Fototerapi untuk merawat ulser kaki bagi pengidap kencing manis
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. Follow‐up time: 15 days. | |
| Participants | Setting: KLES Dr. Prabhakar Kore Hospital and Medical Research Centre, Belgaum. Number: 34 in the intervention group, 34 in the control group. Inclusion criteria: Type 2 diabetes mellitus patients with Meggitt‐Wagner grade I diabetic foot ulcer of at least 4 weeks' duration. Exclusion criteria: Patients with clinical signs of ischaemia and ankle brachial pressure index less than 0.9. Mean age: 50.9 years. Male/Female: 51/17. Average BMI: Not reported. Ulcer duration: 4 to 5 weeks. Ulcer size: Not reported. Duration of diabetes: Not reported. Peripheral neuropathy: 9 (26.47%) participants in study group; 6 (17.64%) participants in control group | |
| Interventions | Intervention group: LLLT + conventional therapy. Control group: Conventional therapy. LLLT was carried out with a multidiode cluster probe (Thor International Ltd). On the basis of the ulcer size, the duration of exposure was calculated to deliver 2 to 4 J/cm2 at 60 mW, 5 kHz, daily for 15 days. The ulcer floor and edge were irradiated. The ulcer was then covered with conventional moist dressing. Conventional treatment included daily wet saline or povidone‐iodine (Betadine) dressings, antibiotic treatment, contact cast immobilisation, and slough excision as and when required. Duration of treatment: 15 days. | |
| Outcomes | Change in ulcer size. Ulcer area was calculated by obtaining the impression of ulcer floor on a sheet of cellophane paper and then transferring the imprint onto graph paper. The ulcer size was measured on day 0 and day 15. | |
| Notes | No funding resources and declaration of conflicts of interests were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups of 34 each on the basis of computer generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of how blinding of participants and personnel was implemented. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 68 participants were randomised and all were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to permit judgement of low or high risk of bias. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. Follow‐up time: 20 weeks. | |
| Participants | Setting: Iranian Center for Medical Laser (ICML) clinic of the Academic Center for Education, Culture and Research (ACECR) Number of participants: 13 in the intervention group, 10 in the control group. Inclusion criteria: Patients with a diabetic foot ulcer for a minimum of 12 weeks with ulcer stage I and II according to the Wagner classification who were capable of giving informed consent, understanding instructions, and co‐operating with study protocol completely were enrolled in this study. Exclusion criteria: The presence of active infection requiring hospitalisation, gangrene, systemic diseases such as collagen‐vascular diseases, renal failure, evidence of ischaemia, pregnancy, and history of photosensitivity. Mean age: 60.2 year in intervention group, 59.4 in control group. Male/Female: 12/6. Ulcer duration: 11.4 months in intervention group, 8.8 in control group. Ulcer size: 10.7 cm2 in intervention group, 7.8 cm2 in control group. | |
| Interventions | Intervention group: LLLT + conventional therapy. Control group: Conventional therapy. LLLT was performed with a laser device (BTL; 685 nm, 50 mW) at a fluence of 10 J/cm2 (with 200 sec of illumination) with a special head in non‐contact mode at a distance of 1 cm from the skin surface (irradiation area was approximately 1 cm2). Participants received illuminations over the ulcers 6 times per week for at least 2 successive weeks and then every other day up to complete healing. Conventional therapy included revision of dead and infected tissue and off‐loading when necessary, individualised topical treatment and dressings, and oral antibiotics when necessary. Treatment duration: 20 weeks. | |
| Outcomes | Primary outcome: Proportion of wounds completely healed during follow‐up. Secondary outcome: Change in ulcer size. The ulcer size was determined in square centimeters through digital imaging and tracing with engineered software. The ulcer size was measured at week 2 and week 4. | |
| Notes | This study was approved and granted by Endocrinology & Metabolism Research Center (EMRC), Tehran University of Medical Sciences, Tehran, Iran. The authors declared that there were no conflicting financial interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was prepared by an independent statistician by the method of computerized random numbers for each treatment." |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were examined by a physician blinded to treatment at baseline and follow‐up sessions, and demographic and clinical data were documented". "Patients in the placebo treatment group similarly received sham irradiation under strictly controlled double‐blinded conditions". "For the purpose of safety, all patients were instructed to wear safety goggles. Since LLLT provides no sensory cues such as thermal or acoustic effects, wearing goggles blinded the patients to the procedure of LLLT at the same time" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The image was processed by engineered software, AutoCAD 2002, by two physicians blinded to treatment." |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of 23 patients enrolled in the study, five patients could not complete follow‐up sessions till 20 weeks. Two patients from the placebo group needed to be hospitalized and amputated due to extended gangrene. One patient in the LLLT group was hospitalized for treatment of infection. One patient from each group died due to myocardial infarction." Comment: The reasons for missing outcome data were likely to be related to the outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: Published reports include all expected outcomes. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. Follow‐up time: 12 weeks. | |
| Participants | Setting: Kaplan Medical Center, Rehovot, Israel. Number of participants: 10 in the intervention group, 6 in the control group. Inclusion criteria:
Exclusion criteria:
Mean age: 62.9 years. Male/Female: 11/5. Average BMI: 28.5. Ulcer duration: Not reported. Ulcer size: Not reported. Duration of diabetes: Not reported. Peripheral neuropathy: Not reported. | |
| Interventions | Intervention group: Phototherapy + usual wound care. Control group: Sham phototherapy (phototherapy at non‐therapeutic light intensity) + usual wound care. Phototherapy was performed with a prototype of the Vireo device (180 mW/cm2 twice a day, wavelength: 400 to 800 nm). Usual wound care included wound cleaning, debridement, daily application of a pad of gauze soaked with saline, and wound dressing. Treatment duration: 12 weeks. | |
| Outcomes | Proportion of wounds completely healed during follow‐up, change in wound size, and adverse events. Wounds were measured once a week for 12 weeks. | |
| Notes | The study was financed by "Qray LTD", Kiryat‐Ata, Israel. The authors declared that one of the authors is the scientific advisor for Qray. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization of patients to treatment with the therapeutic or non‐therapeutic device was performed by a person who was not involved in the evaluation of the study. A simple random allocation generated a number for each device." |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:"The patients and the investigators were blind to the number allocation, as both the placebo and the treatment devices were identical in design and both emitted light." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Four patients were excluded from analysis; 3 patients (2 from the placebo and 1 from the treatment group) were excluded because of noncompliance, as these patients did not use the device at home as instructed. One patient was excluded because of pre‐existing renal failure." Comment: The reasons for missing outcome data were likely to be related to the outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: Published reports include all expected outcomes. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre trial. Follow‐up time: 20 weeks. | |
| Participants | Setting: Not reported. Number of participants: 9 in the intervention group, 5 in the control group. Inclusion criteria: Diabetic patients with non‐ischaemic Wagner grade 1 or 2 ulcers at or below the ankle. Exclusion criteria: Use of immunosuppressive treatment including steroids (> 7.5 mg prednisone/day), creatine level > 250 μg/L, use of antibiotic treatment 2 weeks before inclusion, and > 40% ulcer area reduction during the 4‐week run‐in‐period. Mean age: Not reported. Male/Female: Not reported. Average BMI: Not reported. Ulcer duration: 46 weeks. Ulcer size: Not reported. Duration of diabetes: Not reported. Peripheral neuropathy: Not reported. | |
| Interventions | Intervention group: Phototherapy. Control group: Placebo treatment. Phototherapy was performed with a non‐invasive, non‐thermal CE‐marked medical device (BioLight; wavelengths of between 637 and 956 nm and a pulse repetition frequency between 8 and 9900 Hz). Treatment was given 3 times a week for the first 2 weeks, and twice a week thereafter for up to 20 weeks. | |
| Outcomes | Time to 50% ulcer area reduction, and adverse events. | |
| Notes | No funding resources and declaration of conflict of interests were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "9 patients were randomised to active and 5 to placebo treatment". Comment: Randomisation method was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of participants and personnel was implemented. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Double‐blind, randomised controlled trial. Follow‐up time: 16 weeks. | |
| Participants | Setting: Not reported. Number of participants: 9 in the LLLT group, 10 in the high‐voltage pulsed current group, 9 in the control group. Inclusion criteria: 30 to 75 years old with confirmed diagnosis of diabetes mellitus (diagnosis based on WHO criteria), with ulcers localised on the distal legs or feet, classified as category I or II according to the Wagner classification system. Exclusion criteria: Patients with uncontrolled diabetes, local infection in the ulcer site, ulcer grades III through V (Wagner classification), lower limb amputation, and neuromuscular or musculoskeletal disease were excluded. Mean age: 59.3 years. Male/Female: 42/56. Average BMI: Not reported. Ulcer duration: 16.2 months. Ulcer size: Not reported. Duration of diabetes: 11.2 years. Peripheral neuropathy: Not reported. | |
| Interventions | LLLT group: LLLT + standard wound care. HVPC group: High‐voltage pulsed current + standard wound care. Control group: Standard wound care. LLLT: semiconductor laser diode (DMC, Brazil) with 685 nm wavelength emitted 30 mW in continuous mode, 0.0028 cm2 beam area applied punctually at 2 J/cm2 (0.18 s) every centimetre along the edges of the ulcer in light contact and 1.5 J/cm2 (0.14 s) in the wound bed in non‐contact mode, 3 times a week for 16 weeks or until the wound closed. High‐voltage pulsed current: Wounds were treated with an electrical stimulator (Intelect 340 Stim model; Chattanooga Group), which produced a twin peak pulse having the following parameters: continuous mode, sub‐motor voltage level, 100 pulses per second pulse frequency, and 100 μs pulse duration. The treatment was performed at 45 min 3 times a week for 16 weeks or until the wound closed. Standard wound care: The procedure included irrigation with physiological saline solution, selective sharp debridement of necrotic tissue, and maintaining a moist environment by applying an appropriate wound dressing. Participants were also taught diabetic foot self care and pressure off‐loading in the affected foot. All participants received standard wound care 7 days a week for 16 weeks or until wound closure occurred. | |
| Outcomes | Primary outcome: Proportion of wounds completely healed during follow‐up. Secondary outcomes: Healing proportion, measurement obtained as the percentage of baseline area. Data were evaluated at week 4, week 8, week 12, and week 16. | |
| Notes | The study was funded by COLCIENCIAS. The authors declared that they have no conflict of interest with participants or entities that contributed to this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization process was performed according to a pre‐established order, using randomized blocks" Comment: Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: "the care provider, and investigator were masked." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "evaluators were blinded regarding group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Intention to treat analysis was applied" |
| Selective reporting (reporting bias) | Low risk | Comment: Published reports include all expected outcomes. |
| Other bias | High risk | Comment: There was likely to be an imbalance in total ulcer size, and ulcer duration between intervention group and control group. Ulcer duration, Median (IQR): LLLT 4.1 (1.2 to 4.7); HVPC 2.9 (1.2 to 12.1); Control 12.2 (8.4 to 18.1). Ulcer size, Median (IQR): LLLT 62.9 (23.1 to 172.2); HVPC 20.0 (11.7 to 131.0); Control 41.6 (17.5 to 398.3). The authors did not do any analyses to adjust for the potential influence. |
| Methods | Randomised controlled trial. Follow‐up time: 4 weeks. | |
| Participants | Setting: The inpatients treated in the Sichuan Provincial People's Hospital, Chengdu, Sichuan, China. Number of participants: 30 in the intervention group, 30 in the control group. Inclusion criteria: People diagnosed with a diabetic foot ulcer. Mean age: 63.5 years Male/Female: 18/12. Average BMI: Not reported. Ulcer duration: Not reported. Ulcer size: 4 to 6 cm2 Duration of diabetes: Not reported. Peripheral neuropathy: Not reported. | |
| Interventions | Intervention group: Phototherapy + usual care. Control group: Usual care. Phototherapy was performed with Carnation‐66 red laser device (wavelength: 640 ± 10 nm). The treatment was performed at 10 min 2 times daily for 4 weeks. Usual care included wound cleaning, debridement, and wound dressing. | |
| Outcomes | Proportion of wounds completely healed during follow‐up. | |
| Notes | No funding resources and declaration of conflicts of interests were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation method was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of how blinding of participants and personnel was implemented. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: The study randomised 30 participants to each group, and all participants were included in data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Randomised controlled trial. Follow‐up time: 21 days. | |
| Participants | Setting: The inpatients treated in The First Hospital of Nantong, Nantong, Henan, China. Number of participants: 42 in the intervention group, 42 in the control group. Inclusion criteria: Patients diagnosed with a diabetic foot ulcer, Meggitt‐Wagner grade II to IV. Exclusion criteria: Hepatic or renal insufficiency, serious malnutrition, serious arteriosclerosis. Mean age: 57.7 years. Male/Female: Not reported. Average BMI: Not reported. Ulcer duration: Not reported. Ulcer size: 18.3 cm2. Duration of diabetes: 18.1 years. Peripheral neuropathy: Not reported. | |
| Interventions | Intervention group: Phototherapy + usual care. Control group: Usual care. Phototherapy was performed with Carnation‐22 red laser device. The treatment was performed at 10 min 2 times daily for 21 days. Usual care included wound cleaning, debridement, wound dressing, and standard management for diabetes. | |
| Outcomes | Change in ulcer area, measured at day 7, 14, and 21. | |
| Notes | No funding resources and declaration of conflicts of interests were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation method was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of how blinding of participants and personnel was implemented. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Methods | Randomised controlled trial. Follow‐up time: 4 weeks. | |
| Participants | Setting: The inpatients treated in The Rehabilitation Center of PLA, Dalian, China. Number of participants: 12 in the intervention group, 11 in the control group. Inclusion criteria: Patients diagnosed with a diabetic foot ulcer. Mean age: Intervention group 66.8 years, control group 68.5 years. Male/Female: 8/15. Average BMI: Not reported. Ulcer duration: 7 years. Ulcer size: Not reported. Duration of diabetes: Not reported. Peripheral neuropathy: Not reported. | |
| Interventions | Intervention group: Phototherapy + usual care. Conctrol group: Usual care. Phototherapy was performed with YS‐50 far‐infrared phototherapy device and YS‐2 ultraviolet light phototherapy device. The far‐infrared phototherapy (wavelength 9.6 μm) was performed at 30 min per day for 4 weeks. The ultraviolet light phototherapy (253.7 nm) was performed once daily or once every other day. Usual care included wound cleaning, debridement, wound dressing, and standard management for diabetes. Treatment duration: 4 weeks. | |
| Outcomes | Proportion of wounds completely healed during follow‐up (4 weeks). | |
| Notes | No funding resources and declaration of conflicts of interests were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation method was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of how the randomisation sequence was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: No mention of how blinding of participants and personnel was implemented. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: No mention of how blinding of outcome assessment was implemented. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: The study randomised 12 participants to the phototherapy group and 11 to the control group, all of which were included in data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
| Other bias | Unclear risk | Comment: Insufficient information to assess whether an important risk of bias exists. |
BMI: body mass index
HVPC: high‐voltage pulsed current
IQR: interquartile range
LLLT: low‐level laser therapy
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| Participants were not eligible (wounds) | |
| Participants were not eligible (chronic wounds and ulcers on the lower leg) | |
| Participants were not eligible (diabetic leg ulcers) | |
| Intervention was not eligible (sulphadiazine or phyto therapy cream) | |
| Participants were not eligible (diabetic patients with peripheral sensory neuropathy) | |
| Not a randomised controlled trial | |
| Participants were not eligible (diabetic patients with disease‐related skin lesions including dryness, diabetic bullae, nail changes and alopecia, infections, pruritus, and frank eczema) | |
| Participants were not eligible (diabetic ulcers or gangrenes were included) | |
| Participants were not eligible (diabetic microangiopathy) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT (possible) |
| Participants | Diabetic patients with foot ulcers |
| Interventions | LLLT |
| Outcomes | Unclear |
| Notes | We judged from study title. Abstract and full text were not available |
LLLT = Low level laser therapy
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Low level laser effect on improving the blood flow in diabetic foot ulcers |
| Methods | RCT |
| Participants | Diabetic patients with ischaemic diabetic foot ulcers (of both sexes) aged 40 to 60 years |
| Interventions | Intervention 1: In placebo group, participants will be treated with standard therapy and laser every other day, for 12 sessions. Intervention 2: In laser therapy group, participants will be treated with Ga‐As laser, 2 J/cm2, 90 mW, and standard therapy every other day, for 12 sessions. |
| Outcomes | Primary outcomes:
Secondary outcome:
|
| Starting date | June 2013 |
| Contact information | Name: Dr Gity Torkaman Address: Physiotherapy Department, Tarbiat Modares University, Jalal Ale Ahmad Highway Tehran, Islamic Republic of Iran Email: [email protected] Affiliation: Tarbiat Modares University |
| Notes | Identified from WHO International Clinical Trials Registry Platform |
| Trial name or title | CO2‐Laser Treatment in Patients With Diabetic Infected Foot Ulcers (DULCIS) |
| Methods | RCT |
| Participants | Diabetic patients with foot ulcers |
| Interventions | CO2 laser |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | January 2016 |
| Contact information | Edoardo Mannucci, MD, University of Florence |
| Notes | Identified from WHO International Clinical Trials Registry Platform |
RCT: randomised controlled trial
WHO: World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks) Show forest plot | 4 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 1.57 [1.08, 2.28] |
| Analysis 1.1 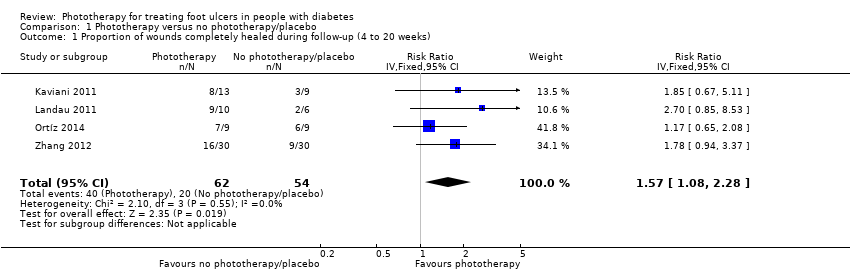 Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks). | ||||
| 2 Change in ulcer size in relative terms (percentage change in wound area) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 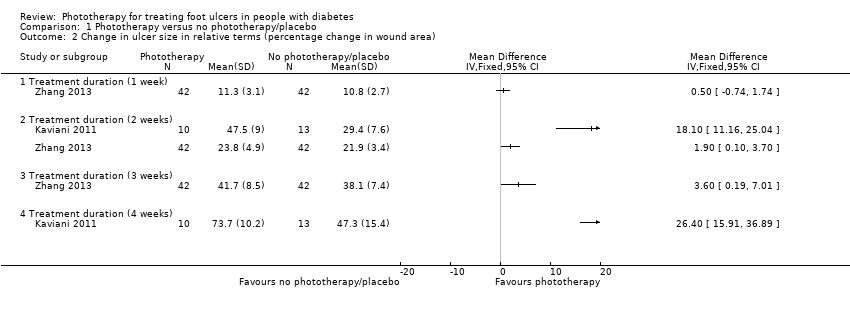 Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 2 Change in ulcer size in relative terms (percentage change in wound area). | ||||
| 2.1 Treatment duration (1 week) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Treatment duration (2 weeks) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Treatment duration (3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Treatment duration (4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in ulcer size in absolute terms (mean change in wound area) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 3 Change in ulcer size in absolute terms (mean change in wound area). | ||||
| 3.1 Treatment duration (2 weeks) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 720.76 [626.61, 814.91] |
| 4 Number of amputations at study end (20 weeks) Show forest plot | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 0.16 [0.01, 2.95] |
| Analysis 1.4 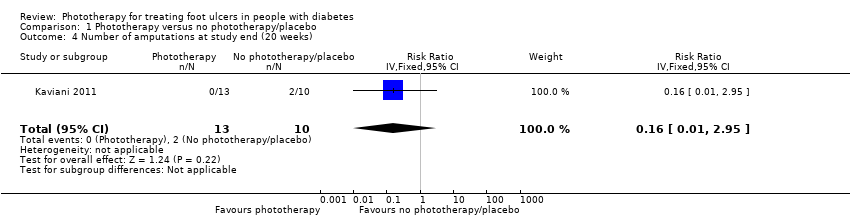 Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 4 Number of amputations at study end (20 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Phototherapy versus high‐voltage pulsed current, Outcome 1 Proportion of wounds completely healed during follow‐up. | ||||
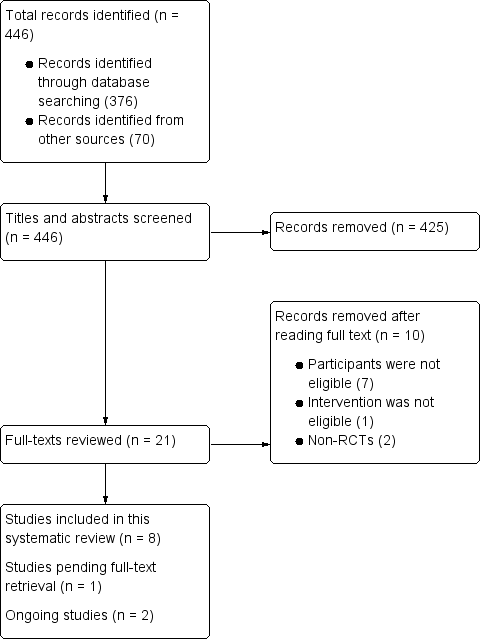
Flow chart of study selection.
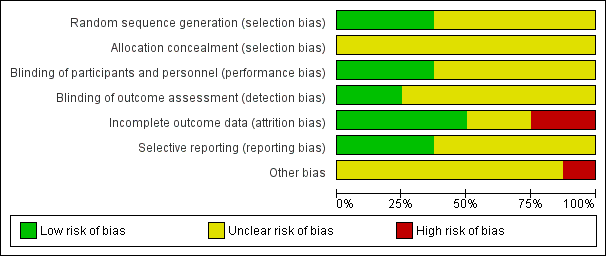
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks).
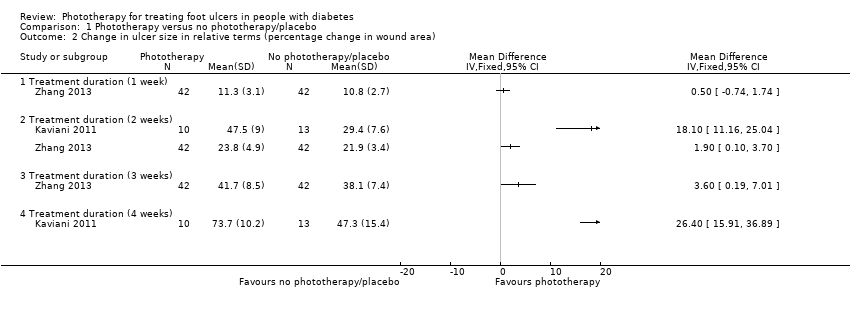
Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 2 Change in ulcer size in relative terms (percentage change in wound area).
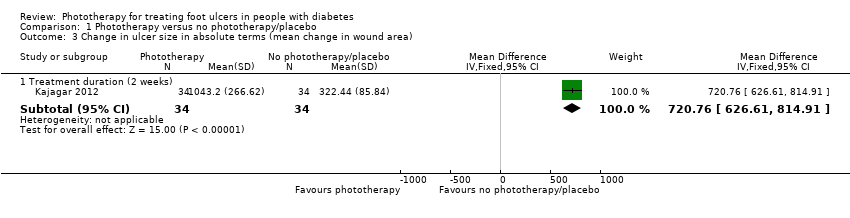
Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 3 Change in ulcer size in absolute terms (mean change in wound area).

Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 4 Number of amputations at study end (20 weeks).
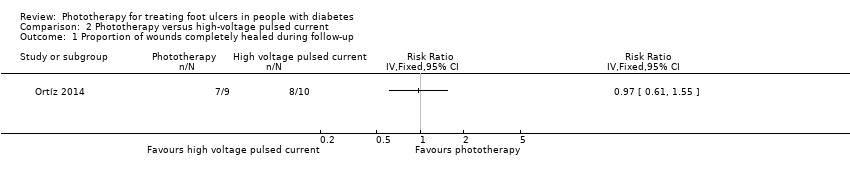
Comparison 2 Phototherapy versus high‐voltage pulsed current, Outcome 1 Proportion of wounds completely healed during follow‐up.
| Phototherapy compared with placebo/no phototherapy for foot ulcers in people with diabetes | ||||||
| Patient or population: Diabetes with foot ulcers Settings: Clinics and hospitals Intervention: Phototherapy Comparison: Placebo/no phototherapy | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed absolute effect | Corresponding absolute effect | |||||
| Placebo/no phototherapy | Phototherapy | |||||
| Wound healing ‐ time to complete wound healing (weeks) | No study provided reliable data for this outcome. | |||||
| Wound healing ‐ proportion of wounds completely healed during follow‐up | 330 per 1000 | 568 per 1000 | RR 1.57 (1.08 to 2.28) | 116 (4 studies) | ⊕⊕⊝⊝ | |
| Adverse events | See comment | See comment | See comment | See comment | See comment | In Landau 2011, there were no device‐related adverse events. In Londahl 2013, the authors suggested that there was no difference in adverse events between intervention and control groups, but the number of adverse events was not reported. |
| *The basis for the assumed absolute effect (e.g. the median control group risk across studies) is provided in footnotes. The corresponding absolute effect (and its 95% confidence interval) is based on the assumed absolute effect in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for study limitations (high risk of bias for incomplete outcome data in two studies and potential influence of imbalance in baseline characteristics in one study) and one level for imprecision (small sample size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks) Show forest plot | 4 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 1.57 [1.08, 2.28] |
| 2 Change in ulcer size in relative terms (percentage change in wound area) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Treatment duration (1 week) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Treatment duration (2 weeks) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Treatment duration (3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Treatment duration (4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in ulcer size in absolute terms (mean change in wound area) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Treatment duration (2 weeks) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 720.76 [626.61, 814.91] |
| 4 Number of amputations at study end (20 weeks) Show forest plot | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 0.16 [0.01, 2.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |

