رواسکولاریزاسیون کامل در برابر رواسکولاریزاسیون رگ درگیر به تنهایی در انفارکتوس میوکارد همراه با بالا رفتن قطعه ST در موارد درگیری چند عروق با هم
Appendices
Appendix 1. Search strategies
| CENTRAL, DARE, and HTA (Wiley) 1 MeSH descriptor: [Myocardial Infarction] explode all trees 2 ((myocard* or heart) near/3 infarct*):ab,ti,kw 3 (heart next/1 attack*):ab,ti,kw 4 ((stun* or hibernat*) near/3 myocard*):ab,ti,kw 5 'cardiogenic shock':ab,ti,kw 6 (st near/2 elevat* near/4 ('myocardial infarction' or 'myocardial infarctions' or mi)):ab,ti,kw 7 stemi:ab,ti,kw 8 {or #1‐#7} 9 MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees 10 pci:ab,ti,kw or ppci:ab,ti,kw 11 ('percutaneous coronary' near/6 (intervention* or revascularization*)):ab,ti,kw 12 ((transluminal or 'trans luminal') near/6 coronary):ab,ti,kw 13 angioplast*:ab,ti,kw 14 atherectom*:ab,ti,kw 15 (balloon near/2 (coronary or dilat*)):ab,ti,kw 16 MeSH descriptor: [Stents] explode all trees 17 stent*:ab,ti,kw 18 {or #9‐#17} 19 (multi* near/4 vessel):ab,ti,kw 20 (multivessel or 'multi‐vessel'):ab,ti,kw 21 ('infarct related' or IRA or 'non infarct related' or 'non‐IRA'):ab,ti,kw 22 (culprit or 'culprit‐only' or 'non‐culprit' or nonculprit or bystander):ab,ti,kw 23 {or #19‐#22} 24 #8 and #18 and #23 Ovid MEDLINE(R) 1946 to December Week 1 2016, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations January 03, 2017 and Ovid MEDLINE(R) Epub Ahead of Print January 03, 2017 1. exp Myocardial Infarction/ 2. ((myocard* or heart) adj3 infarct*).tw. 3. heart attack*.tw. 4. ((stun* or hibernat*) adj3 myocard*).tw. 5. cardiogenic shock.tw. 6. (ST adj2 elevat* adj4 (myocardial infarction* or MI)).tw. 7. stemi.tw. 8. or/1‐7 9. exp Percutaneous Coronary Intervention/ 10. (PCI or PPCI).tw. 11. (percutaneous coronary adj6 (intervention* or revascularization*)).tw. 12. ((transluminal or trans‐luminal) adj6 coronary).tw. 13. angioplast*.tw. 14. atherectom*.tw. 15. (balloon adj2 (coronary or dilat*)).tw. 16. exp Stents/ 17. stent*.tw. 18. or/9‐17 19. (multi* adj4 vessel).tw. 20. (multivessel or multi‐vessel).tw. 21. (infarct related or IRA or non infarct related or non‐IRA).tw. 22. (culprit or culprit‐only or non‐culprit or nonculprit or bystander).tw. 23. or/19‐22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomized.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. exp animals/ not humans.sh. 34. 32 not 33 35. 8 and 18 and 23 36. 34 and 35 EMBASE (embase.com) #28 #26 AND #27 #27 #8 AND #18 AND #23 #26 #24 NOT #25 1122006 #25 'animal'/exp OR 'nonhuman'/exp NOT 'human'/exp #24 random*:ab,ti OR placebo* OR (double NEXT/1 blind*):ab,ti #23 #19 OR #20 OR #21 OR #22 #22 culprit:ab,ti OR 'culprit‐only':ab,ti OR 'non‐culprit':ab,ti OR nonculprit:ab,ti OR bystander:ab,ti #21 'infarct related':ab,ti OR ira:ab,ti OR 'non infarct related':ab,ti OR 'non‐ira':ab,ti #20 multivessel:ab,ti OR 'multi‐vessel':ab,ti #19 (multi* NEAR/4 vessel):ab,ti #18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #17 stent*:ab,ti #16 'stent'/exp #15 (balloon NEAR/2 (coronary OR dilat*)):ab,ti #14 atherectom*:ab,ti #13 angioplast*:ab,ti #12 ((transluminal OR 'trans luminal') NEAR/6 coronary):ab,ti #11 ('percutaneous coronary' NEAR/6 (intervention* OR revascularization*)):ab,ti #10 pci:ab,ti OR ppci:ab,ti #9 'interventional cardiovascular procedure'/exp #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #7 stemi:ab,ti #6 (st NEAR/2 elevat* NEAR/4 ('myocardial infarction' OR 'myocardial infarctions' OR mi)):ab,ti #5 'cardiogenic shock':ab,ti #4 ((stun* OR hibernat*) NEAR/3 myocard*):ab,ti #3 (heart NEXT/1 attack*):ab,ti #2 ((myocard* OR heart) NEAR/3 infarct*):ab,ti #1 'heart infarction'/exp Conference Proceedings Citation Index‐ Science (CPCI‐S)‐‐1990‐present (Web of Science) # 21 #20 AND #15 AND #7 # 20 #19 OR #18 OR #17 OR #16 # 19 TS=(culprit or "culprit‐only" or "non‐culprit" or nonculprit or bystander) # 18 TS=("infarct related" or IRA or "non infarct related" or "non‐IRA") # 17 TS=(multivessel or "multi‐vessel") # 16 TS=(multi* near/4 vessel) # 15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 # 14 TS=(stent*) # 13 TS=(balloon near/2 (coronary or dilat*)) # 12 TS=(atherectom*) # 11 TS=(angioplast*) # 10 TS=((transluminal or "trans luminal") near/6 coronary) # 9 TS=(PCI or PPCI) # 8 TS=("percutaneous coronary" near/6 (intervention* or revascularization*)) # 7 #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 6 TS=(stemi) # 5 TS=(st near/2 elevat* near/4 ("myocardial infarction" or "myocardial infarctions" or mi)) # 4 TS=("cardiogenic shock") # 3 TS=((stun* or hibernat*) near/3 myocard*) # 2 TS=("heart attack" OR "heart attacks") # 1 TS=((myocard* or heart) near/3 infarct*) ClinicalTrials.gov (Expert search) multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (Standard search) multivessel OR multi vessel OR infarct related OR non infarct related OR culprit OR non culprit European (EU) Clinical Trials Register multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" Epistemonikos (http://www.epistemonikos.org) (Advance search) multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" |
Appendix 2. Survey of authors providing information on included trials
| Characteristic | Date trial author asked for additional information | Date trial author replied | Trial author provided data |
| 26 May 2016 | No reply | ||
| 26 May 2016 | No reply | ||
| 26 May 2016 | 27 May 2016 | 1 July 2016 | |
| 26 May 2016 | No reply | ||
| 26 May 2016 | No reply | ||
| 26 May 2016 | No reply | ||
| 26 May 2016 | No reply | ||
| 26 May 2016 | 31 May 2016 | Did not provide additional data | |
| 26 May 2016 | No reply |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term all‐cause mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from proportion event in control (Pc) group of 6.3% with an alpha of 2% and beta of 10%.
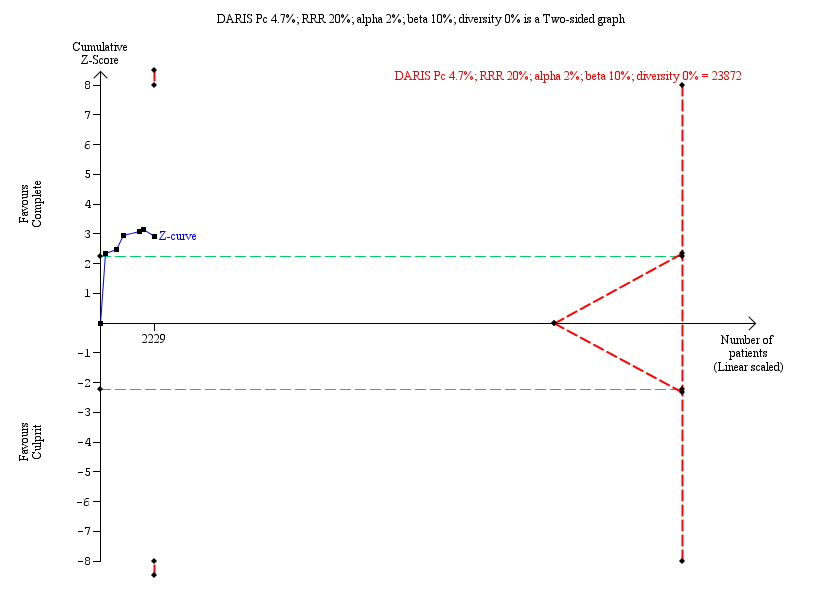
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term cardiovascular mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 4.7% with an alpha of 2% and beta of 10%.

Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term non‐fatal myocardial infarction. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 7.0% with an alpha of 2% and beta of 10%.
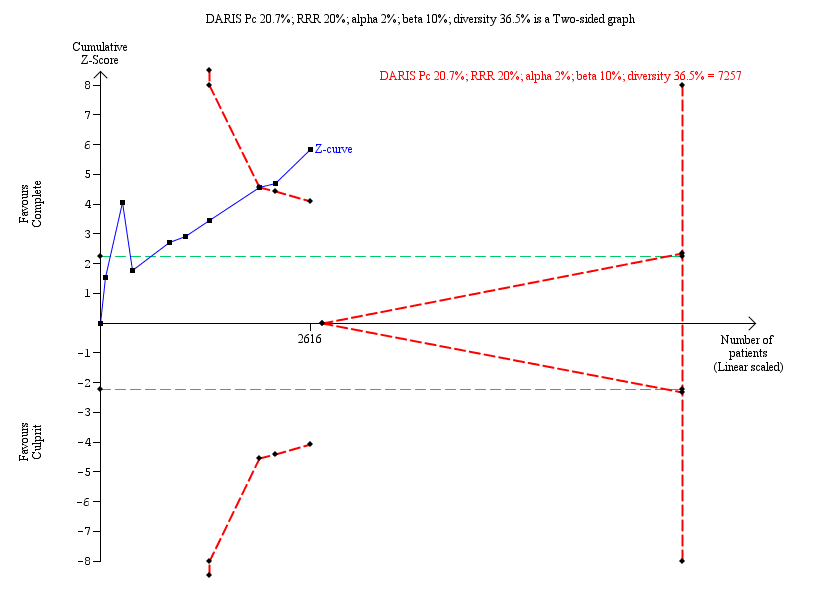
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term revascularisation. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 20.7% with an alpha of 2% and beta of 10%.

Comparison 1 Primary outcomes, Outcome 1 Long‐term all‐cause mortality.
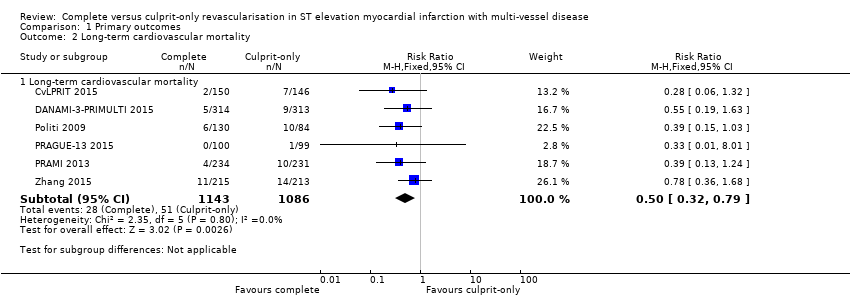
Comparison 1 Primary outcomes, Outcome 2 Long‐term cardiovascular mortality.

Comparison 1 Primary outcomes, Outcome 3 Long‐term non‐fatal myocardial infarction.
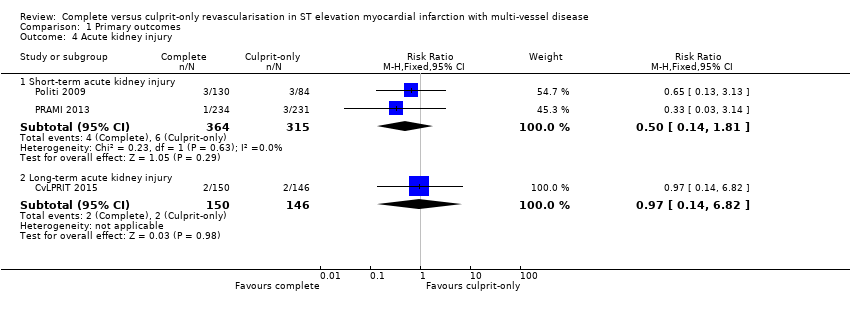
Comparison 1 Primary outcomes, Outcome 4 Acute kidney injury.
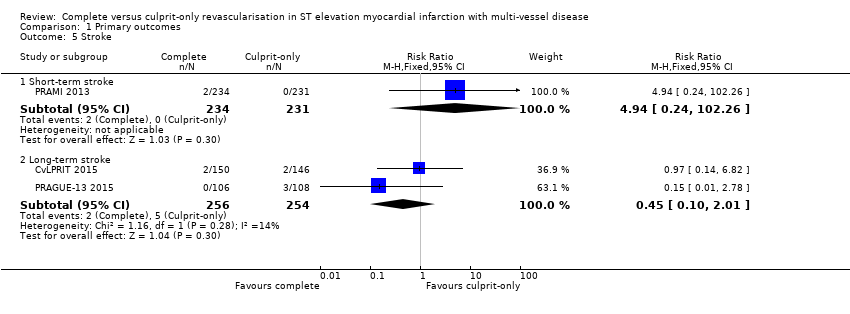
Comparison 1 Primary outcomes, Outcome 5 Stroke.

Comparison 1 Primary outcomes, Outcome 6 Bleeding.
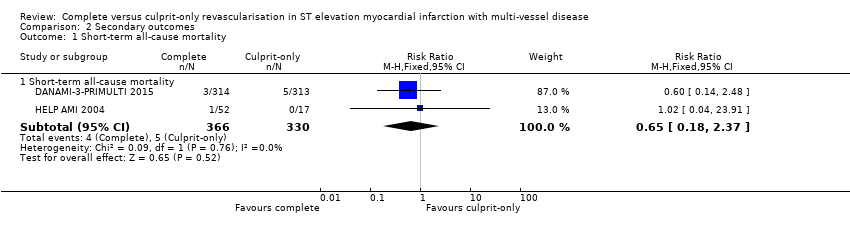
Comparison 2 Secondary outcomes, Outcome 1 Short‐term all‐cause mortality.
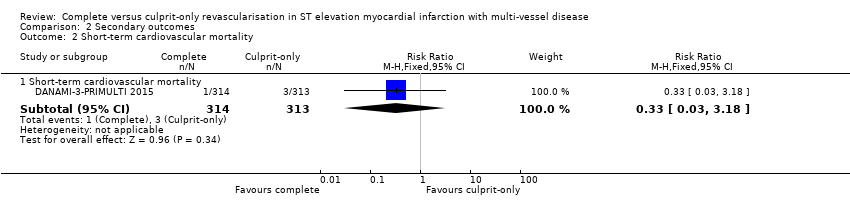
Comparison 2 Secondary outcomes, Outcome 2 Short‐term cardiovascular mortality.

Comparison 2 Secondary outcomes, Outcome 3 Short‐term non‐fatal myocardial infarction.
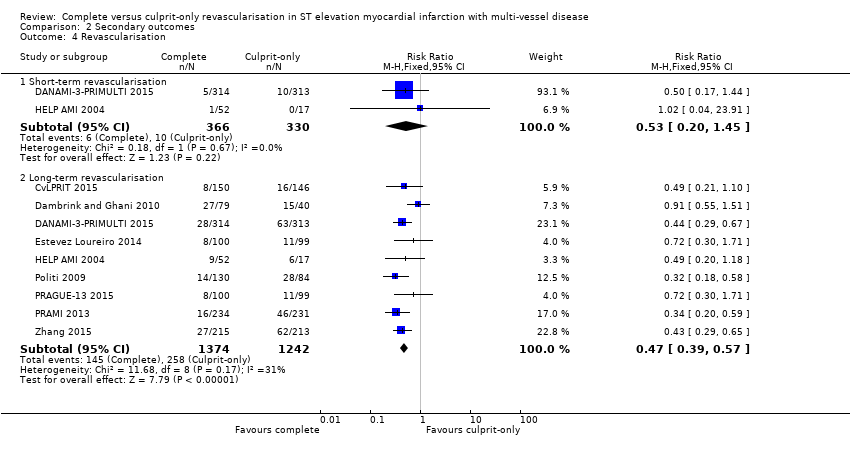
Comparison 2 Secondary outcomes, Outcome 4 Revascularisation.

Comparison 2 Secondary outcomes, Outcome 5 Cost ≥ 1 year.
| Complete revascularisation compared to culprit‐only revascularisation in ST elevated myocardial infarction with multi‐vessel disease | ||||||
| Patient or population: people with STEMI and MVD. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with culprit only | Risk with complete revascularisation | |||||
| Long‐term all‐cause mortality (≥ 1 year after the intervention) | Study population | RR 0.80 | 2417 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 63 per 1000 | 50 per 1000 | |||||
| Long‐term cardiovascular mortality (≥ 1 year after the intervention) | Study population | RR 0.50 | 2229 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 47 per 1000 | 23 per 1000 | |||||
| Long‐term myocardial infarction (≥ 1 year after the intervention) | Study population | RR 0.62 | 2099 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 70 per 1000 | 43 per 1000 | |||||
| Overall adverse events (pooled short and long term) | Study population | OR 0.84 | 4086 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 29 per 1000 | 24 per 1000 | |||||
| Short‐term all‐cause mortality (within the first 30 days after the intervention) | Study population | RR 0.65 | 696 | ⊕⊝⊝⊝ | HELP‐AMI trial did not describe in detail their methodology to analyse for bias. | |
| 15 per 1000 | 10 per 1000 | |||||
| Long‐term revascularisation (≥ 1 year after the intervention) | Study population | RR 0.47 | 2616 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 208 per 1000 | 98 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MVD: multi‐vessel disease; RCT: randomised controlled trial; RR: risk ratio; STEMI: ST elevated myocardial infarction. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to publication (reporting) bias. 2 Downgraded due to study limitations (largely risk of attrition bias and selection bias). 3 Downgraded because of indirectness: black and Hispanic people, as well as women were under‐represented. 4 Downgraded due to imprecision. | ||||||
| Study | Dates | Complete revascularisation (staged vs 1 time) | Intervention criteria in non‐culprit vessel | Mean follow‐up (years) | Description multi‐vessel disease | Country | Number of centres |
| May 2011 to May 2013 | At index procedure or before discharge. 65% of participants in invasive group had at index procedure. | > 70% diameter stenosis in 1 plane or > 50% in 2 planes. | 2.5 | Culprit vessel plus ≥ 1 non‐infarct‐related epicardial artery with ≥ 1 lesion deemed angiographically significant (> 70% stenosis in 1 plane or > 50% in 2 planes). | UK | 7 | |
| June 2004 to February 2007. | Staged 7.5 days after P‐PCI. | FFR < 0.75 and in stenosis > 90%, PCI was performed without FFR measurement. PCI was with BMS or DES. | 3 | ≥ 1 significant stenosis (> 50% stenosis in ≥ 1 view) in ≥ 2 major epicardial coronary arteries, or the combination of a side branch and a main epicardial vessel provided that they supplied different territories. | The Netherlands | 1 | |
| March 2011 to February 2014 | Staged 2 days after P‐PCI. | FFR < 0.8 and those > 90% stenotic arteries visually. | 2.2 | Significant stenosis (> 50% stenosis visually in arteries > 2 mm diameter) in ≥ 1 of the non‐culprit epicardial coronary arteries or their major side branches in addition to the infarct‐related artery. | Denmark | 2 | |
| 2010 to 2013 | Staged. | Complete. Criteria not described in study. | 1 | NR. | Spain | NR | |
| NR | Index procedure. | Not described. | 1 | NR. | Not described | NR | |
| January 2003 to December 2007 | At index procedure or staged mean 56 days after P‐PCI. 50% participants of complete revascularisation had at intervention of the non‐culprit lesions at index procedure. | > 70% diameter stenosis. | 2.5 | > 70% diameter stenosis of ≥ 2 epicardial coronary arteries or their major branches by visual estimation. | Not described | NR | |
| September 2008 to December 2014 | Staged between 3 and 40 days after P‐PCI. | > 70% stenosis of non‐culprit coronary artery. | 3 | ≥ 1 vessel, beside the culprit vessel, with significant stenosis (> 70% stenosis). | Czech Republic | 6 | |
| April 2008 to January 2013 | At index procedure. | Stenosis ≥ 50%. | 2 | The presence of stenosis ≥ 50% in ≥ 1 coronary artery other than the culprit vessel. | UK | 5 | |
| January 2009 to June 2012 | Staged between 7 and 10 days after P‐PCI. | 75% to 90%. | 2 | Non‐culprit vessel with significant stenosis (75% to 90% stenosis). | China | NR | |
| BMS: bare‐metal stent; DES: drug‐eluting stent; FFR: fractional flow reserve; NR: not reported in the article; PCI: percutaneous coronary intervention; P‐PCI: primary percutaneous coronary intervention. | |||||||
| Study | Group | Sample size (n) | Participants (n (%)) | Dropouts (n (%)) | % Male | Mean age (years) | % HTN | % DM | % HLD | % Prior MI | % Anterior STEMI |
| Complete | 150 | 139 (92.7) | 11 (7.3) | 85.3 | 64.6 | 36 | 12.7 | 27.3 | 4.7 | 36 | |
| Culprit‐only | 146 | 139 (95.2) | 8 (5.5) | 76.7 | 65.3 | 35 | 13.7 | 23.3 | 3.4 | 35.6 | |
| Complete | 80 | 71 (88.8) | 1 (1.3) | 80 | 62 | 26.3 | 6.3 | 15 | 6.3 | 21.3 | |
| Culprit‐only | 41 | 41 (100) | 1 (2.4) | 80.5 | 61 | 42.5 | 5 | 30 | 4.9 | 23.3 | |
| Complete | 314 | 294 (93.6) | 1 (0.3) | 80 | 64 | 41.4 | 9.2 | NR | 5.4 | 33.4 | |
| Culprit‐only | 313 | 313 (100) | 0 | 81.5 | 63 | 46.6 | 13.4 | NR | 8.6 | 35.8 | |
| Complete | 100 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | 99 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Complete | 52 | NR | NR | 88.5 | 63.5 | 36.5 | 11.5 | 41.2 | NR | 52 | |
| Culprit‐only | 17 | NR | NR | 82.4 | 65.3 | 58.8 | 41.2 | 53 | NR | 59 | |
| Complete | 130 | NR | NR | 78.5 | 64 | 57 | 16.2 | NR | NR | 45.4 | |
| Culprit‐only | 84 | NR | NR | 76.2 | 66.5 | 60 | 23.8 | NR | NR | 41.7 | |
| Complete | 106 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | 108 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Complete | 234 | 223 (95.3) | 10 (4.3) | 75.6 | 62 | 40.2 | 15 | NR | 8.1 | 28.6 | |
| Culprit‐only | 231 | 229 (99) | 8 (3.5) | 80.5 | 62 | 40.3 | 20.8 | NR | 7 | 38.5 | |
| Complete | 215 | NR | NR | 61 | 62.3 | 64.2 | 36.7 | 35.3 | NR | 36.7 | |
| Culprit‐only | 213 | NR | NR | 67.1 | 62 | 61 | 35.2 | 36.6 | NR | 40 | |
| DM: diabetes mellitus; HLD: hyperlipidaemia; HTN: hypertension; MI: myocardial infarction; n: number of participants; NR: not reported in the article; STEMI: ST elevated myocardial infarction. | |||||||||||
| Study | Group | Symptoms to PCI time (minute) | PCI without stenting (n (%)) | DES (n (%)) | BMS (n (%)) | 2‐Vessel disease (n (%)) | 3‐Vessel disease (n (%)) | Received PCI non‐culprit (n (%)) | DAPT | DAPT duration |
| Complete | 182 | NR | 141 (94) | NR | 119 (79.3) | 31 (20.7) | 139 (92.7) | Yes | NR | |
| Culprit‐only | 159 | NR | 127 (87) | NR | 110 (75.3) | 36 (24.7) | 0 | |||
| Complete | NR | 6 (7.5) | 18 (22.5) | 56 (70) | 60 (75) | 20 (25) | 48 (60) | Yes | 1 month | |
| Culprit‐only | NR | 7 (17.1) | 7 (7.1) | 27 (66) | 33 (80.5) | 8 (19.5) | 0 | |||
| Complete | NR | 12 (3.8) | 298 (95) | 0 | NR | 97 (31) | 193 (61.5) | Yes | 1 year | |
| Culprit‐only | NR | 18 (5.8) | 290 (92.7) | 0 | NR | 100 (32) | 0 | |||
| Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| Complete | 210 | 0 | 52 (100) | 0 | 36 (69) | 16 (30.8) | NR | Yes | 1 month | |
| Culprit‐only | 236 | 0 | 17 (100) | 0 | 9 (53) | 8 (47) | NR | |||
| Complete | NR | NR | 11 (8.5) | NR | NR | 48 (37) | NR | NR | NR | |
| Culprit‐only | NR | NR | 10 (12) | NR | NR | 21 (25) | NR | |||
| Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| Complete | NR | 1 (< 1) | 147 (63) | 86 (37) | 143 (61.1) | 91 (39) | 223 (95.3) | Yes | 1 month | |
| Culprit‐only | NR | 0 | 135 (58) | 96 (42) | 155 (67.1) | 76 (33) | 2 (1) | |||
| Complete | 214 | 0 | 215 (100) | 0 | NR | NR | NR | NR | NR | |
| Culprit‐only | 227 | 0 | 213 (100) | 0 | NR | NR | NR | |||
| BMS: bare‐metal stent; DAPT: dual antiplatelet therapy; DES: drug‐eluting stent; n: number of participants; NR: not reported in the article; PCI: percutaneous coronary intervention. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term all‐cause mortality Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Long‐term all‐cause mortality | 8 | 2417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.58, 1.11] |
| 2 Long‐term cardiovascular mortality Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Long‐term cardiovascular mortality | 6 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.79] |
| 3 Long‐term non‐fatal myocardial infarction Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Long‐term non‐fatal myocardial infarction | 6 | 2099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.89] |
| 4 Acute kidney injury Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term acute kidney injury | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.81] |
| 4.2 Long‐term acute kidney injury | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.82] |
| 5 Stroke Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Short‐term stroke | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [0.24, 102.26] |
| 5.2 Long‐term stroke | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.01] |
| 6 Bleeding Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short‐term bleeding | 3 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.86] |
| 6.2 Long‐term bleeding | 2 | 923 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term all‐cause mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term all‐cause mortality | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.18, 2.37] |
| 2 Short‐term cardiovascular mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Short‐term cardiovascular mortality | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.18] |
| 3 Short‐term non‐fatal myocardial infarction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term non‐fatal myocardial infarction | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.52, 5.90] |
| 4 Revascularisation Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term revascularisation | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.45] |
| 4.2 Long‐term revascularisation | 9 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.39, 0.57] |
| 5 Cost ≥ 1 year Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1948.0 [‐9171.85, 5275.85] |

