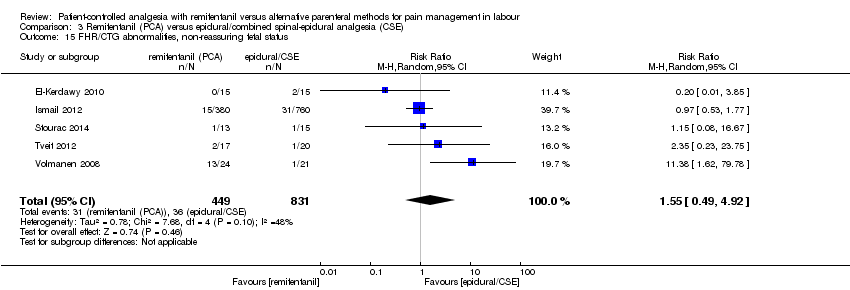
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 15 FHR/CTG abnormalities, non‐reassuring fetal status.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 16 Pain intensity 'early' (1 h).
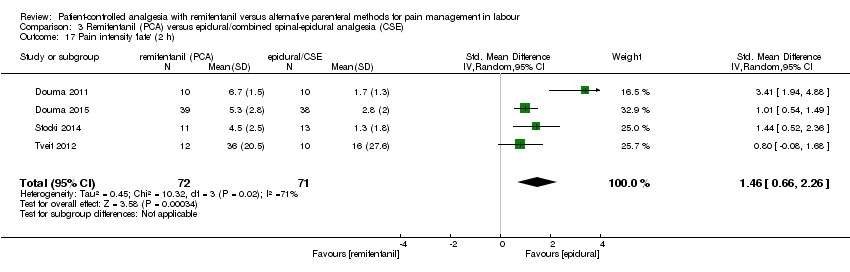
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 17 Pain intensity 'late' (2 h).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 18 Additional analgesia required.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 19 Rate of caesarean delivery.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 20 Rate of assisted birth.

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 21 Augmented labour.
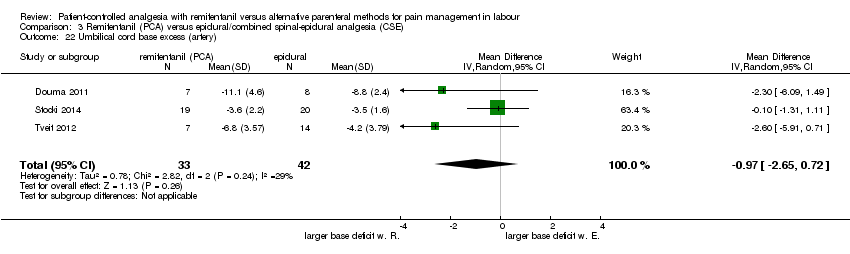
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 22 Umbilical cord base excess (artery).
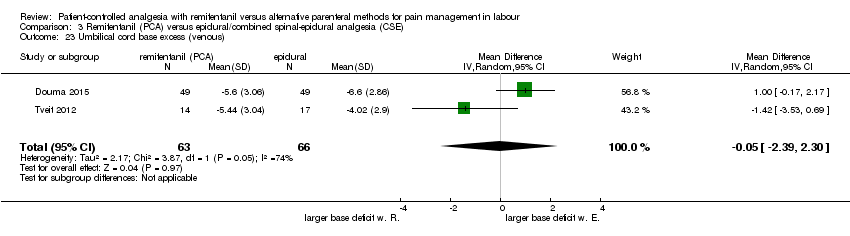
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 23 Umbilical cord base excess (venous).
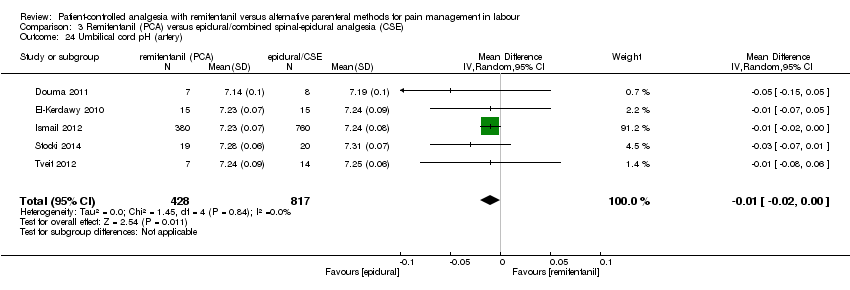
Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 24 Umbilical cord pH (artery).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 25 Umbilical cord pH (venous).

Comparison 3 Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE), Outcome 26 Neonatal resuscitation.

Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 1 Respiratory depression (< 8 breaths/min).
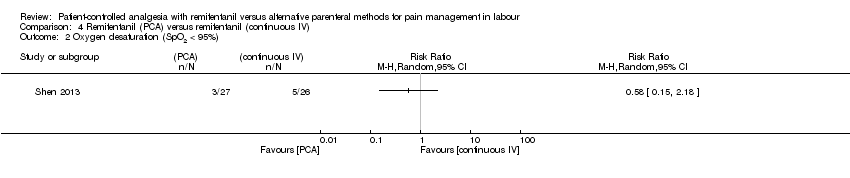
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 2 Oxygen desaturation (SpO2 < 95%).
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 3 Hypotension.
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 4 Bradycardia.
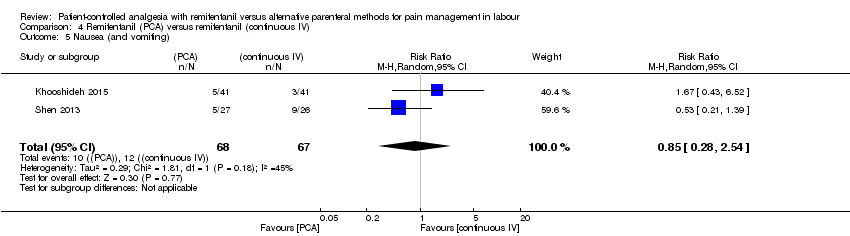
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 5 Nausea (and vomiting).
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 6 Pruritus.
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 7 Sedation (1 h).
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 8 Need for naloxone.

Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 9 FHR/CTG abnormalities, non‐reassuring fetal status.
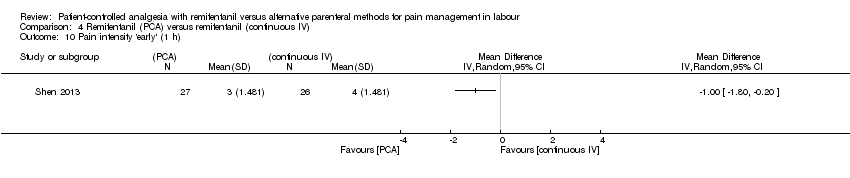
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 10 Pain intensity 'early' (1 h).
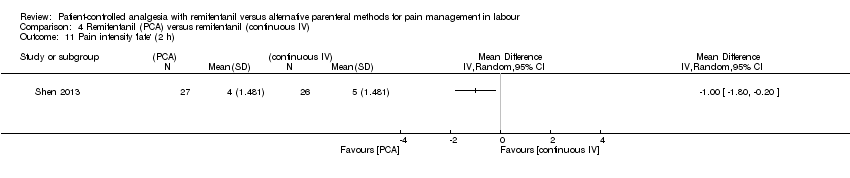
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 11 Pain intensity 'late' (2 h).
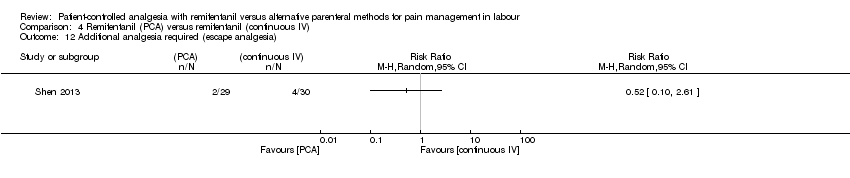
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 12 Additional analgesia required (escape analgesia).
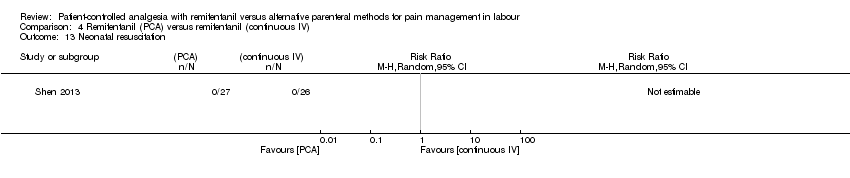
Comparison 4 Remifentanil (PCA) versus remifentanil (continuous IV), Outcome 13 Neonatal resuscitation.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 1 Satisfaction with pain relief.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 2 Oxygen desaturation (SpO2 < 95%).
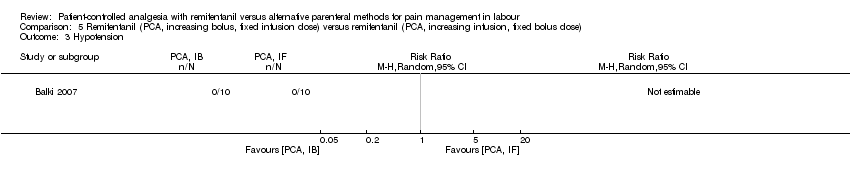
Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 3 Hypotension.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 4 Bradycardia.
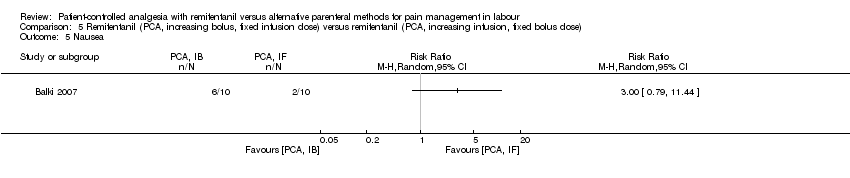
Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 5 Nausea.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 6 Vomiting.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 7 Pruritus.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 8 Apgar score < 7 at 5 min.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 9 Need for naloxone.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 10 FHR/CTG abnormalities, non‐reassuring fetal status.

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 11 Additional analgesia required (escape analgesia).
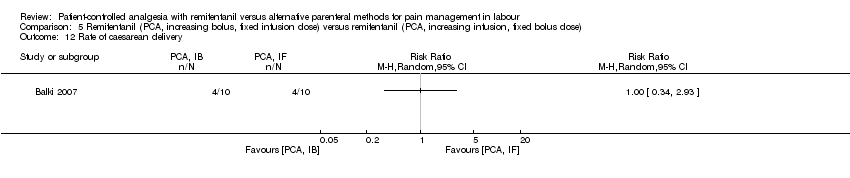
Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 12 Rate of caesarean delivery.
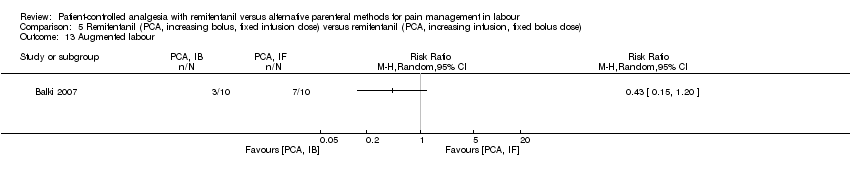
Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 13 Augmented labour.
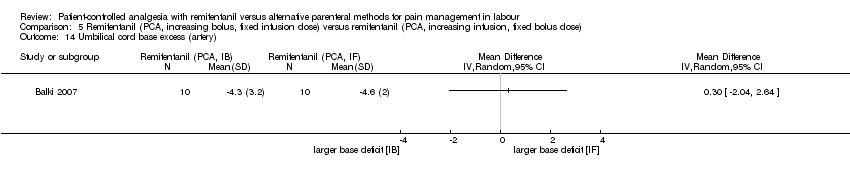
Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 14 Umbilical cord base excess (artery).

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 15 Umbilical cord base excess (venous).

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 16 Umbilical cord pH (artery).

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 17 Umbilical cord pH (venous).

Comparison 5 Remifentanil (PCA, increasing bolus, fixed infusion dose) versus remifentanil (PCA, increasing infusion, fixed bolus dose), Outcome 18 Neonatal resuscitation.
| Remifentanil (PCA) compared to another opioid (IV/IM) for pain management in labour | ||||||
| Patient or population: women in labour with planned vaginal delivery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with another opioid (IV/IM) | Risk with remifentanil (PCA) | |||||
| Satisfaction (overall) with pain relief (VAS 0 to 10 cm, NRS 1 to 4, NRS 0 to 10, VRS 0 to 5) | see comment | The standardised mean satisfaction score in the intervention group was 2.11 higher (0.72 higher to 3.49 higher)** | ‐ | 216 | ⊕⊝⊝⊝ | A SMD of 2.11 higher is equivalent to a range of 2.74 cm higher (SD = 1.3) to 4.68 cm higher (SD = 2.22) on a VAS 0 to 10 cm scale in the intervention group. The mean satisfaction scores in the control group range from 4.23 to 6.0 cm.# ** |
| Pain intensity 'early' (30 min/1 h) (VAS 0 to 10 cm, VAS 0 to 100 cm) | see comment | The standardised mean pain score 'early' in the intervention group was 1.58 fewer (2.69 fewer to 0.48 fewer)*** | ‐ | 180 | ⊕⊝⊝⊝ | A SMD of 1.58 fewer is equivalent to a range of 1.26 cm fewer (SD = 0.8) to 2.8 cm fewer (SD = 1.77) on a VAS 0 to 10 cm scale in the intervention group. The mean pain scores in the control group range from 3.56 to 6.3 cm (VAS 0 to 10 cm).# *** |
| Additional analgesia required (escape analgesia) | Study population | RR 0.57 | 190 | ⊕⊕⊕⊝ | ||
| 621 per 1.000 | 354 per 1.000 | |||||
| Rate of caesarean delivery | Study population | RR 0.63 | 215 | ⊕⊕⊝⊝ | Two studies includes zero events in one arm (constant continuity correction of 0.01).7 | |
| 148 per 1.000 | 93 per 1.000 | |||||
| Maternal apnoea | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Maternal respiratory depression (< 8 breaths/min) | None out of 18 women in the control group and three out of 18 in the remifentanil group had a respiratory depression. | not estimable | 36 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. | |
| Apgar score < 7 at 5 min | None of the newborns in both groups had an Apgar score < 7 at 5 min. | not estimable | 88 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 RoB ‐ downgrading (very serious): Substantial information is derived from studies at high risk of bias. After exclusion of high risk trials the CI crosses the line of no effect. 2 Inconsistency ‐ downgrading (serious): I2 > 50%. 3 Imprecision ‐ downgrading (serious): The number of women is insufficient to demonstrate the anticipated effect (OIS not reached). 4 RoB ‐ downgrading (serious): Substantial information is derived by high risk of bias studies (If more than one study: Exclusion of high risk of bias trials has no substantial effect on robustness of the results). 5 Imprecision ‐ downgrading (serious): The number of women is insufficient to demonstrate the anticipated effect (RIS not reached). The result is imprecise including appreciable benefit and harm. 6 Imprecision ‐ downgrading (very serious): Only one study with small sample size (< 150 participants) reported this outcome. 7 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 1.14: RR = 0.70 [0.34, 1.41], I2 = 1%. # The SMD was back‐transformed into the VAS 0 to 10 cm scale to facilitate the interpretation. The smallest as well as the largest SD of the studies were used for back‐transformation to reflect the range of effect. ** Higher values indicate higher levels of satisfaction. *** Lower values indicate less pain. | ||||||
| Remifentanil (PCA) compared to another opioid (PCA) for pain management in labour | ||||||
| Patient or population: women in labour with planned vaginal delivery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with another opioid (PCA) | Risk with remifentanil (PCA) | |||||
| Satisfaction (overall) with pain relief (VRS 1 to 10) | The mean satisfaction in the combined (meperidine + fentanyl) control group was 7.1 on a VRS 1 to 10 scale | Mean satisfaction in the remifentanil group was 0.92 VRS higher (0.46 to 1.39 higher).** | ‐ | 110 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. |
| Pain intensity 'early' (30 min/1 h) (VAS 0 to 10 cm, VAS 0 to 100 cm) | see comment | The standardised mean pain score 'early' in the intervention group was 0.51 fewer (1.01 fewer to 0)*** | ‐ | 215 | ⊕⊝⊝⊝ | A SMD of 0.51 fewer is equivalent to a range of 1.13 cm fewer (SD = 2.22) to 1.46 cm fewer (SD = 2.875) on a VAS 0 to 10 cm scale in the intervention group. Mean pain scores in the control groups range from 5.13 cm to 7.0 cm (VAS 0 to 10 cm).# *** |
| Additional analgesia required (escape analgesia) | Study population | RR 0.76 | 215 | ⊕⊕⊝⊝ | ||
| 381 per 1.000 | 289 per 1.000 | |||||
| Rate of caesarean delivery | Study population | RR 2.78 | 143 | ⊕⊝⊝⊝ | ||
| 56 per 1.000 | 156 per 1.000 | |||||
| Maternal apnoea | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Maternal respiratory depression | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Apgar score ≤ 7 (< 7) at 5 min | Three out of eight newborns in the control group and none out of nine in the remifentanil group had an Apgar score < 7 at 5 min. | not estimable | 17 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 RoB ‐ downgrading (serious): Information is derived from a high risk of bias trial. 2 RoB ‐ downgrading (serious): After exclusion of 1 high risk of bias trial (blinding) the estimated effect with CI reached clinically relevance ‐0.73 [‐1.05, ‐0.40] 3 Inconsistency ‐ downgrading (serious): I2 > 50% 4 Imprecision ‐ downgrading (serious): The number of women is insufficient to demonstrate the anticipated effect (RIS/OIS not reached). The result is imprecise including appreciable and no appreciable effect. 5 RoB ‐ downgrading (very serious): Substantial information is derived from studies at high risk of bias. Exclusion of high risk of bias trials widened the CI including appreciable benefit and harm. 6 Imprecision ‐ downgrading (very serious): Only one study with small sample size (< 150 participants) reported this outcome. 7 RoB ‐ downgrading (serious): Information is derived from a trial with unclear risk of bias. # The SMD was back‐transformed into the VAS 0 to 10 cm scale to facilitate the interpretation. The smallest as well as the largest SD of the studies were used for back‐transformation to reflect the range of effect. ** Higher values indicate higher levels of satisfaction. *** Lower values indicate less pain. | ||||||
| Remifentanil (PCA) compared to epidural/CSE for pain management in labour | ||||||
| Patient or population: women in labour with planned vaginal delivery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with epidural analgesia/central neuraxial blocks (CSE) | Risk with remifentanil (PCA) | |||||
| Satisfaction (overall) with pain relief (NRS 0 to 4, 1 to 4, 0 to 10, 1 to 10, VRS 1 to 4) | see comment | The standardised mean satisfaction score in the intervention group was 0.22 fewer (0.40 fewer to 0.04 fewer)** | ‐ | 2135 | ⊕⊝⊝⊝ | A SMD of 0.22 fewer is equivalent to a range of 0.15 cm fewer (SD = 0.7) to 0.61 cm fewer (SD = 2.78) on a VAS 0 to 10 cm scale in the intervention group. Mean satisfaction scores in the control group range from 6.7 to 9.1 cm (VAS 0 to 10 cm).# ** |
| Pain intensity 'early' (1 h) (VAS 0 to 10 cm, VAS 0 to 100 cm, NRS 0 to 10) | see comment | The standardised mean pain score 'early' in the intervention group was 0.57 higher (0.31 higher to 0.84 higher)*** | ‐ | 235 | ⊕⊕⊝⊝ | A SMD of 0.57 higher is equivalent to a range of 0.57 cm higher (SD = 1.0) to 1.43 cm higher (SD = 2.5) on a VAS 0 to 10 cm scale in the intervention group. The mean pain scores in the control group range from 1.6 to 4.14 cm (VAS 0 to 10 cm).# *** |
| Additional analgesia required | Study population | RR 9.27 | 1037 | ⊕⊕⊕⊝ | One study includes zero events in both arms; two studies include zero events in one arm (constant continuity correction of 0.01). 8 | |
| 10 per 1.000 | 93 per 1.000 | |||||
| Rate of caesarean delivery | Study population | RR 1.0 | 1578 | ⊕⊕⊕⊝ | One study includes zero events in one arm (constant continuity correction of 0.01). 9 | |
| 215 per 1.000 | 215 per 1.000 | |||||
| Maternal apnoea | None out of 19 women in the control group and nine out of 19 in the remifentanil group had an apnoea. | not estimable | 38 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. | |
| Maternal respiratory depression (< 9, < 8 breaths/min) | Study population | RR 0.91 | 687 | ⊕⊕⊝⊝ | One study includes zero events in both arms; one study includes zero events in one arm (constant continuity correction of 0.01). 10 | |
| 38 per 1.000 | 35 per 1.000 | |||||
| Apgar score ≤ 7 (< 7) at 5 min | Study population | RR 1.26 | 1322 | ⊕⊕⊝⊝ | Two studies include zero events in both arms; two studies include zero events in one arm (constant continuity correction of 0.01). 11 | |
| 23 per 1.000 | 30 per 1.000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 RoB ‐ downgrading (very serious): Substantial information is derived from studies at high risk of bias. After exclusion of high risk trials the CI crosses the line of no effect. 2 Inconsistency ‐ downgrading (serious): I2 > 50% 3 RoB ‐ downgrading (serious): Substantial information is derived from studies at high risk of bias. Exclusion of high risk of bias trials has no substantial impact on robustness of the results. 4 Imprecision ‐ downgrading (serious): The number of women is insufficient to demonstrate the anticipated effect (OIS not reached). 5 RoB ‐ downgrading (serious): Information is derived from a high risk of bias trial. 6 Imprecision ‐ downgrading (serious): The number of women is insufficient do demonstrate the anticipated effect (RIS/OIS not reached). The result is imprecise including appreciable benefit and harm. 7 Imprecision ‐ downgrading (very serious): Only one study with small sample size (< 150 participants) reported this outcome. 8 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 3.18: RR = 8.1 [3.5, 18.75], I2 = 0%. 9 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 3.19: RR = 0.99 [0.81, 1.21], I2 = 0%. 10 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 3.3: RR = 1.52 [0.23, 9.90], I2 = 50%. 11 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 3.12: RR = 1.28 [0.65, 2.51], I2 = 0%. # The SMD was back‐transformed into the VAS 0 to 10 cm scale to facilitate the interpretation. The smallest as well as the largest SD of the studies were used for back‐transformation to reflect the range of effect. ** Higher values indicate higher levels of satisfaction. *** Lower values indicate less pain. | ||||||
| Remifentanil (PCA) compared to remifentanil (continuous IV) for pain management in labour | ||||||
| Patient or population: women in labour with planned vaginal delivery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with remifentanil (continuous IV) | Risk with remifentanil (PCA) | |||||
| Satisfaction (overall) with pain relief | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Pain intensity 'early' (30 min/1 h) (VAS 0 to 10 cm) | The mean pain score in the remifentanil (continuous IV) group was 4.0 cm on a VAS 0 to 10 cm scale. | Mean pain score in the remifentanil (PCA) group was 1.0 cm fewer (1.8 fewer to 0.2 fewer).*** | not estimable | 53 | ⊕⊝⊝⊝ | Only one trial assessed this outcome. |
| Additional analgesia required (escape analgesia) | Two out of 29 women in the remifentanil (PCA) group and four out of 30 participants in the remifentanil (continuous IV) group required additional epidural analgesia. | not estimable | 59 (1 RCT) | ⊕⊝⊝⊝ | Only one trial assessed this outcome. | |
| Rate of caesarean delivery | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Maternal apnoea | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Maternal respiratory depression (< 8 breaths/min) | see comment | see comment | RR 0.98 | 135 | ⊕⊕⊝⊝ | All study arms include zero events (constant continuity correction of 0.01). 5 |
| Apgar score < 7 at 5 min | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 RoB ‐ downgrading (serious): Information is derived from a high risk of bias trial. 2 Imprecision ‐ downgrading (very serious): Only one study with small sample size (< 150 participants) reported this outcome. 3 RoB ‐ downgrading (serious): Substantial information is derived from studies at high risk of bias. Exclusion of high risk of bias trials has no substantial impact on robustness of the results. 4 Imprecision ‐ downgrading (serious): The number of women is insufficient to demonstrate the anticipated effect (RIS/OIS not reached). The result is imprecise including appreciable benefit and harm. 5 Estimated effect with zero/zero event handling (constant continuity correction of 1.0), Analysis 4.1: RR = not estimable *** Lower values indicate less pain. | ||||||
| Remifentanil (PCA, increasing bolus dose) compared to remifentanil (PCA, increasing infusion dose) for pain management in labour | ||||||
| Patient or population: women in labour with planned vaginal delivery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with remifentanil (continuous IV) | Risk with remifentanil (PCA) | |||||
| Satisfaction (overall) with pain relief (VNRS 0 to 10) | The mean satisfaction scores in the remifentanil (PCA, IF) group was 8.4 on a VNRS 0 to 10 scale. | Mean satisfaction scores in the remifentanil (PCA, IB) group was 0.2 higher (0.81 fewer to 1.21 higher).** | not estimable | 20 (1 RCT) | ⊕⊕⊝⊝ | Only one trial assessed this outcome. |
| Pain intensity 'early' (30 min/1 h) | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Additional analgesia required (escape analgesia) | Only one out of 10 woman in the remifentanil (PCA, IF) group crossed over to the epidural group. | not estimable | 20 (1 RCT) | ⊕⊕⊝⊝ | Only one trial assessed this outcome. | |
| Rate of caesarean delivery | Four out of 10 women in each group delivered by caesarean section. | not estimable | 20 (1 RCT) | ⊕⊕⊝⊝ | Only one trial assessed this outcome. | |
| Maternal apnoea | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Maternal respiratory depression (< 8 breaths/min) | see comment | see comment | ‐ | (0 studies) | ‐ | No trial assessed this outcome. |
| Apgar score < 7 at 5 min | None of the newborns in both groups had an Apgar score < 7 at 5 min. | not estimable | 20 (1 RCT) | ⊕⊕⊝⊝ | Only 1 trial assessed this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision ‐ downgrading (very serious): Only one study with small sample size (< 150 participants) reported this outcome. ** Higher values indicate higher levels of satisfaction. | ||||||
| Study | No. randomised (Remifentanil/ control) | No. analysed (Remifentanil/ control) | Overall assessment for risk of attrition bias | Outcome level_Risk of bias | |||||
| Satisfaction with pain relief | AE for women | AE for newborns | Pain intensity | Additional analgesia | Rate of CS | ||||
| 10/ 10 | 10/ 10 | Low | Low | Low | Low | Low | Low | Low | |
| 20/ 20 | 20/ 19 | High | High | High | High | Unclear | Unclear | ||
| 12/ 12 | 12/ 12 | Low | Low | Low | Low | Low | Low | ||
| 60/ 60/ 60 | 52/ 53/ 54 | High | High | High | High | Low | Low | High | |
| 14/ 12 | 10/ 10 | High | High | Low | High | High | Low | Low | |
| 57/ 59 | 49/ 49 | High | High | High | High | Unclear | Unclear | High | |
| 15/ 15 | 15/ 15 | Low | Low | Low | Low | Low | Low | ||
| 43/ 45 | 43/ 45 | Unclear | Low | High | Low | Low | Low | Low | |
| 213 NA/ NA/ NA/ NA | 192 44/ 50/ 49/ 49 | Low | Low | Low | Low | ||||
| 709/ 705 | 687/ 671 | High | High | High | High | High | High | High | |
| 380/ 380/ 380 | 380/ 380/ 380 | Low | Low | Low | Low | Low | Low | ||
| 41/ 41 | 41/ 41 | Low | Low | Low | Low | Low | |||
| 34/ 34 | 34/ 34 | Low | Low | Low | Low | Low | Low | Low | |
| 30/ 30 | 27/ 26 | High | High | High | High | High | High | ||
| 20/ 20 | 19/ 20 | Low | Low | Low | Low | Low | Low | Low | |
| 13/ 15 | 12/ 12 | High | High | High | Low | High | Low | ||
| 18/ 18 | 18/ 18 | Unclear | Low | Low | Low | High | High | ||
| 19/ 20 | 17/ 20 | High | High | High | High | High | Low | High | |
| 9/ 8 | 9/ 8 | Low | Low | Low | Low | Low | Low | ||
| 27/ 25 | 24/ 21 | High | High | High | High | High | High | High | |
| Abbreviations: AE: adverse events, CS: caesarean section | |||||||||
| Sensitivity analysis: Selection bias | Statistical method | All studies | 'high risk of bias'‐studies excluded | Impact on robustness (95% CI) | ||
| n | Effect estimate | n | Effect estimate | |||
| 1. Remifentanil (PCA) versus another opioid (IV/IM) | ||||||
| 1.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 4, all at low risk of bias | ||||
| 1.3 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 1.4 Nausea (and vomiting) | RR (MH, Random), 95% CI | 4, all at low risk of bias | ||||
| 1.6 Pruritus | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 1.10 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 1.11 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 1.13 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3, all at low risk of bias | ||||
| 1.14 Rate of caesarean delivery | RR (MH, Random), 95% CI | 4, all at low risk of bias | ||||
| 2. Remifentanil (PCA) versus another opioid (PCA) | ||||||
| 2.2 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 2.10 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 2.12 NACS at 15/30 min | MD (IV, Random), 95% CI | 2, all at low risk of bias | ||||
| 2.13 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 2.15 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3, all at low risk of bias | ||||
| 2.16 Rate of caesarean delivery | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 3. Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE) | ||||||
| 3.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 7 | ‐0.22 [‐0.40, ‐0.04] | 6 | ‐0.20 [‐0.46, 0.07] | Yes (CI includes 0) |
| 3.3 Respiratory depression (< 9, < 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 3 | 0.91 [0.51, 1.62] | 2 | 0.91 [0.52, 1.61] | No |
| 3.4 Oxygen desaturation (SpO2 < 92%) | RR (MH, Random), 95% CI | 3 | 3.24 [1.66, 6.32] | 2 | 5.83 [0.40, 84.06] | Yes (CI includes 1) |
| 3.5 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 3 | 3.27 [2.32, 4.61] | 2 | 5.44 [2.11, 14.02] | Yes (effect and CI increased) |
| 3.6 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 4 | 0.59 [0.37, 0.94] | 3 | 0.57 [0.00, 2.4E7] | Yes (CI includes 1) |
| 3.7 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 3.8 Nausea | RR (MH, Random), 95% CI | 8 | 1.49 [1.19, 1.86] | 7 | 1.41 [1.09, 1.83] | No |
| 3.9 Vomiting | RR (MH, Random), 95% CI | 6 | 1.63 [1.25, 2.13] | 5 | 1.82 [1.29, 2.57] | No |
| 3.10 Pruritus | RR (MH, Random), 95% CI | 7 | 0.75 [0.48, 1.18] | 6 | 0.81 [0.45, 1.45] | No |
| 3.11 Sedation (1 h) | MD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 3.12 Apgarscore ≤ 7 (< 7) at 5 min | RR (IV, Random), 95% CI, 0/0 cell counts | 5, all at low risk of bias | ||||
| 3.13 Apgarscore at 5 min | MD (IV,), 95% CI | 3, all at low risk of bias | ||||
| 3.14 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 3.15 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 5, all at low risk of bias | ||||
| 3.16 Pain intensity 'early' (1 h) | SMD (IV, Random), 95% CI | 6, all at low risk of bias | ||||
| 3.18 Additional analgesia required | RR (IV, Random), 95% CI, 0/0 cell counts | 6 | 9.27 [3.73, 23.03] | 5 | 5.29 [1.2, 23.3] | No |
| 3.19 Rate of caesarean delivery | RR (MH, Random), 95% CI | 9, all at low risk of bias | ||||
| 4. Remifentanil (PCA) versus remifentanil (continuous IV) | ||||||
| 4.1 Respiratory depression (< 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 4.3 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 4.4 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 4.5 Nausea (and vomiting) | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 4.8 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| All RR for outcomes including 0/0 cell counts (zero/zero event trials) were calculated using TSA (constant continuity correction, 0.01). Review Manager 5 produces computational errors when both the intervention and control group have zero events. By using TSA there is no possibility to choose the MH method (only IV) which may cause small deviations within results. Abbreviations: [95% CI]: 95% confidence interval; IV: Inverse Variance; MD: mean difference; MH: Mantel‐Haenszel; n: number of participants; RPCA: Remifentanil PCA; RR: risk ratio; SMD: standardised mean difference | ||||||
| Sensitivity analysis: Blinding (performance and detection bias) | Statistical method | All studies | 'high risk of bias'‐studies excluded | Impact on robustness (95% CI) | ||
| n | Effect estimate | n | Effect estimate | |||
| 1. Remifentanil (PCA) versus another opioid (IV/IM) | ||||||
| 1.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 4 | 2.11 [0.72, 3.49] | 2 | 2.46 [‐0.34, 5.26] | Yes (CI includes 0) |
| 1.3 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2 | 0.48 [0.00, 47.37] | 1 | 0.05 [0.00, 0.82] | Yes (CI < 1: favours RPCA) |
| 1.4 Nausea (and vomiting) | RR (MH, Random), 95% CI | 4 | 0.54 [0.29, 0.99] | 2 | 0.36 [0.06, 2.29] | Yes (CI includes 1) |
| 1.6 Pruritus | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 1.10 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 1.11 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3 | ‐1.58 [‐2.69, ‐0.48] | 2 | ‐1.28 [‐2.62, 0.07] | Yes (CI includes 0) |
| 1.13 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3 | 0.57 [0.40, 0.81] | 2 | 0.48 [0.25, 0.91] | No |
| 1.14 Rate of caesarean delivery | RR (MH, Random), 95% CI | 4 | 0.70 [0.34, 1.41] | 2 | 0.63 [0.30, 1.31] | No |
| 2. Remifentanil (PCA) versus another opioid (PCA) | ||||||
| 2.2 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2 | 1.28 [0.49, 3.30] | 1 | 1.64 [1.25, 2.15] | Yes (CI > 1: favours opioid) |
| 2.10 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.03 [0.00, 1.8E8] | 1 | 0.00 [0.00, 0.06] | Yes (CI < 1: favours RPCA) |
| 2.12 NACS at 15/30 min | MD (IV, Random), 95% CI | 2 | 1.11 [‐0.65, 2.87] | 1 | 0.20 [‐0.93, 1.33] | Yes (direction of effect changed, CI decreased) |
| 2.13 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3 | ‐0.51 [‐1.01, ‐0.00] | 2 | ‐0.73 [‐1.05, ‐0.40] | Yes (lower CI: clinically relevant moderate effect) |
| 2.15 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3 | 0.76 [0.45, 1.28] | 2 | 0.65 [0.39, 1.09] | No |
| 2.16 Rate of caesarean delivery | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 3. Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE) | ||||||
| 3.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 7 | ‐0.22 [‐0.40, ‐0.04] | 1 | 0.27 [‐0.31, 0.86] | Yes (CI includes 0) |
| 3.3 Respiratory depression (< 9, < 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 3 | 0.91 [0.51, 1.62] | 0 | Not estimable | All studies at high risk |
| 3.4 Oxygen desaturation (SpO2 < 92%) | RR (MH, Random), 95% CI | 3 | 3.24 [1.66, 6.32] | 0 | Not estimable | All studies at high risk |
| 3.5 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 3 | 3.27 [2.32, 4.61] | 1 | 11.38 [1.62, 79.78] | Yes (effect and CI increased) |
| 3.6 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 4 | 0.59 [0.37, 0.94] | 0 | Not estimable | All studies at high risk |
| 3.7 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 1.0 [0.00, 1.0E12] | 0 | Not estimable | All studies at high risk |
| 3.8 Nausea | RR (MH, Random), 95% CI | 8 | 1.49 [1.19, 1.86] | 1 | 3.94 [0.96, 16.22] | Yes (CI includes 1) |
| 3.9 Vomiting | RR (MH, Random), 95% CI | 6 | 1.63 [1.25, 2.13] | 0 | Not estimable | All studies at high risk |
| 3.10 Pruritus | RR (MH, Random), 95% CI | 7 | 0.75 [0.48, 1.18] | 0 | Not estimable | All studies at high risk |
| 3.11 Sedation (1 h) | MD (IV, Random), 95% CI | 3 | 0.71 [0.03, 1.39] | 0 | Not estimable | All studies at high risk |
| 3.12 Apgarscore ≤ 7 (< 7) at 5 min | RR (IV, Random), 95% CI, 0/0 cell counts | 5 | 1.26 [0.62, 2.57] | 0 | Not estimable | All studies at high risk |
| 3.13 Apgarscore at 5 min | MD (IV,), 95% CI | 3 | 0.06 [‐0.27, 0.39] | 0 | Not estimable | All studies at high risk |
| 3.14 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.02 [0.00, 1.6E8] | 0 | Not estimable | All studies at high risk |
| 3.15 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 5 | 1.55 [0.49, 4.92] | 1 | 11.38 [1.62, 79.78] | Yes (CI > 1: favours epidural) |
| 3.16 Pain intensity 'early' (1 h) | SMD (IV, Random), 95% CI | 6 | 0.57 [0.31, 0.84] | 0 | Not estimable | All studies at high risk |
| 3.18 Additional analgesia required | RR (IV, Random), 95% CI, 0/0 cell counts | 6 | 9.27 [3.73, 23.07] | 0 | Not estimable | All studies at high risk |
| 3.19 Rate of caesarean delivery | RR (MH, Random), 95% CI | 9 | 0.99 [0.81, 1.21] | 1 | 0.88 [0.06, 13.14] | Yes (CI increased) |
| 4. Remifentanil (PCA) versus remifentanil (continuous IV) | ||||||
| 4.1 Respiratory depression (< 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.3 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.4 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.5 Nausea (and vomiting) | RR (MH, Random), 95% CI | 2 | 0.85 [0.28, 2.54] | 1 | 0.53 [0.21, 1.39] | No |
| 4.8 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| All RR for outcomes including 0/0 cell counts (zero/zero event trials) were calculated using TSA (constant continuity correction, 0.01). Review Manager 5 produces computational errors when both the intervention and control group have zero events. By using TSA there is no possibility to choose the MH method (only IV) which may cause small deviations within results. Abbreviations: [95% CI]: 95% confidence interval; IV: Inverse Variance; MD: mean difference; MH: Mantel‐Haenszel; n: number of participants; RPCA: Remifentanil PCA; RR: risk ratio; SMD: standardised mean difference | ||||||
| Sensitivity analysis: Attrition bias | Statistical method | All studies | 'high risk of bias'‐studies excluded | Impact on robustness (95% CI) | ||
| n | Effect estimate | n | Effect estimate | |||
| 1. Remifentanil (PCA) versus another opioid (IV/IM) | ||||||
| 1.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 4, all at low risk of bias | ||||
| 1.3 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2 | 0.48 [0.00, 47.37] | 1 | 3.50 [0.84, 14.61] | Yes (CI + effect moved to favour of opioid) |
| 1.4 Nausea (and vomiting) | RR (MH, Random), 95% CI | 4, all at low risk of bias | ||||
| 1.6 Pruritus | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 1.10 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 2, all at low risk of bias | ||||
| 1.11 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 1.13 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3 | 0.57 [0.40, 0.81] | 2 | 0.48 [0.25, 0.91] | No |
| 1.14 Rate of caesarean delivery | RR (MH, Random), 95% CI | 4 | 0.70 [0.34, 1.41] | 3 | 0.60 [0.29, 1.24] | No |
| 2. Remifentanil (PCA) versus another opioid (PCA) | ||||||
| 2.2 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 2 | 1.28 [0.49, 3.30] | 0 | Not estimable | All studies at high risk |
| 2.10 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.03 [0.00, 1.8E8] | 1 | 0.00 [0.00, 0.06] | Yes (CI moved to favour RPCA) |
| 2.12 NACS at 15/30 min | MD (IV, Random), 95% CI | 2 | 1.11 [‐0.65, 2.87] | 0 | Not estimable | All studies at high risk |
| 2.13 Pain intensity 'early' (30 min/1 h) | SMD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 2.15 Additional analgesia required (escape analgesia) | RR (MH, Random), 95% CI | 3, all at low risk of bias | ||||
| 2.16 Rate of caesarean delivery | RR (MH, Random), 95% CI | 2 | 2.78 [0.99, 7.82] | 1 | 1.78 [0.20, 16.10] | Yes (CI increased) |
| 3. Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE) | ||||||
| 3.1 Satisfaction with pain relief | SMD (IV, Random), 95% CI | 7 | ‐0.22 [‐0.40, ‐0.04] | 3 | ‐0.27 [‐0.64, 0.10] | Yes (CI includes 0) |
| 3.3 Respiratory depression (< 9, < 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 3 | 0.91 [0.51, 1.62] | 1 | 0.91 [0.39, 2.10] | No |
| 3.4 Oxygen desaturation (SpO2 < 92%) | RR (MH, Random), 95% CI | 3 | 3.24 [1.66, 6.32] | 0 | Not estimable | All studies at high risk |
| 3.5 Oxygen desaturation (SpO2 < 95%) | RR (MH, Random), 95% CI | 3 | 3.27 [2.32, 4.61] | 1 | 4.33 [1.47, 12.79] | Yes (effect and CI increased) |
| 3.6 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 4 | 0.59 [0.37, 0.94] | 2 | 0.01 [0.00, 7.8E7] | Yes (CI includes 1) |
| 3.7 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 1.0 [0.00, 1.0E12] | 1 | 1.0 [0.00, ∞] | No |
| 3.8 Nausea | RR (MH, Random), 95% CI | 8 | 1.49 [1.19, 1.86] | 4 | 1.27 [0.82, 1.98] | Yes (CI includes 1) |
| 3.9 Vomiting | RR (MH, Random), 95% CI | 6 | 1.63 [1.25, 2.13] | 3 | 1.54 [0.75, 3.14] | Yes (CI includes 1) |
| 3.10 Pruritus | RR (MH, Random), 95% CI | 7 | 0.75 [0.48, 1.18] | 5 | 0.86 [0.48, 1.56] | No |
| 3.11 Sedation (1 h) | MD (IV, Random), 95% CI | 3, all at low risk of bias | ||||
| 3.12 Apgarscore ≤ 7 (< 7) at 5 min | RR (IV, Random), 95% CI, 0/0 cell counts | 5 | 1.26 [0.62, 2.57] | 3 | 1.26 [0.62, 2.57] | No |
| 3.13 Apgarscore at 5 min | MD (IV,), 95% CI | 3 | 0.06 [‐0.27, 0.39] | 0 | Not estimable | All studies at high risk |
| 3.14 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2, all at low risk of bias | ||||
| 3.15 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH, Random), 95% CI | 5 | 1.55 [0.49, 4.92] | 2 | 0.87 [0.41, 1.87] | Yes (CI decreased, effect changed) |
| 3.16 Pain intensity 'early' (1 h) | SMD (IV, Random), 95% CI | 6 | 0.57 [0.31, 0.84] | 3 | 0.57 [0.25, 0.89] | No |
| 3.18 Additional analgesia required | RR (IV, Random), 95% CI, 0/0 cell counts | 6 | 9.27 [3.73, 23.03] | 5 | 5.29 [1.2, 23.3] | No |
| 3.19 Rate of caesarean delivery | RR (MH, Random), 95% CI | 9 | 0.99 [0.81, 1.21] | 6 | 1.02 [0.83, 1.25] | No |
| 4. Remifentanil (PCA) versus remifentanil (continuous IV) | ||||||
| 4.1 Respiratory depression (< 8 breaths/min) | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.3 Hypotension | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.4 Bradycardia | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| 4.5 Nausea (and vomiting) | RR (MH, Random), 95% CI | 2 | 0.85 [0.28, 2.54] | 1 | 1.67 [0.43, 6.52] | No |
| 4.8 Need for naloxone | RR (IV, Random), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 1 | 0.98 [0.00, ∞] | No |
| All RR for outcomes including 0/0 cell counts (zero/zero event trials) were calculated using TSA (constant continuity correction, 0.01). Review Manager 5 produces computational errors when both the intervention and control group have zero events. By using TSA there is no possibility to choose the MH method (only IV) which may cause small deviations within results. Abbreviations: [95% CI]: 95% confidence interval; IV: Inverse Variance; MD: mean difference; MH: Mantel‐Haenszel; n: number of participants; RPCA: Remifentanil PCA; RR: risk ratio; SMD: standardised mean difference | ||||||
| EE [95% CI], P value, I2 (%), n | TSA_Low risk of bias‐based (all low) | |||||
| RRR (%) | CER (%) | H (%) | RIS | evidence | ||
| 1.13 Additional analgesia | 0.58 [0.42, 0.79], 0.0005, 15%, 190 | 51.21 | 58 | 25 | 156 | evidence of effect (intervention) |
| low risk of bias studies: Evron 2005 + Ng 2011 (best) | ||||||
| 1.14 Rate of caesarean delivery | 0.63 [0.30, 1.32], 0.22, 0%,215 | 37.47 | 19 | 25 | 1444 | absence of evidence |
| low risk of bias studies: Evron 2005 + Ng 2011 (best) | ||||||
| 2.15 Additional analgesia | 0.87 [0.74, 1.03], 0.11, 0%, 215 | 35.21 | 28 | 25 | 1024 | absence of evidence |
| low risk of bias studies: Douma 2010 (best) + Volikas 2001 | ||||||
| 2.16 Rate of caesarean delivery | 2.78 [0.99, 7.82], 0.05, 0%, 143 | ‐77.76 | 12.5 | 25 | 852 | absence of evidence |
| only low risk of bias study: Volikas 2001 | ||||||
| 3.3 Respiratory depression | 0.91 [0.51, 1.62], 0.75, 0%,687 | 9.09 | 58 | 25 | 4986 | absence of evidence |
| best study (high risk): Stocki‐2014 | ||||||
| 3.12 Apgarscore < 7 at 5 min | 1.26 [0.62, 2.57], 0.52, 0%, 1322 | ‐26.33 | 3 | 25 | 2.9E4 | absence of evidence |
| not best study (0/0 events), but largest (high risk): Ismail 2012 | ||||||
| 3.18 Additional analgesia | 9.27 [3.73, 23.03], < 0.0001, 0%, 1037 | ‐218.8 | 5 | 25 | 449 | evidence of effect (control) |
| Not best study (0/0 events), but second best (high risk): Stocki 2014 | ||||||
| 3.19 Rate of caesarean delivery | 1.0 [0.82, 1.22], 0.9857, 0%, 1578 | ‐12.5 | 8 | 25 | 4.4E4 | absence of evidence |
| best study (high risk): Evron 2008 clinically relevant (RRR) assumptions: RRR = ‐ 50%, CER (empirical) = 22%, H (empirical) = 0% → IS = 924 (lack of effect) | ||||||
| 4.1 Respiratory depression | 0.98 [0.06, 15.37], 0.9896, 0%, 135 | 4 | 1 | 25 | 3.4E6 | absence of evidence |
| best study (high risk): Shen 2013 | ||||||
| TSA (trial sequential analysis): random‐effects modelling; IV (inverse variance); (α = 0.05, power = 90% (ß = 0.10); zero event handling = constant continuity correction, 0.01; H = 25% (mild heterogeneity); calculated with TSA software (http://www.ctu.dk/tsa/) Abbreviations: CER: control event rate; EE [95% CI]: estimated effect with 95% confidence interval; EER: experimental event rate; H: heterogeneity adjustment factor; n: number of participants; NA: not applicable; RIS: required information size; RRR: relative risk reduction = (EER‐CER)/CER; TSMB: trial sequential monitoring boundary | ||||||
| EE [95% CI], P value, I2 (%), n | TSA_Empirical (with all studies) | |||||
| RRR (%) | CER (%) | H (%) | RIS | evidence | ||
| 1.13 Additional analgesia | 0.58 [0.42, 0.79], 0.0005, 15%, 190 | 42.39 | 62 | 21.39 | 194 | evidence of effect, TSMB, (intervention) |
| 1.14 Rate of caesarean delivery | 0.63 [0.30, 1.32], 0.22, 0%,215 | 30.4 | 15 | 0 | 2245 | absence of evidence |
| 2.15 Additional analgesia | 0.87 [0.74, 1.03], 0.11, 0%, 215 | 12.58 | 38 | 0 | 4218 | absence of evidence |
| 2.16 Rate of caesarean delivery | 2.78 [0.99, 7.82], 0.05, 0%, 143 | ‐177.7 | 6 | 0 | 372 | absence of evidence |
| 3.3 Respiratory depression | 0.91 [0.51, 1.62], 0.75, 0%,687 | 2 | 4 | 0 | 2.5E6 | absence of evidence |
| 3.12 Apgarscore < 7 at 5 min | 1.26 [0.62, 2.57], 0.52, 0%, 1322 | ‐26 | 2 | 0 | 3.4E4 | absence of evidence |
| 3.18 Additional analgesia | 9.27 [3.73, 23.03], < 0.0001, 0%, 1037 | ‐665 | 1 | 0 | 394 | evidence of effect (control) |
| 3.19 Rate of caesarean delivery | 1.0 [0.82, 1.22], 0.9857, 0%, 1578 | 1.18 | 22 | 0 | 1.1E6 | absence of evidence |
| 4.1 Respiratory depression | 0.98 [0.06, 15.37], 0.9896, 0%, 135 | 2 | 1 | 0 | 1.0E7 | absence of evidence |
| TSA (trial sequential analysis): random‐effects modelling; IV (inverse variance); (α = 0.05, power = 90% (ß = 0.10); zero event handling = constant continuity correction, 0.01; H = 25% (mild heterogeneity); calculated with TSA software (http://www.ctu.dk/tsa/) Abbreviations: CER: control event rate; EE [95% CI]: estimated effect with 95% confidence interval; EER: experimental event rate; H: heterogeneity adjustment factor; n: number of participants; NA: not applicable; RIS: required information size; RRR: relative risk reduction = (EER‐CER)/CER; TSMB: trial sequential monitoring boundary | ||||||
| EE [95% CI], P value, I2, n | OIS_minimal clinically relevant difference1 | |||||
| mean1 | mean2 | SDlargest | OIS | evidence | ||
| 1.1 Satisfaction with pain relief | 2.11 [0.72, 3.49], 0.003, 93%, 216 | 7 | 6 | 2.22 | 208 | evidence of effect (intervention) |
| best low risk of bias study: Ng 2011 | ||||||
| 1.11 Pain intensity 'early' | ‐1.58 [‐2.69, ‐0.48], 0.005, 89%, 180 | 25.6 | 35.6 | 26.6 | 298 | absence of evidence |
| best low risk of bias study: Ng 2011 | ||||||
| 2.13 Pain intensity 'early' | ‐0.51 [‐1.01, ‐0.00], 0.05, 52%, 215 | 5.282 | 6.282 | 2.414 | 246 | absence of evidence |
| best low risk of bias study: Douma 2010 | ||||||
| 3.1 Satisfaction with pain relief | ‐0.22 [‐0.40, ‐0.04], 0.02, 52%, 2135 | 8.1 | 9.1 | 1.5 | 96 | evidence of effect (control) |
| best study (high risk): Stocki 2014 | ||||||
| 3.16 Pain intensity 'early' | 0.57 [0.31, 0.84], < 0.0001, 0%, 235 | 3.3 | 2.3 | 3.3 | 458 | absence of evidence |
| best study (high risk): Stocki 2014 | ||||||
| The summary statistics for the GRADE‐relevant continuous outcomes was SMD (standardised mean difference). The TSA software (version 0.9 Beta) did not support trial sequential analysis of SMD. Therefore, we conducted OIS (optimal information size) calculations (http://stat.ubc.ca/˜rollin/stats/ssize/n2.html) which corresponds to a sample size calculation for an individual trial with the following general assumptions on α = 0.05 and ß = 0.10 (power = 90%). 1The assumed minimal clinically relevant difference was 1.0 cm (10 mm) on a VAS 0 to 10 cm (0 to 100 mm) scale. The mean2 was derived from the control group (low risk of bias (best) trial). Abbreviations: EE [95% CI]: estimated effect with 95% confidence interval; mean1: intervention group; mean2: control group; n: number of participants; SDlargest: largest standard deviation of the pooled studies was assumed | ||||||
| EE [95% CI], P value, I2, n | OIS_low risk of bias‐based (best) | |||||
| mean1 | mean2 | SDlargest | OIS | evidence | ||
| 1.1 Satisfaction with pain relief | 2.11 [0.72, 3.49], 0.003, 93%, 216 | 8 | 6 | 2.22 | 52 | evidence of effect (intervention) |
| best low risk of bias study: Ng 2011 | ||||||
| 1.11 Pain intensity 'early' | ‐1.58 [‐2.69, ‐0.48], 0.005, 89%, 180 | 22.1 | 35.6 | 26.6 | 164 | evidence of effect (intervention) |
| best low risk of bias study: Ng 2011 | ||||||
| 2.13 Pain intensity 'early' | ‐0.51 [‐1.01, ‐0.00], 0.05, 52%, 215 | 4.56 | 6.282 | 2.414 | 82 | lack of effect |
| best low risk of bias study: Douma 2010 | ||||||
| 3.1 Satisfaction with pain relief | ‐0.22 [‐0.40, ‐0.04], 0.02, 52%, 2135 | 8.6 | 9.1 | 1.5 | 380 | evidence of effect (control) |
| best study (high risk): Stocki 2014 | ||||||
| 3.16 Pain intensity 'early' | 0.57 [0.31, 0.84], < 0.0001, 0%, 235 | 4 | 2.3 | 3.3 | 160 | evidence of effect (control) |
| best study (high risk): Stocki 2014 | ||||||
| The summary statistics for the GRADE‐relevant continuous outcomes was SMD (standardised mean difference). The TSA software (version 0.9 Beta) did not support trial sequential analysis of SMD. Therefore, we conducted OIS (optimal information size) calculations (http://stat.ubc.ca/˜rollin/stats/ssize/n2.html) which corresponds to a sample size calculation for an individual trial with the following general assumptions on α = 0.05 and ß = 0.10 (power = 90%). The mean2 was derived from the control group (low risk of bias (best) trial). Abbreviations: EE [95% CI]: estimated effect with 95% confidence interval; mean1: intervention group; mean2: control group; n: number of participants; SDlargest: largest standard deviation of the pooled studies was assumed | ||||||
| Sensitivity analysis: Random‐effects versus fixed‐effect model | Statistical method | Random‐effects model | Fixed‐effect model | Impact on robustness (95% CI) (fixed‐effect model) | |||
| n | Effect estimate | n | Effect estimate | ||||
| 1. Remifentanil (PCA) versus another opioid (IV/IM) | |||||||
| 1.1 Satisfaction with pain relief | SMD (IV), 95% CI | 4 | 2.11 [0.72, 3.49] | 4 | 1.85 [1.51, 2.19] | Yes (CI decreased, large effect) | |
| 1.3 Oxygen desaturation (SpO2 < 95%) | RR (MH), 95% CI | 2 | 0.48 [0.00, 47.37] | 2 | 0.66 [0.28, 1.57] | Yes (CI decreased) | |
| 1.4 Nausea (and vomiting) | RR (MH), 95% CI | 4 | 0.54 [0.29, 0.99] | 4 | 0.51 [0.28, 0.95] | No | |
| 1.6 Pruritus | RR (IV), 95% CI, 0/0 cell counts | 2 | 1.02 [0.00, 1.1E12] | 2 | 1.02 [0.00, 1.1E12] | No | |
| 1.10 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH), 95% CI | 2 | 0.30 [0.10, 0.90] | 2 | 0.30 [0.10, 0.85] | No | |
| 1.11 Pain intensity 'early' (30 min/1 h) | SMD (IV), 95% CI | 3 | ‐1.58 [‐2.69, ‐0.48] | 3 | ‐1.35 [‐1.68, ‐1.01] | Yes (CI decreased, large effect) | |
| 1.13 Additional analgesia required (escape analgesia) | RR (MH), 95% CI | 3 | 0.57 [0.40, 0.81] | 3 | 0.53 [0.39, 0.71] | No | |
| 1.14 Rate of caesarean delivery | RR (MH), 95% CI | 4 | 0.70 [0.34, 1.41] | 4 | 0.77 [0.39, 1.49] | No | |
| 2. Remifentanil (PCA) versus another opioid (PCA) | |||||||
| 2.2 Oxygen desaturation (SpO2 < 95%) | RR (MH), 95% CI | 2 | 1.28 [0.49, 3.30] | 2 | 1.39 [1.16, 1.67] | Yes (CI > 1: favours opioid) | |
| 2.10 Need for naloxone | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.03 [0.00, 1.8E8] | 2 | 0.01 [0.00, 2.4E6] | No | |
| 2.12 NACS at 15/30 min | MD (IV), 95% CI | 2 | 1.11 [‐0.65, 2.87] | 2 | 1.15 [0.38, 1.93] | Yes (CI > 0: favours RPCA) | |
| 2.13 Pain intensity 'early' (30 min/1 h) | SMD (IV), 95% CI | 3 | ‐0.51 [‐1.01, ‐0.00] | 3 | ‐0.57 [‐0.86, ‐0.29] | Yes (CI < 0: favours RPCA) | |
| 2.15 Additional analgesia required (escape analgesia) | RR (MH), 95% CI | 3 | 0.76 [0.45, 1.28] | 3 | 0.74 [0.55, 1.00] | No | |
| 2.16 Rate of caesarean delivery | RR (MH), 95% CI | 2 | 2.78 [0.99, 7.82] | 2 | 2.78 [0.99, 7.77] | No | |
| 3. Remifentanil (PCA) versus epidural/combined spinal‐epidural analgesia (CSE) | |||||||
| 3.1 Satisfaction with pain relief | SMD (IV), 95% CI | 7 | ‐0.22 [‐0.40, ‐0.04] | 7 | ‐0.29 [‐0.38, ‐0.20] | No | |
| 3.3 Respiratory depression (< 9, < 8 breaths/min) | RR (IV), 95% CI, 0/0 cell counts | 3 | 0.91 [0.51, 1.62] | 3 | 1.2 [0.67, 2.17] | No | |
| 3.4 Oxygen desaturation (SpO2 < 92%) | RR (MH), 95% CI | 3 | 3.24 [1.66, 6.32] | 3 | 3.46 [2.32, 5.16] | No | |
| 3.5 Oxygen desaturation (SpO2 < 95%) | RR (MH), 95% CI | 3 | 3.27 [2.32, 4.61] | 3 | 3.30 [2.43, 4.49] | No | |
| 3.6 Hypotension | RR (IV,), 95% CI, 0/0 cell counts | 4 | 0.59 [0.37, 0.94] | 4 | 0.57 [0.36, 0.89] | No | |
| 3.7 Bradycardia | RR (IV,), 95% CI, 0/0 cell counts | 2 | 1.0 [0.00, 1.0E12] | 2 | 1.0 [0.00, 1.0E12] | No | |
| 3.8 Nausea | RR (MH), 95% CI | 8 | 1.49 [1.19, 1.86] | 8 | 1.53 [1.22, 1.91] | No | |
| 3.9 Vomiting | RR (MH), 95% CI | 6 | 1.63 [1.25, 2.13] | 6 | 1.62 [1.24, 2.10] | No | |
| 3.10 Pruritus | RR (MH), 95% CI | 7 | 0.75 [0.48, 1.18] | 7 | 0.76 [0.54, 1.07] | No | |
| 3.11 Sedation (1 h) | MD (IV), 95% CI | 3 | 0.71 [0.03, 1.39] | 3 | 0.91 [0.57, 1.25] | No | |
| 3.12 Apgarscore ≤ 7 (< 7) at 5 min | RR (IV,), 95% CI, 0/0 cell counts | 5 | 1.26 [0.62, 2.57] | 5 | 1.22 [0.67, 2.62] | No | |
| 3.13 Apgarscore at 5 min | MD (IV,), 95% CI | 3 | 0.06 [‐0.27, 0.39] | 3 | 0.06 [‐0.27, 0.39] | No | |
| 3.14 Need for naloxone | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.02 [0.00, 1.6E8] | 2 | 0.01 [0.00, 4.6E5] | No | |
| 3.15 FHR/CTG abnormalities, non‐reassuring fetal status | RR (MH), 95% CI | 5 | 1.55 [0.49, 4.92] | 5 | 1.38 [0.84, 2.25] | No | |
| 3.16 Pain intensity 'early' (1 h) | SMD (IV), 95% CI | 6 | 0.57 [0.31, 0.84] | 6 | 0.57 [0.31, 0.84] | No | |
| 3.18 Additional analgesia required | RR (IV,), 95% CI, 0/0 cell counts | 6 | 9.27 [3.73, 23.03] | 6 | 10.86 [4.37, 26.95] | No | |
| 3.19 Rate of caesarean delivery | RR (MH), 95% CI | 9 | 0.99 [0.81, 1.21] | 9 | 0.96 [0.79, 1.18] | No | |
| 4. Remifentanil (PCA) versus remifentanil (continuous IV) | |||||||
| 4.1 Respiratory depression (< 8 breaths/min) | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 2 | 0.98 [0.00, 1.0E12]] | No | |
| 4.3 Hypotension | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 2 | 0.98 [0.00, 1.0E12] | No | |
| 4.4 Bradycardia | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 2 | 0.98 [0.00, 1.0E12] | No | |
| 4.5 Nausea (and vomiting) | RR (MH), 95% CI | 2 | 0.85 [0.28, 2.54] | 2 | 0.81 [0.38, 1.73] | No | |
| 4.8 Need for naloxone | RR (IV,), 95% CI, 0/0 cell counts | 2 | 0.98 [0.00, 1.0E12] | 2 | 0.98 [0.00, 1.0E12] | No | |
| All RR for outcomes including 0/0 cell counts (zero/zero event trials) were calculated using TSA (constant continuity correction, 0.01). Review Manager 5 produces computational errors when both the intervention and control group have zero events. By using TSA there is no possibility to choose the MH method (only IV) which may cause small deviations within results. Abbreviations: [95% CI]: 95% confidence interval; IV: Inverse Variance; MD: mean difference; MH: Mantel‐Haenszel; n: number of participants; RPCA: Remifentanil PCA; RR: risk ratio; SMD: standardised mean difference | |||||||
| Data | 0‐ and 0/0‐event trials included (TSA) | 0‐event trials included and 0/0‐event trials excluded (RevMan)1 | |||||||
| Outcome (n, studies) | 0‐events, 0/0‐events, imbalance (Yes/No) | Summary statistic | Reciprocal (1.0) | Reciprocal (0.01) | Empirical (1.0) | Empirical (0.01) | Constant (1.0) | Constant (0.01) | Constant (1.0) |
| 1.3 Oxygen desaturation (2) | 1, 0 (Yes) | RR [95% CI], P value, I2 | 0.51 [0.01, 30.22], 0.7471, 86% | 3.41 [0.82, 14.22] 0.0918, 0% | 0.57 [0.01, 24.87] 0.7699, 87% | 3.39 [0.81, 14.10], 0.0938, 0% | 0.5 [0.01, 31.95], 0.7421, 86% | 3.42 [0.82, 14.25], 0.0914, 0% | 0.5 [0.01, 31.95], 0.7421, 86% |
| 1.4 Nausea (and vomiting) (4) | 1, 0 (No) | RR [95% CI], P value, I2 | 0.54 [0.29, 0.99], 0.0460, 0% | 0.56 [0.30, 1.04], 0.0665, 0% | 0.54 [0.29, 0.99], 0.0463, 0% | 0.56 [0.30, 1.04], 0.0667, 0% | 0.54 [0.29, 0.99], 0.0461, 0% | 0.56 [0.30, 1.04], 0.0664, 0% | 0.54 [0.29, 0.99], 0.0461, 0% |
| 1.6 Pruritus (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.06, 15.71], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 1.02 [0.07, 16.06], 0.9874, 0% | 1.02 [0.00, 1.1E12], 0.9987, 0% | NA |
| 1.14 Rate of caesarean delivery (4) | 2, 0 (No) | RR [95% CI], P value, I2 | 0.69 [0.34, 1,40], 0.3084, 0% | 0.63 [0.30, 1.32], 0.2164, 0% | 0.7 [0.34, 1.43], 0.3268, 1% | 0.63 [0.30, 1.32], 0.2182, 0% | 0.70 [0.35, 1.40], 0.3103, 0% | 0.63 [0.30, 1.32], 0.2165, 0% | 0.70 [0.35, 1.40], 0.3103, 0% |
| 2.10 Need for naloxone (2) | 1, 1 (No) | RR [95% CI], P value, I2 | 0.49 [0.05, 5.29], 0.5580, 0%, | 0.03 [0.00, 1.1E8], 0.7484, 0% | NA | NA | 0.48 [0.04, 5.30], 0.5473, 0% | 0.03 [0.00, 1.8E8], 0.7549, 0% | 0.3 [0.03, 2.72], 0.2847, 0% |
| 3.3 Respiratory depression (3) | 1, 1 (Yes) | RR [95% CI], P value, I2 | 0.97 [0.56, 1.70] 0.9206, 0% | 0.91 [0.51, 1.62] 0.7506, 0% | 0.98 [0.57, 1.71] 0.9550, 0% | 0.91 [0.51, 1.62] 0.7532, 0% | 0.98 [0.56, 1.71] 0.9424, 0% | 0.91 [0.51, 1.62] 0.7518, 0% | 1.35 [0.30, 6.18], 0.6967, 37% |
| 3.4 Oxygen desaturation (3) | 1, 0 (Yes) | RR [95% CI], P value, I2 | 3.2 [1.72, 5.94], 0.0002, 46% | 2.88 [1.94, 4.27], < 0.0001, 0% | 3.04 [1.70, 5.43], 0.0002, 38% | 2.88 [1.94, 4.27], < 0.0001, 0% | 3.19 [1.72, 5.91], 0.0002, 46% | 2.88 [1.94, 4.27], < 0.0001, 0% | 3.19 [1.72, 5.91], 0.0002, 46% |
| 3.6 Hypotension (4) | 2, 1 (No) | RR [95% CI], P value, I2 | 0.59 [0.38, 0.93], 0.0225, 0% | 0.59 [0.37, 0.94], 0.0271, 0% | 0.59 [0.38, 0.93], 0.0219, 0% | 0.59 [0.38, 0.94], 0.0273, 0% | 0.59 [0.38, 0.93], 0.0225, 0% | 0.59 [0.37, 0.94], 0.0271, 0% | 0.58 [0.23, 1.48], 0.2517, 16% |
| 3.7 Bradycardia (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.07, 15.07], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 1.0 [0.07, 15.07], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA |
| 3.10 Pruritus (7) | 1, 0 (Yes) | RR [95% CI], P value, I2 | 0.75 [0.48, 1.18], 0.2182, 29% | 0.78 [0.51, 1.18], 0.2366, 21% | 0.75 [0.48, 1.18], 0.2170, 29% | 0.78 [0.51, 1.18], 0.2368, 21% | 0.75 [0.48, 1.18], 0.2154, 29% | 0.78 [0.51, 1.18], 0.2370, 21% | 0.75 [0.48, 1.18], 0.2154, 29% |
| 3.12 Apgarscore < 7 at 5 min (5) | 2, 2 (No) | RR [95% CI], P value, I2 | 1.26 [0.65, 2.43], 0.4944, 0% | 1.26 [0.62, 2.57], 0.5193, 0% | 1.28 [0.66, 2.47], 0.4596, 0% | 1.26 [0.62, 2.57], 0.5209, 0% | 1.26 [0.65, 2.43], 0.4904, 0% | 1.26 [0.62, 2.57], 0.5197, 0% | 1.28 [0.65, 2.51], 0.4801, 0% |
| 3.14 Need for naloxone (2) | 1, 1 (Yes) | RR [95% CI], P value, I2 | 0.34 [0.03, 3.82], 0.3846, 0% | 0.02 [0.00, 1.6E8], 0.7247, 0% | NA | NA | 0.46 [0.04, 4.88], 0.5200, 0% | 0.02 [0.00, 1.6E8], 0.7447, 0% | 0.2 [0.03, 1.15], 0.0720, 0% |
| 3.15 FHR/CTG abnormalities (5) | 1, 0 (No) | RR [95% CI], P value, I2 | 1.54 [0.50, 4.75], 0.4499, 46% | 1.88 [0.63, 5.61], 0.2578, 35% | 1.53 [0.52, 4.54], 0.4410, 44% | 1.88 [0.63, 5.64], 0.2600, 35% | 1.54 [0.50, 4.75], 0.4499, 46% | 1.88 [0.63, 5.61], 0.2578, 35% | 1.54 [0.50, 4.75], 0.4499, 46% |
| 3.18 Additional analgesia required (6) | 2, 1 (No) | RR [95% CI], P value, I2 | 7.47 [3.28, 16.99] < 0.0001, 0% | 9.26 [3.73, 23.03] < 0.0001, 0% | 9.66 [3.97, 23.52] < 0.0001, 0% | 9.23 [3.71, 22.95] < 0.0001, 0% | 7.65 [3.37, 17.38] < 0.0001, 0% | 9.27 [3.73, 23.03] < 0.0001, 0% | 8.1 [3.5, 18.75], < 0.0001, 0% |
| 3.19 Rate of caesarean delivery (9) | 1, 0 (No) | RR [95% CI], P value, I2 | 0.99 [0.81, 1.21], 0.9076, 0% | 1.0 [0.82, 1.22], 0.9858, 0% | 0.99 [0.81, 1.21], 0.9058, 0% | 1.0 [0.82, 1.22], 0.9857, 0% | 0.99 [0.81, 1.21], 0.9067, 0% | 1.0 [0.82, 1.22], 0.9857, 0% | 0.99 [0.81, 1.21], 0.9067, 0% |
| 3.20 Rate of assisted birth (8) | 1, 0 (No) | RR [95% CI], P value, I2 | 0.92 [0.66, 1.26], 0.5914, 0% | 0.94 [0.68, 1.30], 0.6917, 0% | 0.92 [0.66, 1.26], 0.5926, 0% | 0.94 [0.68, 1.30], 0.6918, 0% | 0.92 [0.66, 1.26], 0.5914, 0% | 0.94 [0.68, 1.30], 0.6917, 0% | 0.92 [0.66, 1.26], 0.5914, 0% |
| 3.26 Neonatal resuscitation (2) | 2, 0 (No) | RR [95% CI], P value, I2 | 1.01 [0.04, 24.25], 0.9933, 57% | 1.09 [0.00, 3.1E8], 0.9929, 0% | NA | NA | 1.02 [0.04, 25.09], 0.9901, 57% | 1.03 [0.00, 3.4E8], 0.9980, 0% | 1.02 [0.04, 25.09], 0.9901, 57% |
| 4.1 Respiratory depression (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.06, 15.66], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 0.98 [0.06, 15.37], 0.9896, 0% | 0.98 [0.00, 1.0E12], 0.9989, 0% | NA |
| 4.3 Hypotension (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.06, 15.66], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 0.98 [0.06, 15.37], 0.9896, 0% | 0.98 [0.00, 1.0E12], 0.9989, 0% | NA |
| 4.4 Bradycardia (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.06, 15.66], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 0.98 [0.06, 15.37], 0.9896, 0% | 0.98 [0.00, 1.0E12], 0.9989, 0% | NA |
| 4.8 Need for naloxone (2) | 0, 2 (No) | RR [95% CI], P value, I2 | 1.0 [0.06, 15.66], 1.0, 0% | 1.0 [0.00, 1.0E12], 1.0, 0% | NA | NA | 0.98 [0.06, 15.37], 0.9896, 0% | 0.98 [0.00, 1.0E12], 0.9989, 0% | NA |
| 1Review Manager 5 ignores zero/zero events trials and uses a constant continuity correction of 0.5 for studies with zero events in 1 arm. For the reciprocal, the empirical, and the constant approach including zero/zero‐event trials we used the TSA software. By using TSA there is no possibility to choose the Mantel‐Haenszel method (only inverse variance possible) which may cause small deviations within results. We performed sensitivity analyses by using different approaches for handling of zero event trials (reciprocal, empirical, and constant approach) in meta‐analysis with two or more studies. A) reciprocal approach ; value (k): 1.0, 0.01 Adds a factor of the reciprocal of the size of the opposite treatment arm to the cells which accounts for imbalance in group sizes. B) empirical approach ; value (k): 1.0, 0.01 All studies without zero events are used to calculate a pooled effect estimate. Using this effect estimate a continuity correction factor can be calculated which produces an estimated effect close to the pooled estimated effect in the studies with zero events in both arms. C) constant approach ; value (k): 1.0, 0.01 A value of 0.5 or 0.005, respectively, is added to each group in a 2 x 2 table; thus 1 participant is added to each intervention arm. Abbreviations: NA: not applicable; RR: risk ratio | |||||||||
| Study | Comparator | Analgesia duration (mean ± SD, median (range)) [min] | Background infusion [µg/(kg*min)] | Bolus dose | Bolus application speed (calculated) | Bolus dose escalation on request | Lockout time [min] | Maximum dose | Total dose administered (mean ± SD, median (range [IQR]) |
| Remifentanil variable infusion, fixed bolus | 463 | 0.025 | 0.25 µg/kg | NA | 0.5 ‐ 1 µg/kg, every 15 min | 2 | 3000 µg in 4 h | 474 (188 ‐ 925) µg/h | |
| Pethidine PCA | 147.5 ± 79 | no | 40 µg | 133.33 µg/min | no | 2 | NA | NA | |
| Meperidine IM | 280 ± 55 | 0.025 | 50 µg | 2 µg/min | no | 30 | NA | NA | |
| (1) Meperidine PCA (2) Fentanyl PCA | 234 ± 136 | no | 40 µg | NA | no | 2 | 1200 µg/h | 1840 ± 1090 µg | |
| epidural | 286 ± 145 | no | 40µg | 66.67 µg/min | no | 2 | 1200 µg/h | 2817 ± 1564 µg | |
| epidural | 192 ± 116 | no | 40µg | 66.67 µg/min | no | 2 | 1200 µg/h | 1417 µg | |
| epidural | NA | 0.0 | 0.25 µg/kg | 1.5 µg/(kg*min) | no | 5 | 3000 µg in 4 h | NA | |
| Meperidine IV | NA | no | 20 µg | NA | 5 µg increments, every 15 ‐ 20 min | 3 | 1500 µg/h | 1034.5 (133 ‐ 4021) µg | |
| epidural | NA | 0.025 | 20 µg | NA | 25% increase every 15 ‐ 20 min | 3 | NA | 8.5 ± 2.2 µg/(kg*h) | |
| epidural | 236 (128 ‐ 376) | no | 30 µg | NA | increase to 40 µg or decrease to 20 µg | 3 | 40 µg per bolus | NA | |
| epidural/CSE | NA | no | 25 µg | 25 µg/min | escalation scheme (0.1 – 0.2 – 0.3 – 0.5 – 0.7 – 0.9 µg/kg) until the maximum dose of 0.9 µg/kg | 1 | 25 µg/mL + 0.9 µg/kg per bolus | NA | |
| Remifentanil IV | NA | no | 0.25 µg/kg | NA | increased to 0.4 µg/kg (if VNRS ≥ 7) | 4 | 0.4 µg/kg per bolus | 942.6 ± 86.4 µg | |
| Pethidine IM | NA | no | 25 µg (< 60 kg) or 30 µg (≥ 60 kg) | 6.67 µg/min | no | 3.75‐4.50 | 500 µg/h (calculated) | NA | |
| Remifentanil IV | 1511 | no | 0.1 µg/kg | 0.2 µg/(kg*min) | increments of 0.1 µg/kg to 0.4 µg/kg | 2 | 0.4 µg/kg per bolus | 1340 (1220 ‐ 1480 [890 ‐ 1680]) µg | |
| epidural | NA | no | 20 µg | NA | up to 60 µg | 2 min, 1 min on request | 60 µg per bolus | 1725 ± 1392 µg | |
| epidural | 162.75 ± 77.15 | no | 20 µg | NA | 10 µg increments (if VAS decrease < 2) | 3 | NA | NA | |
| Meperidine IM | NA | no | 20 µg | 60 µg/min | NA | 3 | NA | NA | |
| epidural | 225 ± 117.2 | no | 0.15 µg/kg | 100 µg/min | 0.15 µg/kg increments every 15 min | 2 | No limit | NA | |
| Pethidine PCA | 334 ± 260 | no | 0.5 µg/kg | NA | no | 2 | No limit | 3670 (120 ‐ 4880) µg (mean (range)) | |
| epidural | Max. 60 | no | 25 µg | 25 µg/min | escalation scheme (0.1 – 0.2 – 0.33 – 0.5 – 0.7 – 0.9 µg/kg) until the maximum dose of 0.9 µg/kg | 1 | 25 µg/mL + 0.9 µg/kg per bolus | 0.14 (0.08 ‐ 0.18 [0.03 ‐ 0.32]) µg/(kg*min) | |
| 1Time from the start of remifentanil analgesia until the final dose increment, 50% survival (Kaplan‐Meier cumulative event curve) Abbreviations: NA: not applicable | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 4 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 2.11 [0.72, 3.49] |
| 2 Respiratory depression (< 8 breaths/min) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Oxygen desaturation (SpO2 < 95%) Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.00, 47.37] |
| 4 Nausea (and vomiting) Show forest plot | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 0.99] |
| 5 Vomiting Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Pruritus Show forest plot | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Apgar score at 5 min Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.90] |
| 11 Pain intensity 'early' (1 h) Show forest plot | 3 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.69, ‐0.48] |
| 12 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Additional analgesia required (escape analgesia) Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 14 Rate of caesarean delivery Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.34, 1.41] |
| 15 Rate of assisted birth Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.09] |
| 16 Augmented labour Show forest plot | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.29] |
| 17 Breastfeeding initiation (feeding difficulties) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.49, 3.30] |
| 3 Hypotension Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Bradycardia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Nausea (and vomiting) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Apgar score at 5 min Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Need for naloxone Show forest plot | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.47] |
| 11 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12 NACS at 15/30 min Show forest plot | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐0.65, 2.87] |
| 13 Pain intensity 'early' (30 min/1 h) Show forest plot | 3 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.01, ‐0.00] |
| 14 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Additional analgesia required (escape analgesia) Show forest plot | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.28] |
| 16 Rate of caesarean delivery Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.99, 7.82] |
| 17 Rate of assisted birth Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.37] |
| 18 Augmented labour Show forest plot | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.59, 3.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 7 | 2135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.04] |
| 2 Apnoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Respiratory depression (< 9, < 8 breaths/min) Show forest plot | 3 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.23, 9.90] |
| 4 Oxygen desaturation (SpO2 < 92%) Show forest plot | 3 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.66, 6.32] |
| 5 Oxygen desaturation (SpO2 < 95%) Show forest plot | 3 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [2.32, 4.61] |
| 6 Hypotension Show forest plot | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.22, 1.49] |
| 7 Bradycardia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Nausea Show forest plot | 8 | 1909 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.19, 1.86] |
| 9 Vomiting Show forest plot | 6 | 1840 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.25, 2.13] |
| 10 Pruritus Show forest plot | 7 | 1852 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.18] |
| 11 Sedation (1 h) Show forest plot | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.03, 1.39] |
| 12 Apgar score ≤ 7 (< 7) at 5 min Show forest plot | 5 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.65, 2.51] |
| 13 Apgar score at 5 min Show forest plot | 3 | 137 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.27, 0.39] |
| 14 Need for naloxone Show forest plot | 2 | 1170 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.85] |
| 15 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 5 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.49, 4.92] |
| 16 Pain intensity 'early' (1 h) Show forest plot | 6 | 235 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [0.31, 0.84] |
| 17 Pain intensity 'late' (2 h) Show forest plot | 4 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [0.66, 2.26] |
| 18 Additional analgesia required Show forest plot | 6 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 8.10 [3.50, 18.75] |
| 19 Rate of caesarean delivery Show forest plot | 9 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.81, 1.21] |
| 20 Rate of assisted birth Show forest plot | 8 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.26] |
| 21 Augmented labour Show forest plot | 6 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 22 Umbilical cord base excess (artery) Show forest plot | 3 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.65, 0.72] |
| 23 Umbilical cord base excess (venous) Show forest plot | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.39, 2.30] |
| 24 Umbilical cord pH (artery) Show forest plot | 5 | 1245 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 25 Umbilical cord pH (venous) Show forest plot | 4 | 1299 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 26 Neonatal resuscitation Show forest plot | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.04, 25.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Respiratory depression (< 8 breaths/min) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Hypotension Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bradycardia Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Nausea (and vomiting) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.28, 2.54] |
| 6 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Sedation (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Need for naloxone Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Pain intensity 'early' (1 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Pain intensity 'late' (2 h) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Additional analgesia required (escape analgesia) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Neonatal resuscitation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with pain relief Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Oxygen desaturation (SpO2 < 95%) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Hypotension Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Bradycardia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Nausea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Vomiting Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Pruritus Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Apgar score < 7 at 5 min Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Need for naloxone Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 FHR/CTG abnormalities, non‐reassuring fetal status Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Additional analgesia required (escape analgesia) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12 Rate of caesarean delivery Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Augmented labour Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Umbilical cord base excess (artery) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Umbilical cord base excess (venous) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Umbilical cord pH (artery) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Umbilical cord pH (venous) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18 Neonatal resuscitation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

