乳房温存術後の女性に対する腫瘍床ブースト照射
Abstract
Background
Breast‐conserving therapy, involving breast‐conserving surgery followed by whole‐breast irradiation and optionally a boost to the tumour bed, is a standard therapeutic option for women with early‐stage breast cancer. A boost to the tumour bed means that an extra dose of radiation is applied that covers the initial tumour site. The rationale for a boost of radiotherapy to the tumour bed is that (i) local recurrence occurs mostly at the site of the primary tumour because remaining microscopic tumour cells are most likely situated there; and (ii) radiation can eliminate these causative microscopic tumour cells. The boost continues to be used in women at high risk of local recurrence, but is less widely accepted for women at lower risk. Reasons for questioning the boost are twofold. Firstly, the boost brings higher treatment costs. Secondly, the potential adverse events are not negligible. In this Cochrane Review, we investigated the effect of the tumour bed boost on local control and side effects.
Objectives
To assess the effects of tumour bed boost radiotherapy after breast‐conserving surgery and whole‐breast irradiation for the treatment of breast cancer.
Search methods
We searched the Cochrane Breast Cancer Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (January 1966 to 1 March 2017), Embase (1980 to 1 March 2017), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov on 1 March 2017. We also searched the European Society of Radiotherapy and Oncology Annual Meeting, the St Gallen Oncology Conferences, and the American Society for Radiation Oncology Annual Meeting for abstracts.
Selection criteria
Randomised controlled trials comparing the addition and the omission of breast cancer tumour bed boost radiotherapy.
Data collection and analysis
Two review authors (IK and CW) performed data extraction and assessed risk of bias using Cochrane's 'Risk of bias' tool, resolving any disagreements through discussion. We entered data into Review Manager 5 for analysis and applied GRADE to assess the quality of the evidence.
Main results
We included 5 randomised controlled trials analysing a total of 8325 women.
Local control appeared to be better for women receiving a tumour bed boost compared to no tumour bed boost (hazard ratio (HR) 0.64, 95% confidence interval (CI) 0.55 to 0.75; 5 studies, 8315 women, low‐quality evidence). Overall survival did not differ with or without a tumour bed boost (HR 1.04, 95% CI 0.94 to 1.14; 2 studies, 6342 women, moderate‐quality evidence). Disease‐free survival did not differ with or without a tumour bed boost (HR 0.94, 95% CI 0.87 to 1.02; 3 studies, 6549 women, low‐quality evidence). Late toxicity scored by means of percentage of breast retraction assessment did not differ with or without a tumour bed boost (mean difference 0.38, 95% CI ‐0.18 to 0.93; 2 studies, 1526 women, very low‐quality evidence). Cosmesis scored by a panel was better (i.e. excellent or good compared to fair or poor) in the no‐boost group (odds ratio (OR) 1.41, 95% CI 1.07 to 1.85; 2 studies, 1116 women, low‐quality evidence). Cosmesis scored by a physician did not differ with or without a tumour bed boost (OR 1.58, 95% CI 0.93 to 2.69; 2 studies, 592 women, very low‐quality evidence).
We excluded two studies in a sensitivity analysis of local recurrence (because the biological equivalent dose (BED) to the tumour bed was lower, in situ tumours were included, or there was a high risk of selective reporting bias or blinding of outcome assessment bias), which resulted in a HR of 0.62 (95% CI 0.52 to 0.73; 3 studies, 6963 women, high‐quality evidence). Subgroup analysis including women older than 40 years of age yielded a HR of 0.65 (95% CI 0.53 to 0.81; 2 studies, 5058 women, high‐quality evidence).
We found no data for the outcomes of acute toxicity, quality of life, or costs.
Authors' conclusions
It appears that local control rates are increased with the boost to the tumour bed, but we found no evidence of a benefit for other oncological outcomes. Subgroup analysis including women older than 40 years of age yielded similarly significant results. Objective percentage of breast retraction assessment appears similar between groups. It appears that the cosmetic outcome is worse with the boost to the tumour bed, but only when measured by a panel, not when assessed by a physician.
PICOs
一般語訳
乳房温存術後の女性に対する腫瘍床ブースト照射
論点
乳癌は、世界中の女性に最も多いがんである。主な治療戦略としては、乳房温存療法または乳房切除術の2つがある。再発リスクの低減さらに生存率改善のための標準治療としては乳房温存術後の補助放射線療法(電離放射線による治療)がある。補助放射線療法では、主に4〜5週間の全乳房放射線照射に引き続き、腫瘍床(もともと腫瘍があった部位)に対して追加照射(ブースト照射)が行われる。これは、乳癌の場合、癌の摘出部位に再発がみられる傾向があるためである。乳房を維持したまま乳癌を抑制することとは別に、乳房温存療法においては満足のいく見た目(整容性)が重要となる。
重要である理由
腫瘍床に対するブースト照射の適応ガイドラインは、明確でないことが多い。全乳房照射後にすべての女性に対して腫瘍床へのブースト照射を行うことは技術的には可能であるが、その必要性や効果よりも悪影響をもたらす可能性についてははっきりしないままである。腫瘍床へのブースト照射を追加することによって、治療費の増加および治療時間の延長はもちろん、整容性も低下する可能性がある。
本レビューの論点は、全乳房照射および乳房温存術後に腫瘍床へのブースト照射を実施しなくても、実施した場合と同様の結果が得られるかどうかであった。腫瘍床へのブースト照射を省略しても、ブースト照射した場合と同程度に癌を抑制できる必要があるだろう。また、腫瘍床へのブースト照射を省略することで、ブースト照射した場合と比べて副作用が軽減されることも重要と考えられる。
本レビューでは、合計8,325例の女性が含まれた試験5件を特定した。このエビデンスは、2017年3月1日現在のものである。腫瘍床へのブースト照射を行った場合では、行わない場合と比べ局所再発は少なかった(低い質のエビデンス)。腫瘍床へのブースト照射実施の有無によって無病生存期間あるいは全生存期間に違いがあるというエビデンスはなかった(それぞれ低い質と中等度の質のエビデンス)。パネル(外部審査員団)による整容性の評価は、腫瘍床ブースト照射群で悪いように思われた(低い質のエビデンス)。医師による整容性の評価および乳房陥没の割合に差はなかった。
この結果は、腫瘍床へのブースト照射により、整容性が悪化する可能性はあるが、腫瘍の局所制御率が向上することを示している。
Authors' conclusions
Summary of findings
| Boost compared to no boost in breast cancer radiotherapy | ||||||
| Patient or population: breast‐conserving radiotherapy for breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk without boost | Risk with boost | |||||
| Local control: recurrence at 5 years | Study population | HR 0.64 | 8315 | ⊕⊕⊝⊝ | Nice did not report 5‐year local control. | |
| 70 per 1000 | 45 per 1000 | |||||
| Overall survival: mortality at 5 years | Study population | HR 1.04 | 6342 | ⊕⊕⊕⊝ | ||
| 91 per 1000 | 94 per 1000 | |||||
| Disease‐free survival: disease progression/mortality at 5 years | Study population | HR 0.94 | 6549 | ⊕⊕⊝⊝ | EORTC did not report 5‐year disease‐free survival. | |
| 221 per 1000 | 209 per 1000 | |||||
| Late toxicity, pBRA | Mean pBRA 8.17, range 7.55 to 10 | Mean pBRA 8.55, | MD 0.38 higher (0.18 lower to 0.93 higher) | 1526 | ⊕⊝⊝⊝ | |
| Cosmesis, panel scored: fair or poor | Study population | OR 1.41 | 1116 | ⊕⊕⊝⊝ | ||
| 202 per 1000 | 263 per 1000 | |||||
| Cosmesis, physician‐scored: fair or poor | Study population | OR 1.58 | 592 | ⊕⊝⊝⊝ | ||
| 85 per 1000 | 128 per 1000 | |||||
| Sensitivity analysis: local control ‐ recurrence at 5 years | Study population | HR 0.62 | 6963 | ⊕⊕⊕⊕ | ||
| 74 per 1000 | 47 per 1000 | |||||
| Subgroup analysis: local control ‐ > 40 years old, recurrence at 5 years | Study population | HR 0.65 | 5058 | ⊕⊕⊕⊕ | ||
| 59 per 1000 | 39 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1High risk of selective reporting in one study. | ||||||
Background
Description of the condition
Breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012 (Globocan). It represents about 12% of all new cancer cases in both sexes and 25% of all cancer cases in women (Globocan). Data from the World Health Organization (WHO) mortality and population database indicate that breast cancer accounted for the largest number of predicted cancer deaths in women in Europe; the estimated age‐standardised (world) breast cancer mortality rate was 14.6 per 100,000 women in 2013 (Malvezzi 2013). As with any cancer, breast cancer can recur locally or at a distant site. Without (neo‐)adjuvant therapy, up to 30% of node‐negative and up to 70% of node‐positive breast cancers in women will recur locally or spread to another organ after surgery (Darby 2011; EBCTCG 2005).
Several unfavourable risk factors have been reported for local breast cancer recurrence after primary therapy, and predictive tools that can estimate breast cancer risk have been established. Besides nodal status, the reported risk factors are: the omission of radiotherapy/hormonal therapy/chemotherapy, young age, presence of lymphovascular invasion, presence of ductal carcinoma in situ (DCIS) besides invasive tumour, high histologic grade, large tumour diameter, and positive or dubious surgical margins (Darby 2011; Sanghani 2010; van Werkhoven 2011).
Description of the intervention
Breast‐conserving therapy (BCT), involving breast‐conserving surgery (BCS) followed by whole‐breast irradiation (WBI) and optionally a boost to the tumour bed, is a standard therapeutic option for women with early‐stage breast cancer. Randomised controlled trials have shown that local control rates and survival in women with early‐stage disease treated with BCT are comparable to treatment with mastectomy (Fisher 2002; Litière 2012). A boost to the tumour bed means that an extra dose of radiation is applied that covers the initial tumour site where the cancer is most likely to return. The rationale for a boost of radiotherapy to the tumour bed is (i) local recurrence occurs mostly at the site of the primary tumour because remaining microscopic tumour cells are most likely situated there; and (ii) radiation can eliminate these causative microscopic tumour cells.
The main goal of adjuvant radiotherapy (i.e. WBI and an optional boost to the tumour bed) after BCS is to decrease local recurrence and to permit breast conservation with minimal treatment‐induced side effects. The necessity of adjuvant radiotherapy has been demonstrated by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). The meta‐analysis conducted by the EBCTCG showed a reduction in the 10‐year first recurrence rate from 35% to 19.3%, and a breast cancer survival gain of 3.8% at 15 years when adding adjuvant radiotherapy (Darby 2011).
The standard radiotherapy regimen is to treat the whole breast with a total radiation dose of 40 to 50 Gy with an optional tumour bed boost. The boost continues to be used in women at high risk of local recurrence, but is less widely accepted for women at lower risk. Reasons for questioning the boost are twofold. Firstly, the boost brings higher treatment costs. Secondly, the potential adverse events are not negligible. Short‐term side effects of the tumour bed boost include fatigue, breast oedema, skin erythema and hyperpigmentation, while the long‐term risks include fibrosis, scarring of connective tissue, radiation pneumonitis, rib fractures, cardiac toxicity, and radiation‐induced second cancers. The National Comprehensive Cancer Network recommends a boost in women at higher risk for recurrence (NCCN). This can be achieved by external beam radiotherapy with doses of 10 to 16 Gy, or by interstitial brachytherapy. European guidelines advise a boost to the tumour bed in women with at least one of the following risk factors: age up to 50 years, grade 3 tumours, extensive DCIS, vascular invasion, and possibly in cases of non‐radical tumour excision (Senkus 2015).
How the intervention might work
After the initial treatment, a local recurrence can occur where residual microscopic cancerous cells grow back at the original site of the breast cancer. Data state that between 44% and 90% of local recurrences are located in or near the primary tumour bed (Bartelink 2007; Kuerer 2004; Vaidya 2010), and these numbers correlate well with pathological findings from Holland and colleagues (Holland 1985). To eliminate residual microscopic cancerous cells, an additional tumour bed boost is advocated (Bartelink 2015; van Werkhoven 2011).
Ionising radiation is radiation with a high energy whose ionising tracks deposit energy in cells. The total ionising radiation dose, D, is measured in energy per unit mass. Radiation mainly damages chromatin, for example causing DNA double strand breaks (DSBs). An acute dose of 1 Gy makes many thousands of ionisations in the cell's nucleus, of which a small minority quickly induce DSBs. Most DSBs are repaired within the next half hour, and a few are misrepaired. At a typical dose of several Gy, at least one misrepair usually occurs, and this misrepair can be enough to kill the cell at the next cycle of cell division. Irradiation can also form other kinds of lethal lesions, such as lethal point mutations, meaning that the effect is unrepairably lethal and not prone to repair or misrepair.
The effect of radiation on tumours is presented in the linear quadratic (LQ) model, which quantifies the effects of both unrepairable damage and repairable damage susceptible to misrepair (Kellerer 1972). The model is based on the concept of a biologically effective dose (BED) that represents the physical dose required for a given effect. BED = D [1 + d/(α/β)] ‐ γTt/α where d is the dose per fraction, α and β are the LQ parameters, γ = ln(2)/Tpot and Tpot is the potential doubling time, and Tt is the treatment time (Fowler 1989).
Improved tumour control requires an increased radiation dose within the dose‐response range of any residual tumour burden. The effect of radiation on the tumour (based on α/β) in breast tissue is no longer 10 Gy as historically assumed, but around 4.6 Gy (Hennequin 2013; START 2008). With this in mind, existing dose schedules for local tumour control should be reconsidered by calculating all BEDs of the regimens used in studies in breast cancer.
Why it is important to do this review
With the introduction of the computed tomography (CT) scan and 3D‐therapy planning in radiotherapy more than a decade ago, research has focused on optimising treatment techniques. Today, the tumour bed in breast tissue can be irradiated using a whole set of treatment techniques that include conventional radiation, 3D‐conformal radiation, intensity‐modulated radiation, intraoperative radiation, or proton irradiation. The radiation oncologist also has a broad choice of fractionation schemes (i.e. delivering the total dose in different numbers or sizes of doses per fraction) ‐ normofractionation (the standard regimen for breast irradiation equals 50 Gy in 25 fractions of 2 Gy, five days a week), hypofractionation (the total dose of radiation is divided into larger doses per fraction), or even single fraction radiotherapy ‐ and a wide range of treatment times.
Even though research has provided a wide variety of treatment options, no consensus has been reached on the necessity of and consequently the indications for the boost to the tumour bed. The absolute size of the benefit of the tumour bed boost is linked to the baseline risk of local recurrence; the highest benefit is recorded in the highest‐risk groups (Bartelink 2015). The effect of the boost dose seems to be independent of tumour characteristics such as grade or stage and adjuvant systemic treatment (Bartelink 2015). However, it may be dependent on age and surgical margin status (van Werkhoven 2011).
It is important to clearly determine those women who will benefit from having a boost to the tumour bed. In this review, we attempted to help practitioners weigh the benefit of better local control and survival against an increase in side effects (e.g. fibrosis, scarring of connective tissue), the inconvenience of a longer treatment time, and higher cost.
Objectives
To assess the effects of tumour bed boost radiotherapy after breast‐conserving surgery and whole‐breast irradiation for the treatment of breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
All parallel‐group randomised controlled trials comparing the addition and the omission of breast cancer tumour bed boost radiotherapy.
Types of participants
Women with a histological diagnosis of breast cancer for the first time (not recurrent or metastatic disease) with no prior history of malignant disease (other than basal cell carcinoma of the skin).
We only included studies in which women had been treated with BCS. Breast‐conserving surgery could include lumpectomy, wide local excision, quadrantectomy or segmental mastectomy with or without axillary dissection, axillary sampling or sentinel node biopsy. Women were not excluded based on age, race, tumour size or histological type.
Types of interventions
All women with BCS (lumpectomy, wide local excision, quadrantectomy or segmental mastectomy) followed by WBI (any standard schedule). We compared women who had received an additional boost to the tumour bed with women who had not received a boost to the tumour bed. The boost could be delivered with external beam radiation (electrons or photons) or with interstitial brachytherapy.
We excluded women who had received intraoperative radiotherapy.
We allowed systemic treatments such as hormones, chemotherapy, or monoclonal antibodies as long as they were applied equally to women in each arm of the trial.
Types of outcome measures
Primary outcomes
-
Local control, defined as the time (from randomisation) until the development of any local recurrence during follow‐up (time‐to‐event outcome). We defined local recurrence as recurrence in the ipsilateral breast (i.e. the breast in which cancer had been diagnosed), the skin and parenchyma.
-
Acute toxicity related to radiotherapy, i.e. any toxic event occurring in the breast, skin, lung, and heart within six months of completion of radiotherapy. We intended for acute toxicity to be classified according to the scales the authors used and, if possible, converted to the score from the National Cancer Institute Common Toxicity Criteria (NCI‐CTCAE v4.0).
Secondary outcomes
-
Overall survival, defined as the time from randomisation to death from any cause during follow‐up.
-
Disease‐free survival, defined as the time from randomisation to relapse (local or distant) during follow‐up.
-
Late toxicity related to radiotherapy, i.e. any toxic event occurring more than six months after radiotherapy. We classified these according to the scales the authors used, otherwise considering grade 3 or 4 toxic events according to the National Cancer Institute Common Toxicity Criteria (NCI‐CTCAE v4.0). In this review, we extracted data at 5, 10, 15, and 20 years.
-
Cosmesis, scored according to the Harvard scale in four classes: excellent, good, fair, and poor (Harris 1979).
-
Quality of life, to be classified according to validated scales the trial authors used or current scores on the EORTC Quality of Life scale (EORTC QoL).
-
Treatment costs, to be classified according to the scales the trial authors used.
Search methods for identification of studies
Electronic searches
We searched the following databases on 1 March 2017.
-
Cochrane Breast Cancer Group's Specialised Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Trials with the keywords 'randomized controlled trial, breast cancer, breast‐conserving therapy, radiotherapy and boost' were extracted from the Register and considered for inclusion in the review.
-
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2). See Appendix 1.
-
MEDLINE (via OvidSP) (January 1966 to 1 March 2017). See Appendix 2.
-
Embase (via Embase.com) (1980 to 1 March 2017). See Appendix 3.
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials. See Appendix 4.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/). See Appendix 5.
Searching other resources
Bibliographic searching
We searched reference lists of all included trials and relevant (systematic) reviews to identify other studies.
Conference proceedings
We searched the European Society of Radiotherapy and Oncology Annual Meeting, the St Gallen Oncology Conferences, and the American Society for Radiation Oncology Annual Meeting.
Data collection and analysis
Selection of studies
Two review authors (IK and CW) scanned the title, abstract, and keywords of every record identified by the search. We retrieved the full articles of all studies that potentially met our inclusion criteria according to types of studies, participants, and interventions. Two review authors (IK and CW) assessed these full articles for inclusion, resolving any disagreements by discussion. We recorded excluded trials in the 'Characteristics of excluded studies' table with reasons for their exclusion. We included studies in English, French, German, and Dutch.
Data extraction and management
Two review authors (IK and CW) independently conducted data extraction, resolving any disagreements by discussion. We recorded data on data extraction forms that the same two review authors developed and piloted independently. For those studies with more than one publication, we extracted data from all publications, but considered the final or updated version of each study as the primary reference. We entered data into Review Manager 5 software (RevMan).
Where possible, we extracted the following information from the included primary studies to data extraction forms.
-
Publication details: year of publication, authors.
-
Methods: accrual, length of follow‐up, trial setting and design, country.
-
Participants: inclusion and exclusion criteria, population data (i.e. number of women, age of analysed women, time of diagnosis, tumour stage, receptor status, menstrual status, other adjuvant treatments, surgical margins), withdrawals.
-
Interventions: doses, regimen, scheme, length, type of radiotherapy.
-
Outcome measures.
We extracted outcome data on local control, overall survival, disease‐free survival, late toxicity related to radiotherapy, and cosmesis. No data were available for acute toxicity and costs. SGW reported results on quality of life for the whole group without specifying whether or not participants had received a tumour bed boost.
For time‐to‐event outcomes we used the spreadsheet developed by Matthew Sydes (Tierney 2007). We used calculation methods 3 or 7 to derive ln(HR) and standard error using hazard ratios (HRs) and confidence intervals or P values and number of events on each arm.
The Budapest trial reported late toxicity related to radiotherapy according to the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) late radiation morbidity scheme for skin and subcutaneous tissue: grade 0, 1, 2, 3 (Table 1) (Cox 1995). The physician scored fibrosis as none, minimal, moderate, or severe in the EORTC trial. These were both 4‐point scales; the results were dichotomised into 0/1 = none/minimal versus 2/3 = moderate/severe, with occurrence of 2/3 = moderate/severe being counted as 'events'. Breast retraction assessment (BRA) was measured in the SGW trials and percentage of BRA (pBRA) was measured in EORTC and SGW (Figure 1). The Lyon trial scored telangiectasia (spider veins) in the tumour bed as (0) absent, (1) a few visible, (2) covered one quadrant of the breast, and (3) obvious telangiectasia over more than one quadrant; we extracted data on 0 versus 1 and 2, counting 1 and 2 as 'events'. SGW reported on hypopigmentation (loss of skin colour) of the nipple‐areolar complex.
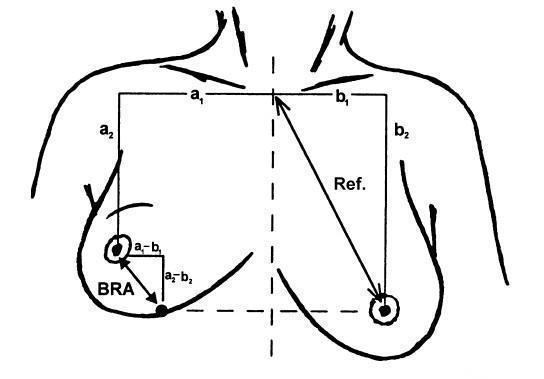
Illustration of the breast retraction assessment (BRA) measurements as reported by EORTC. BRA = √((a1 ‐ b1)² + (a2 ‐ b2)²); reference length = √(b1² + b2²); percentage breast retraction assessment = (BRA/reference length) x 100.
| Organ tissue | 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Skin | None | Slight atrophy; pigmentation change; some hair loss | Patchy atrophy; moderate telangiectasia; total hair loss | Marked atrophy; gross telangiectasia | Ulceration |
| Subcutaneous tissue | None | Slight induration (fibrosis) and loss of subcutaneous fat | Moderate fibrosis but asymptomatic; slight field contracture; < 10% linear reduction | Severe induration and loss of subcutaneous tissue; field contracture > 10% linear measurement | Necrosis |
RTOG/EORTC: Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer
Cosmetic results were scored by a 4‐grade scale (excellent/good/fair/poor) according to Harris and colleagues (Table 2) (Harris 1979). We extracted data comparing excellent and good versus fair and poor, considering fair and poor as an 'event'. Scores were reported by a panel and by the BCCT.core software in the EORTC and SGW trials. Cosmesis was rated by a physician and by the participant in the Lyon and the SGW trials. Cosmesis was rated by the physician in Budapest.
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
To facilitate comparison of radiation doses given at different doses per fraction, we converted the radiation doses to the BED according to the following formula BED = D [1 + d/(α/β)] ‐ γTt/α where d is the dose per fraction, α and β are the LQ parameters, γ = ln(2)/Tpot and Tpot is the potential doubling time, and Tt is the treatment time (Fowler 1989). Values of α/β = 4.6 Gy, Tpot = 15 days, and α = 0.3 were used.
Assessment of risk of bias in included studies
Two authors (IK and CW) independently assessed the risk of bias of the included studies. Objective outcomes were local control, overall survival, disease‐free survival, cosmesis score by the BCCT.core software, pBRA, and treatment costs. Subjective outcomes were acute toxicity, late toxicity, cosmesis scored by the participant, a panel, or the physician, and quality of life. We used Cochrane's 'Risk of bias' tool, which contains domain‐based judgements (Higgins 2011). A judgement of 'low risk' indicated a low risk of bias, 'high risk' indicated a high risk of bias, and 'unclear' indicated an uncertain risk of bias, according to the specific criteria for each domain. These domains were as follows.
-
Sequence generation: was the allocation sequence adequately generated? We rated this domain as 'low risk' if the investigators described a random component in the sequence generation process such as: referring to a random‐number table, using a computer random‐number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, or minimisation.
-
Allocation concealment: was allocation adequately concealed? We rated this domain as 'low risk' if participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal the allocation: central allocation or sequentially numbered, opaque, sealed envelopes.
-
Blinding of participants and personnel, and outcome assessors: was knowledge of the allocated interventions adequately prevented during the study? We rated this domain as 'low risk' if there was no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by the lack of blinding; or if blinding of participants, key study personnel, and outcome assessment was ensured, and it was unlikely that the blinding could have been broken.
-
Incomplete outcome data: were outcome data adequately assessed and accounted for? We rated this domain as 'low risk' if there were no missing outcome data; if the reasons for missing outcome data were unlikely to be related to the true outcome; if missing outcome data ware balanced in numbers across intervention groups with similar reasons for missing data across groups; if for dichotomous data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; if for continuous outcome data, the plausible effect size among missing outcomes was not enough to have a clinically relevant impact on the observed effect size; or if missing data were imputed using appropriate methods.
-
Selective outcome reporting: were reports of the study free of the suggestion of selective outcome reporting? We rated this domain as 'low risk' if the study protocol was available and all of the study's prespecified outcomes that were of interest in the review were reported in the prespecified way; or when the study protocol was not available, if it was clear that the published reports included all expected outcomes, including those that were prespecified.
-
Other potential threats to validity: was the study apparently free of other problems that could put it at risk of bias? We rated this domain as 'low risk' if the study appeared to be free of other sources of bias.
Measures of treatment effect
We presented dichotomous outcomes (two distinct possible outcomes, i.e. acute and late toxicity, cosmetic assessments) as odds ratios (ORs) with 95% confidence intervals (CIs). An OR equal to 1 indicated no difference in the incidence between the experimental group and the control group; an OR greater than 1 indicated that the incidence in the experimental group was higher than in the control group; and an OR less than 1 indicated that the incidence in the experimental group was lower than in the control group.
We summarised continuous outcomes (i.e. pBRA) using the mean difference (MD) with 95% CI. An MD greater than 0 indicated that the level in the experimental group was higher than in the control group; an MD less than 0 indicated that the level in the experimental group was lower than in the control group.
We expressed time‐to‐event outcomes (i.e. local recurrence, overall survival, disease‐free survival) as hazard ratios (HRs) with 95% CIs. If we could not obtain the HR and associated variances directly from the trial publication or from the authors, we obtained these data indirectly using the methods described by Parmar and colleagues by employing the other available summary statistics or data extracted from published Kaplan‐Meier curves (Parmar 1998).
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Missing trial‐level information
In the case of missing or unavailable trial‐level information, as specified under Data extraction and management we contacted trial authors by email with a request for this information.
Missing participant‐level data in published studies
No study reported results based on an inappropriate analysis set (e.g. per protocol where there is a difference in protocol violations between both treatment groups).
Assessment of heterogeneity
We used the I² statistic to assess the percentage of total variation across studies due to heterogeneity rather than chance, considering a value greater than 50% to indicate substantial heterogeneity (Higgins 2011). We also performed visual inspection of the forest plots. Where we identified significant heterogeneity, we explored the reasons for it and made a cautious attempt to explain heterogeneity.
Assessment of reporting biases
The reporting biases included publication, time‐lag, multiple publication, location, citation, and language biases. We contacted trial authors regarding the reasons for the non‐reporting of data for some outcomes.
Data synthesis
We performed meta‐analyses based on summary information from published papers for all outcome variables for which at least two studies provided comparable data. We used the fixed‐effect model for all outcome types because the number of trials was always fewer than 10.
We used the Mantel‐Haenszel method to calculate pooled results for binary outcomes, and the inverse variance method for continuous outcomes. We used the generic inverse‐variance method for time‐to‐event data (DerSimonian 1986).
We conducted meta‐analyses using Review Manager 5 software (RevMan). We summarised the quality of the evidence for local control, overall survival, and disease‐free survival at five years as well as late toxicity and cosmesis using the GRADE approach and developing a 'Summary of findings' table.
To calculate the absolute risk for the control group for time‐to‐event outcomes, we obtained the event rate at five years from reported event rates. We entered these estimated values in GRADEpro (GRADEpro), which automatically calculated the corresponding absolute risks for the intervention group at five years.
Subgroup analysis and investigation of heterogeneity
To correct for differences in dosage per fraction and overall treatment time, we used the BED to compare different radiation fractionation schemes.
EORTC, Nice, and SGW only included women with negative surgical margins. Data were insufficient to perform subgroup analysis according to the margin status. We performed a subgroup analysis for women older than 40 years of age.
Sensitivity analysis
We performed sensitivity analyses on the different levels of risk of bias. We performed the analysis both with and without trials at high risk of bias in order to assess the effect of risk of bias on the results.
Results
Description of studies
See: Characteristics of included studies table.
Results of the search
We identified and screened 5276 references from the major medical databases and 7 additional references from other sources. After exclusion of duplicates and screening of references (by title or abstract), we evaluated 37 full‐text references and excluded two references (relating to the one study) because the study design was not randomised controlled trial (see Characteristics of excluded studies table). We considered one study as ongoing (ChiCTR‐IOR‐17010342). The remaining 34 references related to five studies (Budapest: 2 records; EORTC: 22 records; Lyon: 1 record; Nice: 1 record; SGW: 8 records) (see Figure 2). No studies were awaiting classification.
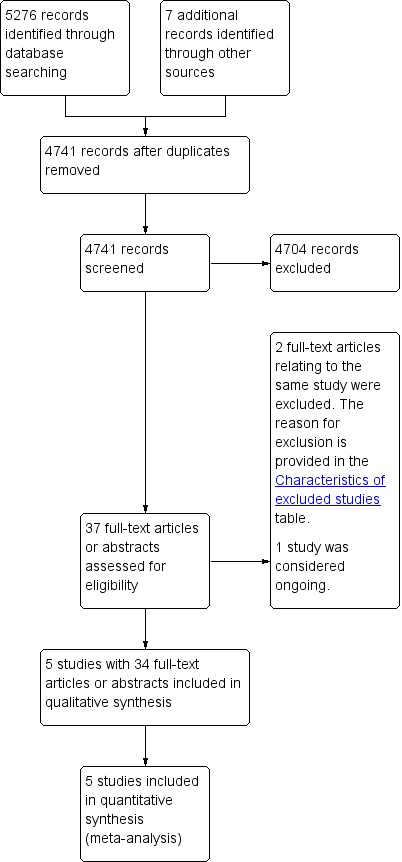
Study flow diagram.
Included studies
Design
Five studies randomised 8325 women (Budapest;EORTC;Lyon;Nice;SGW). These studies enrolled women from August 1995 to October 1998 (Budapest), May 1989 to June 1996 (EORTC), January 1986 to June 1992 (Lyon), 1987 to 1994 (Nice), and accrual was started in September 1996 without reporting an end date (SGW).
Sample size
Budapest enrolled 627 women; EORTC enrolled 5569 participants, of whom 5318 were randomised between a boost and no boost; Lyon enrolled 1028 participants, Nice enrolled 664 participants; and SGW enrolled 688 participants.
Setting
The Budapest and Nice trials were single‐centre trials. The Lyon and SGW were conducted in centres in France and Australia, respectively. The EORTC trial was a multicentre trial in nine countries.
Participants
Budapest included women with stage I‐II breast cancer excluding bilateral cases. EORTC enrolled 5569 patients with stage I or II (T1‐2, N0‐1, M0) invasive breast cancer who were younger than 70 years old. All patients in whom the tumour excision was microscopically complete according to the local pathologist (n = 5318) were randomised to either a boost and no boost to the tumour bed. Patients were excluded if they had pure carcinoma in situ. Lyon included women with invasive ductal carcinoma ≤ 3 cm and "free" pathological margins (absence of detectable cancer cells at the inked margin) and who were younger than 70 years old. SGW included women with T in situ, T1, T2 N0‐3 breast cancer. Nice included patients with invasive breast cancer treated with axillary dissection.
Interventions
Experimental arm
Whole‐breast irradiation:
plus boost irradiation:
-
high‐dose‐rate brachytherapy 12 to 14.25 (Budapest)
-
15 Gy by means of an iridium‐192 implant at a dose rate of 0.5 Gy per hour (EORTC)
-
10 Gy in 4 fractions in 1 week (Lyon)
-
10 Gy in 5 fractions in 1 week (Nice)
The BEDs for the experimental arm were 87.8 Gy in Budapest and EORTC, 86.4 Gy in Lyon, 79.9 Gy in Nice, and 78.6 Gy in SGW.
Control arm
Whole‐breast irradiation:
The BEDs for the control arm were 66.7 Gy in Budapest, EORTC, Nice, and SGW, and 72.1 Gy in Lyon.
Co‐interventions
Budapest only provided detailed population information on the first 207 patients with at least 3 years of follow‐up: 18% received hormonal therapy alone, 18% chemotherapy alone, and 6% hormonal therapy and chemotherapy. There were no significant differences between the boost and no boost group. In EORTC, participants with axillary lymph node involvement received adjuvant systemic therapy (7%): premenopausal women received chemotherapy (38%) and postmenopausal women received tamoxifen (62%). In Lyon, chemotherapy was administered in 22% of women and endocrine therapy in 30%. Premenopausal women with positive lymph nodes received adjuvant chemotherapy. For postmenopausal women, tamoxifen was always started in node‐positive women and sometimes in node‐negative women. In SGW, 20% of women received chemotherapy and 39% received endocrine therapy. Nice did not report numbers on systemic therapy.
Outcomes
Primary outcomes
-
All five studies reported local control.
-
No study reported on acute toxicity related to radiotherapy.
Secondary outcomes
-
Three studies reported disease‐free survival (Budapest; EORTC; Lyon).
-
Four studies reported on late toxicity related to radiotherapy. Budapest described grade 2 to 3 side effects of skin and subcutaneous tissues. EORTC reported the grade of fibrosis as scored by the physician (none, minimal, moderate, severe) and the pBRA. Lyon scored telangiectasias as 0, 1, 2, or 3 and reported the scores for 0 versus 1 or 2. SGW reported the BRA, pBRA, and nipple‐areolar complex hypopigmentation.
-
Cosmesis was rated by a physician in three studies (Budapest; Lyon; SGW). Participant scoring was reported in Lyon and SGW. A panel of physicians scored cosmesis in EORTC and SGW. EORTC and SGW evaluated cosmesis with the BCCT.core software.
-
SGW reported on quality of life, without analysis for boost versus no boost.
-
None of the studies reported treatment costs.
Excluded studies
We excluded two reports based on full‐text analysis (see Characteristics of excluded studies).
Risk of bias in included studies
Refer to Figure 3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged Budapest and EORTC to be at low risk of bias for sequence generation. Lyon, Nice, and SGW did not clearly report random sequence generation or allocation concealment and so were assessed as at unclear risk of bias. We considered allocation concealment as low risk for EORTC. Allocation concealment appears to have been done in Budapest, although the description was incomplete, which contributed to the judgement of unclear risk of bias.
Blinding
Objective outcomes
We judged blinding of participants and personnel and blinding of outcome assessment for objective outcomes as at low risk of bias in Budapest, EORTC, Lyon, and SGW. No study mentioned blinding of participants, which would have been difficult to do with this intervention, but the lack of blinding was unlikely to have introduced bias. The trials also did not mention blinding of physicians. This would have been difficult to do with the intervention, but we considered failure to do so less likely to have introduced bias because the follow‐up (including mammographic screening) interval was prespecified in all four studies. No information on follow‐up was provided in Nice, so we judged assessed this domain as at high risk of bias.
Subjective outcomes
We judged blinding of participants and personnel for subjective outcomes as at low risk of bias in Budapest, EORTC, Lyon, Nice, and SGW.
Colour‐printed photographs were taken at predefined time points in EORTC, and an external review panel scored the cosmetic result. Based on the use of a grading scale and the blinding of assessors, we judged this domain as at low risk of bias. However, since the physician was not blinded to the treatment arm to score fibrosis, we judged this domain as at unclear risk of bias. The clinical oncologist assessed subjective outcomes at follow‐up in Lyon and Budapest; because there was no blinding we judged these studies as at high risk of bias. No information on the blinding of assessors was provided for SGW, therefore we judged this domain as at unclear risk of bias. No information on subjective outcomes was provided in the abstract of the Nice study, so we judged this domain as at high risk of bias.
Incomplete outcome data
In Budapest, two participants were excluded with reasons and no attrition was reported. In Lyon, two participants with bilateral breast cancer were excluded; there was no postrandomisation attrition. Because postrandomisation exclusions were detailed with reasons, we deemed these studies to be at low risk of bias. In EORTC and SGW, no exclusion or attrition was reported, so we judged this domain as at low risk of bias. No information was provided in the abstract of the Nice study, so we judged this domain as at unclear risk of bias.
Selective reporting
We did not have access to the study protocols for Budapest and Lyon, therefore we judged this domain as at unclear risk of bias. All prespecified endpoints in the protocol were reported in the results of EORTC, so we judged this domain as at low risk of bias. The results on local control in Nice were only available in an abstract, and results for SGW were briefly described in Millar 2009 and Graham 2007. Since no protocol was available and no further results on local recurrence have been reported, we judged this domain as at high risk of bias.
Other potential sources of bias
EORTC, Lyon, Nice, and SGW were at low risk of bias for other potential sources of bias. Because Budapest and Nice were interim reports, we judged them as at unclear risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison Boost compared to no boost in breast cancer radiotherapy
See: summary of findings Table for the main comparison.
Primary outcomes
Local control
There were 737 local recurrences in the 8315 women studied in five studies.
Local control appeared to be better with the administration of a tumour bed boost (HR 0.64, 95% CI 0.55 to 0.75; P < 0.00001; 5 studies, 8315 women, low‐quality evidence, Analysis 1.1; Figure 4). There was evidence of heterogeneity on visual inspection and statistical testing (I² = 60%). We performed a sensitivity analysis based on excluding Nice and SGW because BED to the tumour bed was lower compared to the other three studies (79.9 Gy and 78.6 Gy, respectively compared to 86.4 Gy to 87.8 Gy); there was a high risk of selective reporting bias and blinding of outcome assessment bias for Nice; and in situ tumours were included in SGW. We found that a tumour bed boost versus no tumour bed boost was associated with significantly better local control (HR 0.62, 95% CI 0.52 to 0.73; P < 0.00001; 3 studies, 6963 women, high‐quality evidence; Analysis 1.2). There was no evidence of heterogeneity on visual inspection and statistical testing (I² = 24%).

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.1 Local control.
We performed subgroup analysis including only women older than 40 years of age. Local control appeared to be better with the administration of a tumour bed boost in the subgroup analysis (HR 0.65, 95% CI 0.53 to 0.81; P < 0.0001; 2 studies, 5058 women, high‐quality evidence; Analysis 1.3). There was no heterogeneity on either visual inspection or statistical testing (I² = 10%).
Acute toxicity
There were no data on acute toxicity.
Secondary outcomes
Overall survival
We found no difference in overall survival with or without a boost to the tumour bed with data from 1683 deaths in two studies with 6342 women (HR 1.04, 95% CI 0.94 to 1.14; P = 0.44; moderate‐quality evidence; Analysis 1.5; Figure 5). There was no heterogeneity on either visual inspection or statistical testing (I² = 44%).

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.5 Overall survival.
Disease‐free survival
We found no difference in disease‐free survival with or without a boost to the tumour bed with data from 2810 events in three studies with 6549 women (HR 0.94, 95% CI 0.87 to 1.02; P = 0.12; low‐quality evidence; Analysis 1.6). There was no evidence of heterogeneity on visual inspection, but there was on statistical testing (I² = 77%). For sensitivity analysis, no studies were at high risk of bias for the domains of objective outcomes and other bias.
Late toxicity
We found no difference in pBRA with or without a boost to the tumour bed with data from 1526 women in two studies (MD 0.38, 95% CI ‐0.18 to 0.93; P = 0.18; very low‐quality evidence; Analysis 1.7). There was evidence of heterogeneity on visual inspection and statistical testing (I² = 75%).
Cosmesis
Panel‐scored cosmesis appeared to be better without the administration of a boost to the tumour bed with data from 229 women with fair or poor scoring in two studies involving 1116 women (OR 1.41, 95% CI 1.07 to 1.86; P = 0.01; low‐quality evidence; Analysis 1.8; Figure 6). There was evidence of heterogeneity on visual inspection and statistical testing (I² = 96%).

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.8 Cosmesis, Panel.
Treating physician‐scored cosmesis did not differ significantly between boost and no‐boost groups, however the confidence interval was very wide (OR 1.58, 95% CI 0.93 to 2.69; P = 0.09; 2 studies, 592 women; very low‐quality evidence; Analysis 1.9). There was no evidence of heterogeneity on visual inspection and statistical testing (I² = 0%).
There were no data per subgroup in the Lyon study, so a meta‐analysis was not possible.
Quality of life
There were no data on quality of life.
Treatment costs
There were no data on treatment costs.
Discussion
Summary of main results
Local control appeared to be better with a tumour bed boost than without. We found no evidence of a difference in overall survival or disease‐free survival with or without a tumour bed boost. Percentage of breast retraction assessment did not appear affected with a boost to the tumour bed. Panel‐scored cosmesis was significantly better (i.e. good or excellent) if no boost was administered. Physician‐scored cosmesis did not differ significantly between groups.
We found no data for the effects of the boost to the tumour bed on acute toxicity, quality of life, and treatment costs.
Based on these findings, the tumour bed boost appears to be associated with a better local control. Local recurrence was decreased by 25 per 1000 women (range 17 to 41 per 1000) at 5 years.
Overall completeness and applicability of evidence
Overall, the validity of our results was high due to the high number of analysed women and the comprehensive search strategy. However, there were some limitations in completeness and applicability of evidence due to clinical heterogeneity between the studies, duration of follow‐up, and little or no useful information available for some outcome measures.
Clinical heterogeneity between the trials
There was evident clinical heterogeneity in both the study participants and the interventions as follows.
-
The radiotherapy dose varied between trials. The tumour bed boost dose was lower in SGW (78.6 Gy) and Nice (79.9 Gy) compared to the other three studies (86.4 Gy to 87.8 Gy). We conducted the sensitivity analysis for local control excluding these two studies.
-
The radiotherapy boost technique differed, but there was no evidence of a difference in local control related to the boost technique (Verhoeven 2015).
-
The techniques used to define the target volume differed. In EORTC, the placement of a clip in the booster area was advised and a margin of 1.5 cm to 3 cm was taken as clinical target volume. In Budapest, the walls of the excision cavity were marked with four to six titanium clips and a 1‐ to 1.5‐centimetre margin was used to define the clinical target volume. No information was provided on target volume delineation in Lyon, Nice, and SGW. The lack of a definition for target delineation and the omission of robust quality assurance meant that the radiotherapy in some of the studies was not reproducible. We thus cannot be certain of the dose delivered and the irradiated volume.
-
In SGW, 59 out of 688 women had an in situ carcinoma. The other four studies included only women with an invasive breast carcinoma.
-
EORTC included the largest patient population and contributed over 79% of the weight in the meta‐analysis for local control, overall survival, and disease‐free survival.
Duration of follow‐up
The median length of follow‐up differed among all five studies: 60 months in Budapest, 17.2 years in EORTC, 73 months in Nice, 3.3 years in Lyon, and 6 years in SGW. A meta‐analysis by the Early Breast Cancer Trialists’ Collaborative Group confirmed the relationship between tumour control and overall survival. By adding radiotherapy to breast‐conserving surgery, the 10‐year any first‐recurrence rate decreased from 35.0% to 19.3%, and breast cancer survival gains were 3.8% at 15 years. In other words, the prevention of four recurrences at 10 years avoids one breast cancer death at 15 years (Darby 2011). Since four out of the five studies included in this review reported a median follow‐up of less than seven years, the effect on survival might still become apparent with longer follow‐up in the future.
Little or no useful information available for some outcome measures
The Budapest study states that recurrence rates were compared for women younger than 40 years of age, but no P value is provided. In the EORTC study, cosmesis is measured using the BCCT.core software, however no general cosmetic score was provided, so a comparison or meta‐analysis with other studies was impossible. SGW reported quality of life data for the whole group of analysed women and did not provide a subgroup analysis for the boost versus no‐boost group.
There was heterogeneity for the outcome of local control. We performed a sensitivity analysis excluding Nice and SGW because we judged selective reporting or blinding of outcome assessment, or both, as at high risk of bias. We made this assessment because the dose to the tumour bed was significantly lower compared to the other trials and because in situ carcinomas were included in the data set (SGW). In the meta‐analysis for pBRA and for the panel‐scored cosmesis, there was heterogeneity attributed to the lower dose to the tumour bed in SGW, however there were no signs of heterogeneity in physician‐scored cosmesis. As only two studies were included in the meta‐analyses, we could not conduct a sensitivity analysis.
Treatment era
The five studies recruited women two decades ago, and treatment approaches have changed over time. The use of systemic therapy, especially hormonal therapy, in the included studies was low. Also, overall recurrence rates have decreased over time. This treatment era effect is not only based on the increased use of systemic therapy, but also on improvements in preoperative breast imaging and postoperative delineation of the lumpectomy cavity and greater attention to the obtainment of negative surgical margins (Canavan 2014; van Laar 2013). Absolute numbers and effects of the tumour bed boost might thus be lower in the current population.
Quality of the evidence
The available evidence does allow for the drawing of conclusions with respect to the objective of the review: to assess the effects of tumour bed boost radiotherapy after breast‐conserving surgery and whole‐breast irradiation for the treatment of breast cancer. We found five studies with 8325 women.
Local recurrence
We downgraded for risk of bias because there was a high risk of bias for selective reporting in two studies. Regarding inconsistency (I² = 60%), there was considerable clinical heterogeneity with respect to radiotherapy dose and the use of quality assurance procedures, so we downgraded the quality of the evidence. We did not downgrade for indirectness or imprecision. Given our systematic literature search, we did not downgrade for publication bias. The overall GRADE quality of evidence for this outcome was low.
The sensitivity analysis for local recurrence excluded two studies. We did not downgrade for risk of bias, indirectness, inconsistency, imprecision, or publication bias. The overall GRADE quality of evidence for this outcome was high.
We did not downgrade the quality of the evidence for the subgroup analysis for local recurrence in women at least 40 years old based on risk of bias, indirectness, inconsistency, imprecision, or publication bias. The overall GRADE quality of evidence for this outcome was high.
Overall survival
We did not downgrade for risk of bias, inconsistency, indirectness, or publication bias. We downgraded for imprecision because one of the two included studies had a very wide confidence interval. The overall GRADE quality of evidence for this outcome was moderate.
Disease‐free survival
Only two out of the five studies reported five‐year disease‐free survival. We did not downgrade for risk of bias, indirectness, or publication bias. We downgraded for inconsistency due to statistical heterogeneity. We downgraded for imprecision because both included studies had a very wide confidence interval. The overall GRADE quality of evidence for this outcome was low.
pBRA
We downgraded for risk of bias because here was a high risk of bias for selective reporting in one study. Regarding inconsistency (I² = 75%), there was considerable clinical heterogeneity with respect to radiotherapy dose and the use of quality assurance procedures, so we downgraded the quality of the evidence. We downgraded for imprecision because both included studies had a very wide confidence interval. The overall GRADE quality of evidence for this outcome was very low.
Panel‐scored cosmesis
We downgraded for risk of bias because there was a high risk of bias for selective reporting in one study. Regarding inconsistency (I² = 96%), there was considerable clinical heterogeneity with respect to radiotherapy dose and the use of quality assurance procedures, so we downgraded the quality of the evidence. We did not downgrade for indirectness, imprecision, or publication bias. The overall GRADE quality of evidence for this outcome was low.
Physician‐scored cosmesis
We downgraded by two points for risk of bias because there was a high risk of bias for selective reporting in one study and a high risk of bias for blinding of outcome assessment in the other study. We downgraded for imprecision because both included studies had a very wide confidence interval. We did not downgrade for indirectness, inconsistency, or publication bias. The overall GRADE quality of evidence for this outcome was very low.
Potential biases in the review process
We believe we have identified all relevant, completed randomised controlled trials. Follow‐up was relatively short for survival analysis in all but one study. This may improve in future updates, as more information with respect to these outcomes is reported.
Agreements and disagreements with other studies or reviews
We found one systematic review with a meta‐analysis examining the role of boost irradiation in the conservative treatment of stage I‐II breast cancer (Polgár 2001). The authors reported crude local recurrence rates of 6.7% versus 3.9% when adding a boost to the tumour bed (P < 0.0001). Five studies were analysed. Besides the Budapest, EORTC, Lyon, and Nice trials, Polgár 2001 reported data from an abstract presentation by Nagykálnai on the 2nd European Congress on Senology in 1994, but to the best of our knowledge these data are no longer available, and apparently the number of participants was low and no statistical analysis was performed. Polgár 2001 did not include the SGW trial in their review. As in our meta‐analysis, the review by Polgár justifies the use of the tumour bed boost after whole‐breast irradiation. They also conclude that young age (< 50 years), positive or close surgical margins, and extensive intraductal component should be viewed as absolute indications for boost irradiation. A review by Bahadur reports that the significant effect on local control applies to all age groups, but that cosmetic results proved to be worse with the tumour bed boost and should be taken into consideration (Bahadur 2012). European Society for Medical Oncology guidelines report a 50% any‐recurrence risk reduction with boost irradiation, which they indicate for patients who have unfavourable risk factors for local control such as age less than 50 years, grade 3 tumours, extensive ductal carcinoma in situ, vascular invasion, or non‐radical tumour excision (focally, otherwise further surgery should be advocated) (Senkus 2015). NCCN guidelines advise the consideration of a tumour bed boost in the case of high risk of recurrence (such as age less than 50 years, high‐grade disease, or focally positive margins) (NCCN).

Illustration of the breast retraction assessment (BRA) measurements as reported by EORTC. BRA = √((a1 ‐ b1)² + (a2 ‐ b2)²); reference length = √(b1² + b2²); percentage breast retraction assessment = (BRA/reference length) x 100.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.1 Local control.

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.5 Overall survival.

Forest plot of comparison: 1 Tumour bed boost radiotherapy versus no boost, outcome: 1.8 Cosmesis, Panel.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 1 Local control.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 2 Local control ‐ sensitivity analysis.
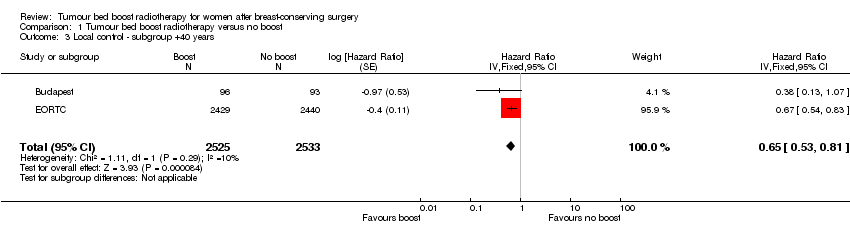
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 3 Local control ‐ subgroup +40 years.
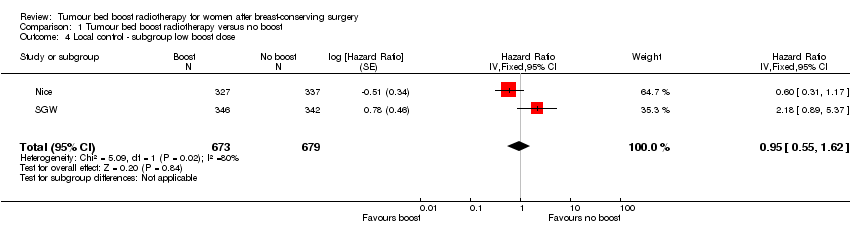
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 4 Local control ‐ subgroup low boost dose.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 5 Overall survival.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 6 Disease‐free survival.

Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 7 Late toxicity, pBRA.
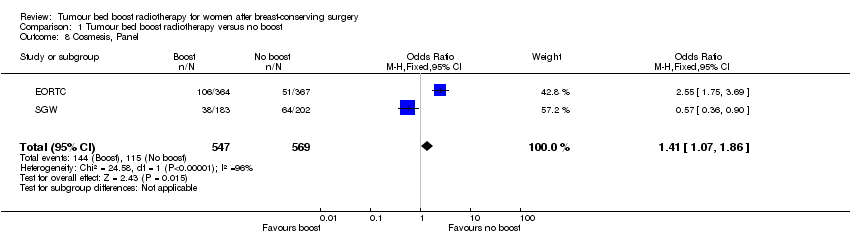
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 8 Cosmesis, Panel.
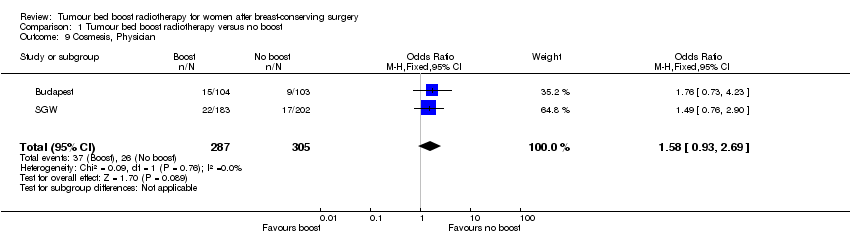
Comparison 1 Tumour bed boost radiotherapy versus no boost, Outcome 9 Cosmesis, Physician.
| Boost compared to no boost in breast cancer radiotherapy | ||||||
| Patient or population: breast‐conserving radiotherapy for breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk without boost | Risk with boost | |||||
| Local control: recurrence at 5 years | Study population | HR 0.64 | 8315 | ⊕⊕⊝⊝ | Nice did not report 5‐year local control. | |
| 70 per 1000 | 45 per 1000 | |||||
| Overall survival: mortality at 5 years | Study population | HR 1.04 | 6342 | ⊕⊕⊕⊝ | ||
| 91 per 1000 | 94 per 1000 | |||||
| Disease‐free survival: disease progression/mortality at 5 years | Study population | HR 0.94 | 6549 | ⊕⊕⊝⊝ | EORTC did not report 5‐year disease‐free survival. | |
| 221 per 1000 | 209 per 1000 | |||||
| Late toxicity, pBRA | Mean pBRA 8.17, range 7.55 to 10 | Mean pBRA 8.55, | MD 0.38 higher (0.18 lower to 0.93 higher) | 1526 | ⊕⊝⊝⊝ | |
| Cosmesis, panel scored: fair or poor | Study population | OR 1.41 | 1116 | ⊕⊕⊝⊝ | ||
| 202 per 1000 | 263 per 1000 | |||||
| Cosmesis, physician‐scored: fair or poor | Study population | OR 1.58 | 592 | ⊕⊝⊝⊝ | ||
| 85 per 1000 | 128 per 1000 | |||||
| Sensitivity analysis: local control ‐ recurrence at 5 years | Study population | HR 0.62 | 6963 | ⊕⊕⊕⊕ | ||
| 74 per 1000 | 47 per 1000 | |||||
| Subgroup analysis: local control ‐ > 40 years old, recurrence at 5 years | Study population | HR 0.65 | 5058 | ⊕⊕⊕⊕ | ||
| 59 per 1000 | 39 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1High risk of selective reporting in one study. | ||||||
| Organ tissue | 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Skin | None | Slight atrophy; pigmentation change; some hair loss | Patchy atrophy; moderate telangiectasia; total hair loss | Marked atrophy; gross telangiectasia | Ulceration |
| Subcutaneous tissue | None | Slight induration (fibrosis) and loss of subcutaneous fat | Moderate fibrosis but asymptomatic; slight field contracture; < 10% linear reduction | Severe induration and loss of subcutaneous tissue; field contracture > 10% linear measurement | Necrosis |
| RTOG/EORTC: Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer | |||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local control Show forest plot | 5 | 8315 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.55, 0.75] |
| 2 Local control ‐ sensitivity analysis Show forest plot | 3 | 6963 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.52, 0.73] |
| 3 Local control ‐ subgroup +40 years Show forest plot | 2 | 5058 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.53, 0.81] |
| 4 Local control ‐ subgroup low boost dose Show forest plot | 2 | 1352 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.55, 1.62] |
| 5 Overall survival Show forest plot | 2 | 6342 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.94, 1.14] |
| 6 Disease‐free survival Show forest plot | 3 | 6549 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 7 Late toxicity, pBRA Show forest plot | 2 | 1526 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.18, 0.93] |
| 8 Cosmesis, Panel Show forest plot | 2 | 1116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.86] |
| 9 Cosmesis, Physician Show forest plot | 2 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.93, 2.69] |

