乳房温存術後の女性に対する腫瘍床ブースト照射
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Accrual: 1995 to 1998 Single‐centre | |
| Participants |
| |
| Interventions | ARM 1: ARM 2: | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization was done by the study coordinator (S.P.)" and "patients were randomly allocated to treatment options by a sealed‐envelope system in blocks of 20".Budapest 2002, page 616, paragraph 2. |
| Allocation concealment (selection bias) | Unclear risk | Quote "patients were randomly allocated to treatment options by a sealed‐envelope system in blocks of 20". Budapest 2002, page 616, paragraph 2. Allocation concealment appears to have been done, although the description was incomplete, which contributed to the judgement of unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants: not mentioned, unlikely to have been done. Personnel: not done, but in view of prespecified protocol of follow‐up with regular mammography, unlikely to have introduced bias. Quote "Patients were seen every 3 months in the first 2 years after RT, and every 6 months thereafter. Mammography, chest X‐ray, breast and abdominal ultrasound examinations, bone scan, and blood tests were performed at least annually. Local recurrence was defined ... proven by histological examination." Budapest 2002, page 618, paragraph 4. Unlikely to be a source of bias in view of the prespecified schedule for follow‐up visits and investigations. Local recurrence required biopsy information, which would reduce the risk of bias in evaluation of this outcome. |
| Blinding of outcome assessment (detection bias) | High risk | Quote "Secondary endpoints were ... late side effects of skin and subcutaneous tissues scored by the RTOG/EORTC late radiation morbidity scheme, and cosmetic results scored by a 4‐grade scale (excellent/good/fair/poor), as suggested by Perez et al." Budapest 2002, page 616, paragraph 4. Outcomes were measured by the treating physician. Because the assessment of subjective outcomes was not blinded, we judged this domain as at high risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions: 2 (ineligibility: early distant metastasis). Quote "One patient refused boost irradiation, but was analysed according to the assigned treatment group." Budapest 2002, page 616, paragraph 4. Postrandomisation exclusions are detailed without information on treatment arm, but with reasons. We deemed this outcome as at low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods section: Primary: ipsilateral breast tumour recurrence; occurrence of local, regional, or distant relapse (whichever came first) for relapse‐free survival. Secondary: death from breast cancer, late side effects of skin and subcutaneous tissues scored by the RTOG/EORTC late radiation morbidity scheme, and cosmetic results scored on a 4‐grade scale (excellent/good/fair/poor), as suggested by Perez and colleagues (Perez 1996). Outcomes reported in paper:
We did not have access to the study protocol, so judged this domain as at unclear risk of bias. |
| Other bias | Unclear risk | Because this was an interim report (prospectively planned however), we judged it as at unclear risk of bias. |
| Methods | Accrual: 1989 to 1996 Multicentre: 31 centres in 9 countries | |
| Participants | 5569 participants randomised in the trial. 5318 participants with stage I or II (T1‐2, N0‐1, M0) breast cancer who had undergone macroscopically complete surgical removal of the tumour load and axillary dissection, with microscopically completely excised tumour.
| |
| Interventions | ARM 1: Intervention details (n = 2661): WBI 50 Gy in 25 fractions in 5 weeks + boost dose of 16 Gy aimed at the tumour bed (in 8 fractions with fast electrons or tangential photon fields; or 15 Gy by means of an iridium‐192 implant at a dose rate of 0.5 Gy per hour). BED 87.8 Gy. ARM 2: Comparator details (n = 2657): WBI 50 Gy in 25 fractions in 5 weeks. BED 66.7 Gy. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Exploratory endpoints:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Patients were centrally randomised at the EORTC Headquarters according to a minimisation algorithm (variance method) in a 1:1 ratio." "Factors used in the minimization were age, menopausal status, presence of extensive ductal carcinoma in situ (ten or more ducts involved), clinical tumour size, nodal status and institute where the patient received treatment." Bartelink 2015, page 48, paragraph 3‐4. We judged this domain as at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote "Patients were centrally randomised at the EORTC Headquarters according to a minimisation algorithm (variance method) in a 1:1 ratio." "Neither patients nor investigators were masked to treatment allocation" Bartelink 2015, page 48, paragraph 3‐4. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants: not mentioned, unlikely to have introduced bias. Personnel: not done, but in view of prespecified protocol of follow‐up with regular mammography, unlikely to have introduced bias. Quote "patients were followed up two or three times per year for 5 years, then once per year by mammography and clinical examination." Bartelink 2015, page 48, paragraph 8. Local recurrence is defined as any recurrence within the treated breast, confirmed by histology or cytology (protocol). Unlikely to be a source of bias in view of the prespecified schedule for follow‐up visits and investigations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote "The cosmetic outcome and assessment of therapy sequelae will be scored regularly. Therefore colour print photographs or slides have to be taken immediately after surgery (before radiotherapy), and thereafter every 3 years. ... An external review panel will score qualitatively the cosmetic result, based on the pictures, using a 4 point grading scale." Protocol, page 26, paragraph 2. Quote "At each visit except baseline, the physician (not blinded for the treatment arm) scored the grade of fibrosis (none/minimal/moderate/severe) for the whole breast and for the boost area" Collette 2008, page 2588, paragraph 8. We would have judged this domain as at low risk of bias based on the use of a grading scale and the blinding of assessors; however, since the physician was not blinded to the treatment arm to score fibrosis, we assessed it as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions: 0 Attritions: 0 Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes in the protocol
Outcomes in the results
We had access to the study protocol and all prespecified endpoints were reported, so we judged this domain as at low risk of bias. |
| Other bias | Low risk | No other sources of bias identified. |
| Methods | Accrual: 1986 to 1992 2 centres: Hospices civils de Lyon and Centre Léon Bérard | |
| Participants |
| |
| Interventions | ARM 1: Intervention details (n = 521): WBI 50 Gy cobalt 60/20 fractions of 2.5 Gy 4 per week + boost 10 Gy 9 or 12 MeV 4 fractions 1 week. BED 86.4 Gy. ARM 2: Comparator details (n = 503): WBI 50 Gy cobalt 60/20 fractions of 2.5 Gy 4 per week. BED 72.1 Gy. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Stratification factors were the pathologic stages of the tumour (T1 v T2) and node (N0 v N1‐2)." Lyon, page 964, paragraph 2. We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Quote "Patients were entered onto the study by the radiation oncologist when first evaluated after surgery, and were randomised at the start of irradiation." Lyon, page 964, paragraph 2. We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants: not mentioned, unlikely to have been done. Personnel: not done, but in view of prespecified protocol of follow‐up with regular mammography, unlikely to have introduced bias. Quote "Clinical follow‐up evaluation was performed every 4 months during the first 2 years, every 6 months up to the fifth year, and then annually. Bilateral mammography was performed every year." Lyon, page 964, paragraph 2. Local recurrence is defined as any recurrence within the treated breast, without further specifying if this required biopsy information. Unlikely to be a source of bias in view of the prespecified schedule for follow‐up visits and investigations. |
| Blinding of outcome assessment (detection bias) | High risk | Quote "Toxicity was evaluated only as teleangiectasias, assessed regularly by the clinical oncologist during follow‐up evaluation as follows: 0, absent; 1, a few visible areas of teleangiectasia in the tumour bed; 2, teleangiectasia that covered one quadrant of the breast; and 3, obvious teleangiectasia over more than one quadrant. The general cosmetic score, assessed by both the clinical oncologist and the patients themselves, was as follows: 1, excellent; 2, good; 3, fair; 4, poor." Lyon, page 965, paragraph 2. Because the assessment of subjective outcomes was not blinded, we judged this domain as at high risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Quote "Two patients presented with bilateral synchronous breast cancer, which corresponded to four cases. Since this situation had not been planned for in the initial protocol, these two patients were excluded after randomization. ... Forty patients had not received the assigned treatment: 31 in the control group who had erroneously received a boost, and nine in the experimental group who had not received the assigned boost. The analysis was performed on an intent‐to‐treat basis." Lyon, page 965, paragraph 4. Quote "Thirty‐two patients (17 in the control group and 15 in the experimental group) were lost to follow‐up after treatment. In 4 years, 194 patients were lost to follow‐up for local recurrence in the control group and 171 in the experimental group." Lyon, page 965, paragraph 6. Postrandomisation exclusions are detailed without information on treatment arm, but with reasons. There was no postrandomisation attrition. We judged this domain as at low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods section Primary outcome: time to local recurrence (any recurrence within the irradiated breast). Secondary outcomes: overall survival; disease‐free survival; local, regional, or metastatic relapse; telangiectasia; general cosmetic score. Outcomes reported in paper
We did not have access to the study protocol, so judged this domain as at unclear risk of bias. |
| Other bias | Low risk | No other sources of bias identified. |
| Methods | Accrual: 1987 to 1994 RCT Nice, France | |
| Participants |
| |
| Interventions | ARM 1: Intervention details: WBI 50 Gy/25 fractions + boost 10 Gy/5 fractions. BED 79.9 Gy. ARM 2: Comparator details: WBI 50 Gy/25 fractions. BED 66.7 Gy. | |
| Outcomes | Primary outcome: local control. | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | High risk | We did not have access to this information in the abstract, so judged this domain as at high risk of bias. |
| Blinding of outcome assessment (detection bias) | High risk | We did not have access to this information in the abstract, so judged this domain as at high risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | No exclusions or attrition of participants reported. We would have judged this domain as at low risk of bias, however since only the abstract was available, we assessed it as at unclear risk of bias. |
| Selective reporting (reporting bias) | High risk | Only the results on local control are described in the abstract. Since no protocol was available and no further results on local recurrence were reported, we judged this domain as at high risk of bias. |
| Other bias | Unclear risk | Because this was an interim report and only the abstract was available, we judged this domain as at unclear risk of bias. |
| Methods | Accrual: 1996 to not reported Multicentre: St George and Wollongong hospitals, NSW and Liverpool Hospital, Sydney | |
| Participants |
| |
| Interventions | ARM 1: Intervention details: WBI 45 Gy/25 fractions + boost 16 Gy/8 fractions. BED 78.6 Gy. ARM 2: Comparator details: WBI 50 Gy/25 fractions. BED 66.7 Gy. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | We did not have access to the study protocol, so judged this domain as at unclear risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel: not mentioned, unlikely to have been done. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "Digital photographs were taken at 1, 2 and 3 years as well as at 5 and 10 years post‐surgery by investigator P.G." SGW 2014, page 230, paragraph 3. Quote "the relative breast retraction assessment was performed by a single observer using another custom written computer software." SGW 2012, page 685, paragraph 2. Also, the BCCT.core software was used. Tumour recurrences were classified as ipsilateral in‐breast recurrence. Quote "Patients were assessed 6 weeks after radiotherapy, every 6 months for 2 years, then annually thereafter with annual breast imaging. We judged this domain as at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Concerning digital photographs: quote "A five‐person panel (two radiation oncologists, one breast surgeon, one breast clinical nurse consultant, one radiotherapy clinical nurse specialist; four women, one man) all with experience in the treatment of breast cancers were asked to evaluate the images" "The treated breast was compared with the untreated breast and scored using a 4‐point scale developed by Harris et al." Evaluation was done independently. SGW 2012, page 683, paragraph 5. Quote "Functional assessments were performed by asking the patient to score the degree of arm swelling, arm pain, limitation of arm movement, loss of feeling in fingers, breast sensitivity and breast tenderness. For the cosmetic assessment, the patient was asked to compare the treated with the untreated breast and score the overall result. SGW 2013, page 116, paragraph 5. EORTC QLQ‐C30 was used for quality of life. Quote "At each follow‐up visit the clinician was asked to assess the cosmetic outcomes. Fibrosis and edema were scored as nil, mild, marked and severe and overall cosmesis was scored as excellent, good, fair and poor. The patient‐self‐assessment .... normal, excellent, good, fair, poor." SGW 2012, page 684, paragraph 2; page 685, paragraph 1. As we had no information on the blinding of assessors, we judged this domain as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | 346 women allocated to the experimental group, 342 to the control group. At 5 years 253 and 260 were alive, respectively, at the St George Hospital and for 183 and 202, respectively, digital photographs were available. (Figure 1) SGW 2012, page 683. Quote: "... randomized 688 patients into the boost and no boost arms. 609, 580, and 428 patients had baseline, 5 and 10 years cosmetic data available, respectively." No exclusions or attrition of participants reported. We judged this domain as at low risk of bias. |
| Selective reporting (reporting bias) | High risk | Outcomes in methods section
Outcomes in results
The results on local control are only briefly described in Millar 2009 and Graham 2007. Since no protocol was available and no further results on local recurrence were reported, we judged this domain as at high risk of bias. |
| Other bias | Low risk | No other sources of bias identified. |
BED: biologically equivalent dose
BRA: breast retraction assessment
ER: oestrogen receptor
HDR‐BT: high‐dose‐rate brachytherapy
MeV: megaelectron volt
N: lymph node
pBRA: percentage breast retraction assessment
PR: progesterone receptor
RCT: randomised controlled trial
RT: radiation therapy
RTOG/EORTC: Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer
RNI: regional nodal irradiation
WBI: whole‐breast irradiation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Prospective randomized controlled clinical trial of external beam radiotherapy + interstitial brachytherapy for breast cancer after breast‐conserving surgery |
| Methods | Accrual: 2017 to 2022 Single‐centre |
| Participants |
|
| Interventions | ARM 1: Intervention details (n = 50): WBI + interstitial brachytherapy boost. ARM 2: Comparator details (n = 50): WBI. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 1 February 2017 |
| Contact information | |
| Notes |
DCIS: ductal carcinoma in situ
WBI: whole‐breast irradiation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local control Show forest plot | 5 | 8315 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.55, 0.75] |
| Analysis 1.1  Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 1 Local control. | ||||
| 2 Local control ‐ sensitivity analysis Show forest plot | 3 | 6963 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.52, 0.73] |
| Analysis 1.2  Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 2 Local control ‐ sensitivity analysis. | ||||
| 3 Local control ‐ subgroup +40 years Show forest plot | 2 | 5058 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.53, 0.81] |
| Analysis 1.3 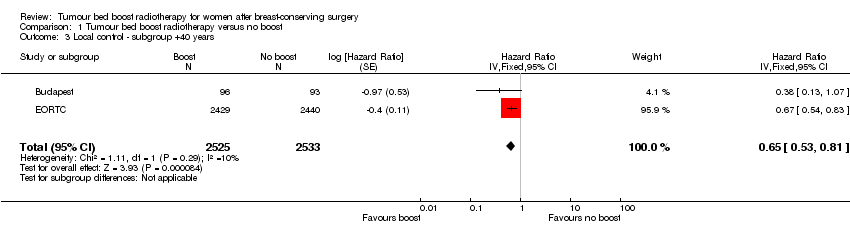 Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 3 Local control ‐ subgroup +40 years. | ||||
| 4 Local control ‐ subgroup low boost dose Show forest plot | 2 | 1352 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.55, 1.62] |
| Analysis 1.4 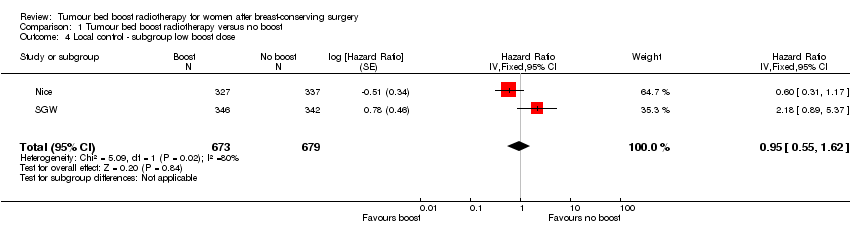 Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 4 Local control ‐ subgroup low boost dose. | ||||
| 5 Overall survival Show forest plot | 2 | 6342 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.94, 1.14] |
| Analysis 1.5  Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 5 Overall survival. | ||||
| 6 Disease‐free survival Show forest plot | 3 | 6549 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| Analysis 1.6  Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 6 Disease‐free survival. | ||||
| 7 Late toxicity, pBRA Show forest plot | 2 | 1526 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.18, 0.93] |
| Analysis 1.7  Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 7 Late toxicity, pBRA. | ||||
| 8 Cosmesis, Panel Show forest plot | 2 | 1116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.86] |
| Analysis 1.8 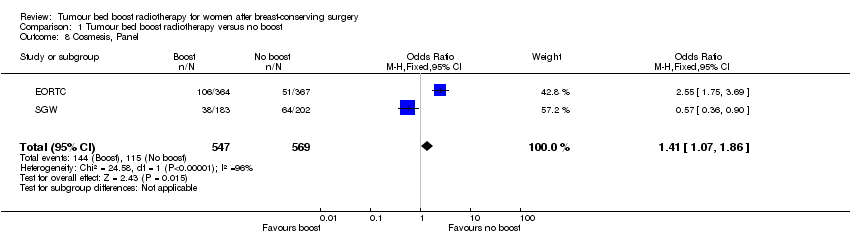 Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 8 Cosmesis, Panel. | ||||
| 9 Cosmesis, Physician Show forest plot | 2 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.93, 2.69] |
| Analysis 1.9 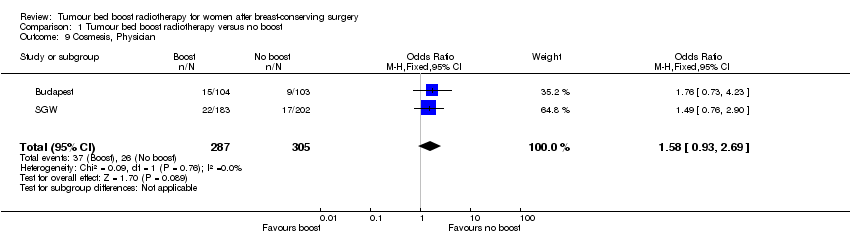 Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 9 Cosmesis, Physician. | ||||

Illustration of the breast retraction assessment (BRA) measurements as reported by EORTC. BRA = √((a1 ‐ b1)² + (a2 ‐ b2)²); reference length = √(b1² + b2²); percentage breast retraction assessment = (BRA/reference length) x 100.
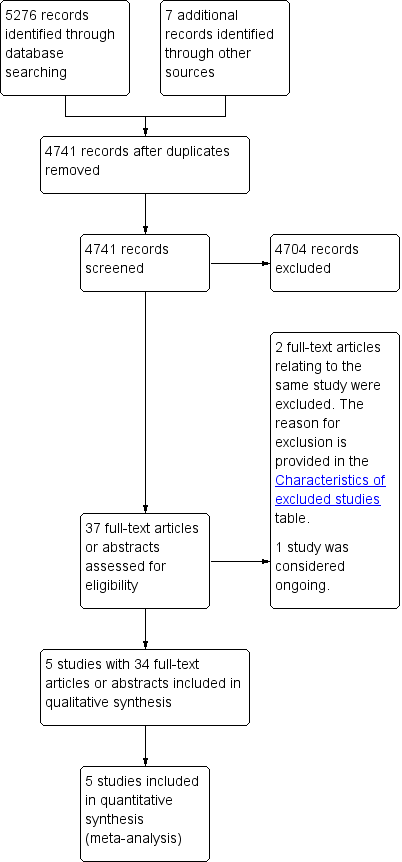
Study flow diagram.
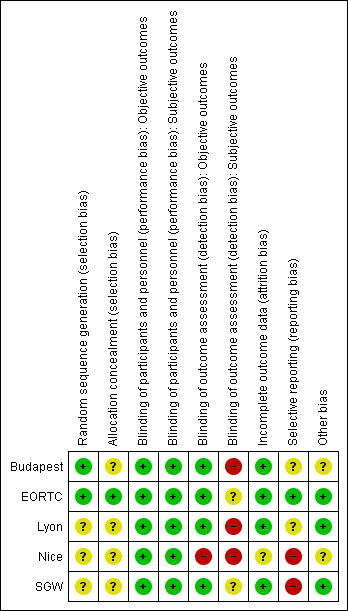
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.1 Local control.
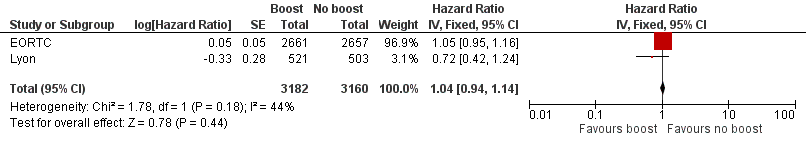
Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.5 Overall survival.
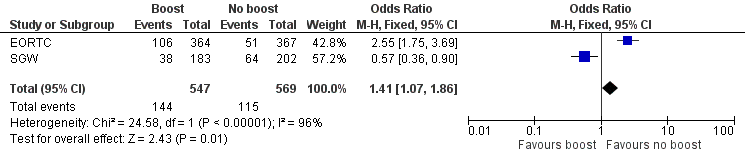
Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.8 Cosmesis, Panel.
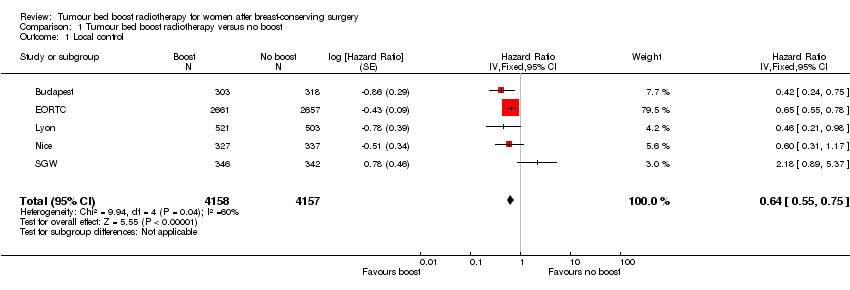
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 1 Local control.
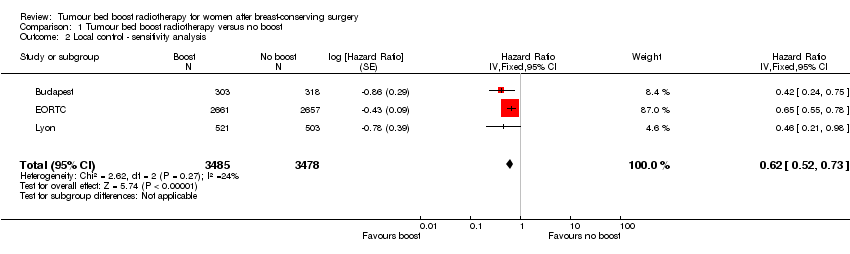
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 2 Local control ‐ sensitivity analysis.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 3 Local control ‐ subgroup +40 years.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 4 Local control ‐ subgroup low boost dose.
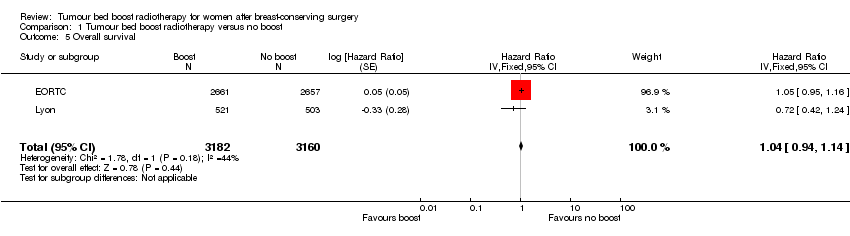
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 5 Overall survival.
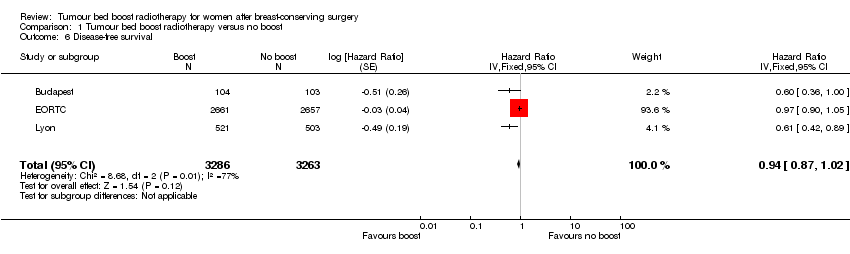
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 6 Disease‐free survival.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 7 Late toxicity, pBRA.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 8 Cosmesis, Panel.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 9 Cosmesis, Physician.
| Boost compared to no boost in breast cancer radiotherapy | ||||||
| Patient or population: breast‐conserving radiotherapy for breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk without boost | Risk with boost | |||||
| Local control: recurrence at 5 years | Study population | HR 0.64 | 8315 | ⊕⊕⊝⊝ | Nice did not report 5‐year local control. | |
| 70 per 1000 | 45 per 1000 | |||||
| Overall survival: mortality at 5 years | Study population | HR 1.04 | 6342 | ⊕⊕⊕⊝ | ||
| 91 per 1000 | 94 per 1000 | |||||
| Disease‐free survival: disease progression/mortality at 5 years | Study population | HR 0.94 | 6549 | ⊕⊕⊝⊝ | EORTC did not report 5‐year disease‐free survival. | |
| 221 per 1000 | 209 per 1000 | |||||
| Late toxicity, pBRA | Mean pBRA 8.17, range 7.55 to 10 | Mean pBRA 8.55, | MD 0.38 higher (0.18 lower to 0.93 higher) | 1526 | ⊕⊝⊝⊝ | |
| Cosmesis, panel scored: fair or poor | Study population | OR 1.41 | 1116 | ⊕⊕⊝⊝ | ||
| 202 per 1000 | 263 per 1000 | |||||
| Cosmesis, physician‐scored: fair or poor | Study population | OR 1.58 | 592 | ⊕⊝⊝⊝ | ||
| 85 per 1000 | 128 per 1000 | |||||
| Sensitivity analysis: local control ‐ recurrence at 5 years | Study population | HR 0.62 | 6963 | ⊕⊕⊕⊕ | ||
| 74 per 1000 | 47 per 1000 | |||||
| Subgroup analysis: local control ‐ > 40 years old, recurrence at 5 years | Study population | HR 0.65 | 5058 | ⊕⊕⊕⊕ | ||
| 59 per 1000 | 39 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1High risk of selective reporting in one study. | ||||||
| Organ tissue | 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Skin | None | Slight atrophy; pigmentation change; some hair loss | Patchy atrophy; moderate telangiectasia; total hair loss | Marked atrophy; gross telangiectasia | Ulceration |
| Subcutaneous tissue | None | Slight induration (fibrosis) and loss of subcutaneous fat | Moderate fibrosis but asymptomatic; slight field contracture; < 10% linear reduction | Severe induration and loss of subcutaneous tissue; field contracture > 10% linear measurement | Necrosis |
| RTOG/EORTC: Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer | |||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local control Show forest plot | 5 | 8315 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.55, 0.75] |
| 2 Local control ‐ sensitivity analysis Show forest plot | 3 | 6963 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.52, 0.73] |
| 3 Local control ‐ subgroup +40 years Show forest plot | 2 | 5058 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.53, 0.81] |
| 4 Local control ‐ subgroup low boost dose Show forest plot | 2 | 1352 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.55, 1.62] |
| 5 Overall survival Show forest plot | 2 | 6342 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.94, 1.14] |
| 6 Disease‐free survival Show forest plot | 3 | 6549 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 7 Late toxicity, pBRA Show forest plot | 2 | 1526 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.18, 0.93] |
| 8 Cosmesis, Panel Show forest plot | 2 | 1116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.86] |
| 9 Cosmesis, Physician Show forest plot | 2 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.93, 2.69] |

