Kanta intraokular penapisan cahaya biru (IOL) untuk melindungi kesihatan makula
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Study grouping: parallel group; no further details provided Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL group 2
Non‐blue‐light filtering IOL group 3
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 2
Non‐blue‐light filtering IOL 3
| |
| Outcomes | Refractive error, visual acuity, IOL tilt, IOL decentration, intraocular pressure, corneal endothelial cell loss and aqueous flare at three months postoperatively | |
| Identification | Sponsorship source: Funding sources: none Declaration of interest: none for all authors Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Date study conducted: not reported First author's name: M Aose Institution: Ophthalmology, Dokkyo University School of Medicine, Tochigi, Japan Email: not reported Address: not reported | |
| Notes | ARVO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting, the outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with 1 eye per participant randomised to 1 of the interventions Exclusions after randomisation: none apparent Losses to follow‐up: none apparent How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1 ‐ yellow
Blue‐light filtering IOL group 2 ‐ orange
Non‐blue‐light filtering IOL group
Inclusion criteria: people with senile cataract Exclusion criteria: people having anomalies or guttata in the corneal endothelium; receiving any ocular treatment within 1 month prior to commencement of the study; taking any medication that can produce somnolence or who are drug addicts or alcoholics; with retinopathies or any other ocular pathology; with any other condition including psychiatric abnormalities that could alter the results; whose pupils were not dilating well with mydriatics Comparison of study groups at baseline: no significant baseline differences | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1 ‐ yellow
Blue‐light filtering IOL 2 ‐ orange
Non‐blue‐light filtering IOL
| |
| Outcomes | BCVA and contrast sensitivity (photopic and mesopic) at one month postoperatively intraoperative complications, and postoperative complications at one month after surgery | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: none Country: India Setting: tertiary care hospital in Kolkata, West Bengal, Eastern India Comments: Date study conducted: enrolments from 1 August 2014‐31 January 2015 Trial registration number: not provided Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Sabayaschi Bandyopadhyay Institution: Department of Ophthalmology, R. G. Kar Medical College Email: [email protected] Address: Department of Ophthalmology, R. G. Kar Medical College, 1 Khudiram Bose Sarani, Kolkata 700 004, India | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking. We assume that in the absence of reporting, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting, assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with bilateral IOL implantation of same IOL type, except for six participants where a blue‐light filtering IOL (AcrySof Natural) was implanted in one eye and a non‐blue‐light IOL (AcrySof MA60BM) was implanted in the other eye. Exclusions after randomisation: none apparent Losses to follow‐up: none apparent How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people having bilateral cataract surgery Exclusion criteria: people with glaucoma, retinal, or any other severe ocular pathology Comparison of study groups at baseline: "There were no significant differences between the two investigated groups regarding age, gender and ocular pathology." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Distance UCVA, measured as the proportion of eyes with UCVA < 0.8 decimal acuity or > 0.8 decimal acuity, after six months Distance BCVA, measured categorically as the number of eyes with BCVA of 0.8, 0.9 or 1.0 decimal acuity, after six months Proportion of people, reported as a dichotomous, who were overall satisfied with their visual outcome (i.e., would have the same type of IOL implanted again), after six months Adverse events:
| |
| Identification | Sponsorship source: Funding source: not reported Declaration of interest: not reported Country: Croatia Setting: University Department of Ophthalmology, General Hospital Sveti Duh, Zagreb, Croatia, and Eye Clinic Svjetlost, Zagreb, Croatia Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Ante Barisic Institution: University Department of Ophthalmology, General Hospital Sveti Duh, Zagreb, Croatia Email: not reported Address: University Department of Ophthalmology General Hospital Sveti Duh Zagreb, Croatia | |
| Notes | Population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided about masking; we assume that in absence of reporting, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking; we assume that in absence of reporting, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: complete reporting for n = 60 participants is implied from the data; from the number of eyes presented in Figures 1, 2 and 3 |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Overall
Inclusion criteria: not reported Exclusion criteria: people with amblyopia or abnormal Ishihara test Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Colour recognition and contrast sensitivity (mesopic and photopic); time point not specified in the abstract | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Wolfgang Behrens‐Baumann MD Institution: not reported Email: not reported Address: not reported | |
| Notes | AAO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open label investigation," Judgement comment: study is described as "open label" |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: study is described as "open label" |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with inter‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL). Exclusions after randomisation: participants with intraoperative complications were excluded from the analysis; no further details were supplied. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people ≥ 60 years with good general and ocular health having bilateral age‐related cataracts with a potential visual acuity of 20/40 or better, indicating cataract extraction and IOL implantation (in both eyes), and who agreed to have surgery in both eyes within a maximum interval of 60 days and were willing to complete a schedule of postoperative follow‐ups. Exclusion criteria: people with pre‐existing systemic disease such as diabetes or hypertension, ocular disease, such as uveitis, or who failed the Farnsworth Munsell 100‐Hue test prior to surgery. To avoid the skew deviation of the results, people with an intraoperative complication such as hyphema, zonular rupture, or posterior capsule were also excluded. Comparison of study groups at baseline: no group differences for the 26 eyes (13 participants) that were reported. However, we are unable to judge any potential group difference for participants who were excluded due to intraoperative complications. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Distance BCVA, colour perception and contrast sensitivity at 18 months postoperatively | |
| Identification | Sponsorship source: Funding sources: Sri Kanchi Sankara Health Educational Foundation, Beltola, Guwahati, Assam, India Declaration of interest: no author had a financial or proprietary interest in any material or method mentioned. Country: India Setting: Sri Sankaradeva Nethralaya Beltola, Assam, India Comments: Date study conducted: June‐August 2003 Trial registration number: not reported Contacting study investigators: we emailed the study authors on 1 September 2017 for the means and standard deviations of the within‐pair differences in distance BCVA at follow‐up; no response was received. As a result, we could not incorporate these data in a meta‐analysis. Corresponding author's name: Harsha Bhattacharjee, MS, Medical Director Institution: Sri Sankaradeva Nethralaya Beltola, Assam, India Email: [email protected] Address: Harsha Bhattacharjee, MS, Medical Director SriSankaradeva Nethralaya Beltola, Guwahati 781028, Assam, India | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On random selection basis in each patient" Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "patient‐masked, examiner‐masked" Judgement comment: clearly states that participants and personnel were not aware of which intervention was received, but surgeon was not masked |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: clearly states that study was "examiner‐masked" |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: appears "patients with an intra‐operative complication... were also excluded" so not intention‐to‐treat and no indication of how many participants were excluded on these grounds |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with bilateral IOL implantation (although no further details provided) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with cataract and no other significant diseases Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Short wavelength chromatic pupillometry, measured using the consensual pupil reaction Circadian rhythm, assessed by direct activity measurements (actigraphy) and by questionnaires (Pittsburgh Sleep Quality Index and Morningness Eveningness Questionnaire) | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for the review Corresponding author's name: Adam Elias Brøndsted Institution: University of Copenhagen Email: not reported Address: not reported | |
| Notes | ARVO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as "randomised" but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: the publication states that "preoperative and postoperative complications with an impact on visual acuity, including ruptured capsule, nucleus drop, and postoperative corneal oedema, led to exclusion." However, no participants developed postoperative complications leading to exclusion. Losses to follow‐up: a total of 76 participants were enrolled from screening 267 candidates; 73 participants were randomised (n = 35 to a non‐blue‐light filtering IOL and n = 38 to a blue‐light filtering IOL) "because oneparticipant changed her mind regarding the operation and one participant dropped out after the baseline examination and another participant was excluded at the day of the operation because of posterior capsule rupture. Further, one participant found the study procedures too comprehensive and dropped out after the first control visit, producing a final number of 72 participants at the three‐week postoperative visit." How missing data were handled: not reported Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: all patients who were referred for bilateral senile cataract surgery to the Department of Ophthalmology, Rigshospitalete Glostrup, Denmark and provided written informed consent Exclusion criteria: ophthalmological disease with an expected effect on the retina, optic disc, or cornea, including advanced AMD, glaucoma, diabetic retinopathy, corneal dystrophy, ocular trauma, and recurrent uveitis; people with severe systemic disease, including diabetes, cancer of any kind, and known sleep disturbances; people with preoperative and postoperative complications with an impact on visual acuity, including ruptured capsule, nucleus drop, and postoperative corneal oedema Comparison of study groups at baseline: there were no significant inter‐group differences at baseline. Participants were similar in terms of age, sex, distance BCVA, cataract severity and circadian type. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Activation of intrinsic photosensitive ganglion cells using post‐illumination pupil response (PIPR) to blue light from 10‐30 s after light exposure as a surrogate measure, before surgery, and at two days and three weeks post‐surgery. Circadian rhythm analysis using actigraphy and 24‐h salivary melatonin measurements before, and at 3 weeks after surgery Objective and subjective sleep quality, as determined by actigraphy and the Pittsburgh Sleep Quality Index before, and at 3 weeks postsurgery | |
| Identification | Sponsorship source: Funding sources: the study was funded by the Danish Association of the Blind and the Velux Foundation. Declaration of interest: the author(s) have no proprietary or commercial interest in any materials discussed in this article. Country: Denmark Setting: Department of Ophthalmology, Rigshospitalete Glostrup, Denmark Comments: Date study conducted: not reported Trial registration number: clinicaltrials.gov (NCT01686308) Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Adam Elias Brøndsted, MD Institution: Department of Ophthalmology, Rigshospitalet, Glostrup, Denmark Email: [email protected] Address: Henrik Ibsens Vej 2, 4.tv, 1813 Frederiksberg C, Denmark | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed on the day of the surgery using automated, computerized block‐randomization lists" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Because of the different colors of the blue‐blocking and neutral IOLs, it was not possible to keep the investigator masked to IOL type." Judgement comment: Participants were masked but not the "investigator". |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: whether the "same physician (A.E.B)" who examined all particpants was masked or unmasked is not stated |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 72 of the 76 participants enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | Judgement comment: main outcome measures are as specified in the relevant entry on clinicaltrials.gov |
| Other bias | Low risk | Judgement comment: no other risks of bias identified |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant contributing to the analyses Exclusions after randomisation: "One participant was excluded at the day of surgery due to posterior capsule rupture" (although group allocation is not described). Losses to follow‐up: "The first eye in 76 participants was recruited for the study. Of these, 72 participants completed the three‐week follow‐up and 67 completed the one‐year follow‐up (31 allocated to neutral IOL and 36 allocated to blue‐blocking IOL). One participant was excluded at the day of surgery due to posterior capsule rupture, one participant dropped out and one participant changed her mind regarding the surgery. Three participants declined participation in the one‐year follow‐up and two participants did not respond to the invitation letter or to telephone calls." Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with bilateral, age‐related cataract eligible for cataract surgery according to the local guidelines. Only the first eye, that is the eye first scheduled for cataract surgery, was included in the study although both eyes were scheduled for surgery. Exclusion criteria: any eye disease with an expected effect on the retina, optic nerve or cornea including advanced AMD, glaucoma, diabetic retinopathy, corneal dystrophy, ocular trauma or recurrent uveitis. People with severe systemic disease, including diabetes, cancer of any kind and known sleep disturbances. Comparison of study groups at baseline: not reported. Note: it is stated that "One participant was excluded at the day of surgery due to posterior capsule rupture" (although group allocation is not described). | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | The main outcome was the intrinsic activation of photosensitive retinal ganglion cells estimated by quantitation of the postillumination pupil response to blue light based on consensual pupil response measurements at one year after surgery. Circadian rhythm, measured by 24‐h melatonin profiles and actigraphy at one year after surgery Self‐evaluated sleep quality, measured using the Danish version of the Pittsburgh Sleep Quality Index (PSQI) at three weeks postoperative and one year after surgery | |
| Identification | Sponsorship source: Funding sources: the study was funded by the Danish Association of the Blind and the Velux Foundation. Declaration of interest: not reported Country: Denmark Setting: Department of Ophthalmology, Rigshospitalete Glostrup, Denmark Comments: Date study conducted: not reported Trial registration number: clinicaltrials.gov (NCT01686308) Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Adam Elias Brøndsted, MD Institution: Department of Ophthalmology, Rigshospitalet, Glostrup, Denmark Email: [email protected] Address: Henrik Ibsens Vej 2, 4.tv, 1813 Frederiksberg C, Denmark | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computerized block randomization lists" Judgement comment: Computer generated list |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomized to implantation" Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Masking of the participants" Judgement comment: "Participants were masked but it is unclear whether study personnel were masked." |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "The first eye in 76 participants was recruited for the study. Of these, 72 participants completed the 3‐week follow‐up and 67 completed the 1‐year follow‐up (31 allocated to neutral IOL and 36 allocated to blue‐blocking IOL). 1 participant was excluded at the day of surgery due to posterior capsule rupture, one participant dropped out and one participant changed her mind regarding the surgery. Three participants declined participation in the 1‐year follow‐up and two participants did not respond to the invitation letter or to telephone calls." Judgement comment: missing data < 20% |
| Selective reporting (reporting bias) | High risk | Judgement comment: results of the "Morningness‐eveningness questionnaire", listed as a Secondary Outcome Measure on the clinicaltrials.gov entry are not reported |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, where both eyes of each participant were randomised to the same type of intervention Exclusions after randomisation: participants with intraoperative complications, IOL tilt or decentration were excluded from the analyses; however, no participant had these complications Losses to follow‐up: none How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1 ‐ SN60AT (Alcon)
Non‐blue‐light filtering IOL group 1 ‐ Tecnis Z9000 (AMO)
Blue‐light filtering IOL group 2 ‐ SN60WF (Alcon)
Non‐blue‐light filtering IOL 2 ‐ Sensar AR40e (AMO)
Non‐blue‐light filtering IOL 3 ‐ Sofport L161AO (Bausch & Lomb)
Inclusion criteria: people aged between 50 and 80 years, bilateral cataracts, potential acuity better than 0.2 logMAR units, preoperative corneal spherical aberration (Z04) values between 0.1 and 0.25μm at 5‐mm pupil diameter, and IOL power between +18.00 and +24.00 diopters (D) Exclusion criteria: people were excluded if any of the following conditions were present: corneal astigmatism ≥ 1.00 D, surgical complications, IOL tilt and decentration estimated by retroillumination, glaucoma, amblyopia, corneal pathology, history of uveitis, diabetic retinopathy, pseudoexfoliation syndrome, macular pathology, and previous intraocular surgery. People using topical medications (apart from lubricants) and taking systemic steroids also were excluded. Comparison of study groups at baseline: there were no statistically significant differences among the groups in terms of participant age (P > 0.05). | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL 2
Non‐blue‐light filtering IOL 3
| |
| Outcomes | BCVA, contrast sensitivity (mesopic and photopic), corneal abberations and wavefront spherical aberration of the whole eye, at two months postoperatively intraoperative complications and postoperative complications at two months postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: "the authors have no proprietary interest in the materials presented herein" Country: Italy Setting: Department of Ophthalmology and Neurosurgery at the University of Siena, Italy Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: authors contacted on 14th August 2017, Re: Table 2 – whether units of error show SD or another unit (as not specified in the paper); no response was received Corresponding author's name: Gianluca Martone, MD Institution: Department of Ophthalmology and Neurosurgery at the University of Siena, Italy Email: [email protected] Address: Gianluca Martone, MD Dipartimento di Scienze Oftalmologiche e NeurochirurgicheUniversità degli Studi di Siena, Viale Bracci 1, 53100 Siena, Italy | |
| Notes | Data reported in this study for the SN60AT, SN60WF, Sensar AR40e, Tecnis Z900 participants (n = 100 people, n = 200 eyes) is the same as for the Caporossi 2009 paper (which reports later follow‐up time points for these participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: participants were masked to which IOL they received; however, there is no comment regarding other personnel, so we assume that in the absence of this information, the study personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: we assume that in absence of reporting, the outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: participants with “surgical complications, IOL tilt and decentration estimated by retroillumination” were excluded, but the paper also states that “no intraoperative or postoperative complications were recorded in the study. In particular no cases of significant IOL decentration and tilt developed during the follow‐up. Further, for the 125 participants enrolled in the study, none of the 125 patients dropped out of the study.” |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, where both eyes of each participant were randomised to the same type of intervention (as per the Caporossi 2007 study) Exclusions after randomisation: 2‐year follow‐up data from participants in the Caporossi 2007 study Losses to follow‐up: none at 2 months postoperatively, 6 participants (12 eyes) at 1 year postoperatively and 11 participants (22 eyes) at 2 years postoperatively How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1 ‐ SN60AT (Alcon)
Non‐blue‐light filtering IOL group 1 ‐ Tecnis Z9000 (AMO)
Blue‐light filtering IOL group 2 ‐ SN60WF (Alcon)
Non‐blue‐light filtering IOL 2 ‐ Sensar AR40e (AMO)
Inclusion criteria: people aged between 50 and 80 years, bilateral cataracts, potential acuity > 0.2 logMAR units, preoperative corneal spherical aberration (Z04) values between 0.1 and 0.25 pm at 5 mm pupil diameter, and IOL power between + 18.00 and + 24.00 diopters (D) Exclusion criteria: people with any of the following present: corneal astigmatism of 1.00 D or more, surgical complications, IOL tilt and decent ration estimated by retroillumination, glaucoma, amblyopia, corneal pathology, history of uveitis, diabetic retinopathy, pseudoexfoliation syndrome, macular pathology, or previous intraocular surgery. People using topical medications (apart from lubricants) or systemic steroids Comparison of study groups at baseline: no statistically significant differences among groups in terms of age (P > 0.05) were noted. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL 2
| |
| Outcomes | BCVA, contrast sensitivity (mesopic and photopic), pupil size, corneal abberations and wavefront spherical aberration of the whole eye, at two months, one year and two years postoperatively Postoperative complications (Nd:YAG capsulotomies) at two years postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: the authors have no proprietary interest in the materials presented herein. Country: Italy Setting: Department of Ophthalmology, University of Siena, Italy Comments: Date study conducted: surgeries performed between March 2004‐July 2005 Trial registration number: not reported Contacting study investigators: authors contacted on 14 August 2017 regarding distance BCVA and contrast sensitivity units of error (specifically, whether it is the SD or a different unit or error). We also asked for clarification with regard to why the longer‐term follow‐up data for individuals fitted with the Sofport L161AO lens (described in the earlier paper by Caporossi 2007, are not reported here). No response was received to the email, and as a consequence we could not incorporate these data in the meta‐analyses. Corresponding author's name: Gianluca Martone, MD Institution: Department of Ophthalmology, University of Siena, Italy Email: [email protected] Address: Gianluca Martone, MDVia Fontenuovu n.2O, 53100, Siena, Italy | |
| Notes | Outcomes:We had already considered the rate of intraoperative complications in this group of participants in Caporossi 2007, so we did not extract the data again from this paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation was administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: we assume that in the absence of reporting, neither participants nor study personnel were masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: we assume that in the absence of reporting, the outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: participants with “surgical complications, IOL tilt and decentration estimated by retroillumination” were excluded, but the paper also states that “no intraoperative or postoperative complications were recorded" in the study. At two years postoperatively, 89 participants (178 eyes) out of 200 participants remained in the study; reasons for incomplete follow‐up are included (Table 2), and this is relatively equal across the four intervention groups. Although, note that the Caporossi 2007 paper, which reported baseline and two‐month follow‐up data for the same participants, included five lens types rather than four lens types (data relating to the Sofport L161AO lens are not included in this paper, without explanation). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, where both eyes of each participant were randomised to the same type of intervention, but the unit of analysis was not reported Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Visual acuity, colour perception and contrast sensitivity; time point not reported | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported. Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Robert J Cionni, MD Institution: not reported Email: not reported Address: not reported | |
| Notes | AAO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: study is described as "patient‐masked" only |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: study is described as "patient masked" |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with inter‐eye comparison (i.e. 1 eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL). Exclusions after randomisation: participants with perioperative or postoperative complications were excluded, but no details were provided about these participants. Losses to follow‐up: people that did not attend follow up or did not use the indicated postoperative medications were excluded, but no details were provided about these participants. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people requiring bilateral cataract surgery with an IOL implant Exclusion criteria: people who didn't attend follow‐up or didn't use the indicated postoperative medications; with systemic diseases that could influence visual function; with postoperative refractive error > 3.00 D; with perioperative or postoperative complications Comparison of study groups at baseline: no baseline information provided on the included participants | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Contrast sensitivity scores with CSV 1000E (®Vistech), for 3, 6, 12 and 18 cycles/degree at 2.6 meters, in scotopic conditions without glare at eight weeks postoperatively Colour vision errors with the Farnsworth test (25 colours) at eight weeks postoperatively participants' subjective measures of self visual function, including colour vision and glare at eight weeks postoperatively BCVA, in decimal scale, at eight weeks postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: JA Cristobal Institution: Hospital Clínico Universitario Lozano Blesa, Zaragoza, España Email: not reported Address: not reported | |
| Notes | Article in Spanish (translated to English for data extraction) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported, and "patients with perioperative or postoperative complications" were excluded from participation; details of those excluded on these grounds are not provided |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: no baseline information available on the included participants |
| Methods | Study design: RCT Study grouping: parallel group, involving the randomisation of 57 people and 61 eyes, with no further details regarding treatment allocation Exclusions after randomisation: participants who were unable to understand or co‐operate with the contrast sensitivity examination were excluded, but no details were provided about these participants. Losses to follow‐up: participants who were lost to follow‐up were excluded, but no details were provided about these participants How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Blue‐light filtering IOL group 2
Non‐blue‐light filtering IOL group
Inclusion criteria: people were included who had age‐related cataract, underwent bilateral cataract surgery at the First Affiliated Hospital, Zhejiang University, ranged in age from 50–80 years, and had low degrees of spherical diopters (≤ 2.5 D) and astigmatism (≤ 1.0 D cylindrical) postoperatively. Exclusion criteria: a history of ocular diseases, such as corneal disease, glaucoma, uveitis, and retinal detachment; a history of systemic diseases, such as diabetes and hyperthyroidism Comparison of study groups at baseline: there was no significant difference in age, sex, laterality, or diopter (power) of the IOLs among the three groups at baseline. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL
| |
| Outcomes | BCVA, higher‐order aberrations, contrast sensitivity pre‐operatively and at one week, one month and two months postoperatively Intraoperative and postoperative complications at two months postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: the authors have no proprietary or commercial interest in any materials discussed in this article. Country: China Setting: Department of Ophthalmology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Dinghua Lou, MD Institution: Department of Ophthalmology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China Email: [email protected] Address: Department of Ophthalmology, First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Rd., Hangzhou, Zhejiang, China | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided in relation to masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided in relation to masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving one eye per participant randomised to an intervention (being a mix of first and second eyes undergoing cataract surgery) Exclusions after randomisation: people with complicated surgery were excluded. One participant developed endophthalmitis and was removed from the trial, being replaced (after re‐randomisation) by another individual. Losses to follow‐up: One participant developed endophthalmitis and was removed from the trial, being replaced (after re‐randomisation) by another individual. There was one anterior capsular rim tear and one posterior capsule tear without vitreous loss. For the purposes of analysis of results, each of the six lens groups contained five participants. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL
Overall
Inclusion criteria: people were recruited from cataract preassessment clinics, and informed consent was obtained. Any person over the age of 18 able to understand English and to give informed consent was eligible for inclusion in the trial. Exclusion criteria: exclusion criteria included coexistent ocular pathology, cylinder > 2 dioptres, complicated surgery and wheelchair‐bound patients. Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOLs
Non‐blue‐light filtering IOLs
| |
| Outcomes | BCVA under standard photopic lighting conditions, contrast sensitivity (under mesopic (6 cd/m2) and photopic (65 cd/m2) conditions with and without glare (CST 1800 digital with Ginsberg Box, Vision Sciences Research Corporation), pupillometry, autorefraction, topography and wavefront analysis preoperatively, at two weeks and three months postoperatively | |
| Identification | Sponsorship source: Funding sources: no financial support received Declaration of interest: no financial interests Country: England Setting: Department of Ophthalmology, Oxford Eye Hospital, West Wing, John Radcliffe Hospital Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: FM Cuthbertson Institution: Department of Ophthalmology, Oxford Eye Hospital Email: [email protected] Address: Department of Ophthalmology, Oxford Eye Hospital, West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DUUK. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: Assessor masked but no mention of participant masking |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The two observers carrying out the pre and postoperative assessments (FC and SD) were masked as to which lens the patient had received." Judgement comment: clearly states that the outcome assessors were masked to the lens allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not explicitly reported. Participants with complicated surgery were excluded from the analysis. "One participant developed endophthalmitis and was removed from the trial, being replaced (after re‐randomisation) by another individual. There was one anterior capsular rim tear and one posterior capsule tear without vitreous loss. For the purposes of analysis of results, each of the six lens groups contained five patients." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other source of bias |
| Methods | Study design: RCT Study grouping: parallel group, where both eyes of each participant were randomised to the same type of intervention Exclusions after randomisation: participants who were unable to understand or co‐operate with the contrast sensitivity examination were excluded, but no details were provided about these participants. Losses to follow‐up: of the 291 participants in the starting sample, 257 participants completed both a baseline HRQoL assessment and at least one of the two assessments following IOL implantation in the second eye and thus were eligible for the HRQoL analyses. Of the 34 ineligible participants, six failed to complete any of the three HRQoL assessments, 14 did not complete a baseline assessment, and 14 completed only a baseline assessment. 19 of the 34 ineligible participants had been implanted with the blue light–filtering IOL, while the other 15 had been implanted with the clear IOL. How missing data were handled: the primary analysis consisted of mean change to the third assessment or early termination, using the last observation carried forward approach to impute missing observations; if a score was missing at the third assessment, the score from the second assessment was carried forward, but no scores were carried forward from the baseline assessment. Analyses were also conducted on mean change to the second and third assessments without last observation carried forward to ensure the robustness of the findings. Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people requiring bilateral extraction of age‐related cataracts with implantation of a posterior chamber IOL; aged ≥ 60 years of age, in good general and ocular health; expected to achieve at least 20/40 postoperative visual acuity, and passed both the Farnsworth D‐15 Panel Test and the Ishihara colour tests Exclusion criteria: people with alcoholism, Alzheimer’s disease, or terminal cancer; people with other eye conditions (including colour blindness or other colour‐vision deficiencies) or taking other medications that could interfere with the results to accurately measure any impact on colour vision and other outcomes postoperatively Comparison of study groups at baseline: as shown in Table 2, there were no treatment group differences at baseline with respect to age, ethnicity, or logMAR visual acuity lines in the first operative eye. However, there was a statistically higher percentage of women in the blue light–filtering IOL group than in the clear (non‐blue light filtering) IOL group. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | The primary treatment comparison analyses focused on the vision‐specific he 39‐item National Eye Institute Visual Functioning Questionnaire (NEI VFQ‐39) score, colour vision, and driving scales, and the generic 2‐item Short Form Health Survey (SF‐12) physical and mental component summary scales. Assessments were made at baseline before implantation in the first eye and 30‐60 days and 120‐180 days after implantation of the lens in the second eye. | |
| Identification | Sponsorship source: Funding sources: Supported by Alcon Laboratories, Inc. Declaration of interest: Dr. Rajagopalan was employed at Alcon Laboratories, Fort Worth, Texas, USA, at the time this research was conducted Country: USA Setting: six clinical sites in the USA Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information provided for review Corresonding author's name: Derek Espindle, MA Institution: Mapi Values Email: [email protected] Address: Derek Espindle, MA, 15 Court Square, Suite 620, Boston, Massachusetts 02108, USA | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation was administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients and HRQoL data collectors were masked to treatment;" Judgement comment: clearly states that participants and personnel not aware of which treatment was received |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients and HRQoL data collectors were masked to treatment; however, clinical investigators were not masked to treatment because the chromophore of the blue light–filtering IOL gives it a visible yellowish tint." Judgement comment: clearly stated that outcome assessors were masked, although investigators were not |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | High risk | Quote: "Supported by Alcon Laboratories, Inc. Dr. Rajagopalan was employed at Alcon Laboratories, Fort Worth, Texas, USA, at the time this research was conducted." Judgement comment: Industry funding and 1 author was an employee of the IOL manufacturer |
| Methods | Study design: RCT Study grouping: parallel group, with two study groups (non‐blue‐light filtering IOL vs different non‐blue‐light filtering IOL, n = 25 eyes each per comparison; non‐blue‐light filtering IOL vs blue‐light filtering IOL, n = 27 eyes each per comparison) Exclusions after randomisation: participants with incomplete follow‐up were excluded from the analyses Losses to follow‐up: participants with incomplete follow‐up were excluded from the analyses How missing data were handled: not reported Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOLs
Non‐blue‐light filtering IOL
Inclusion criteria: people with visually significant bilateral cataract; people with no history of glaucoma Exclusion criteria: people with any ocular disease, such as corneal opacities or irregularity, dry eye, amblyopia, anisometropia, retinal abnormalities, surgical complications, IOL tilt, previous or current use of medications known to cause colour‐vision deficiencies, or incomplete follow‐up Comparison of study groups at baseline: paired eye study; no group differences reported and "no eye had intraoperative complications" | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOLs
| |
| Outcomes | The primary outcome measures were contrast sensitivity (photopic and mesopic) and blue‐on‐yellow perimetry values (mean deviation and pattern standard deviation), at two years postoperatively Safety outcomes were the rate of intraoperative complications and the proportion of participants requiring capsulotomy at two years postoperatively | |
| Identification | Sponsorship source: Funding sources: no specific financial support Declaration of interest: no potential conflicts of interest for any authors Country: Brazil Setting: Study conducted in Ophthalmology Department of the University of São Paulo, São Paulo, Brazil Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Rodrigo F. Espíndola Institution: Ophthalmology Department of the University of São Paulo, São Paulo, Brazil Email: [email protected] Address: Rodrigo F. EspíndolaPraça das Hortências, 70 ‐ Itu (SP) ‐ 13301‐689 ‐ Brazil | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequenced and sealed envelopes containing the first type of IOL (Akreos AO or Akreos Fit; SN60AT or MA60AC) were prepared before surgery. An unscrubbed observer in the operating room opened the envelopes and assigned each patient." Judgement comment: clearly states how the intervention allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All patients and observers were masked about the IOL type implanted." Judgement comment: clearly states that all participants and observers were masked to the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The observers who conducted the postoperative visual evaluations did not have access to the randomization code or information about the surgical procedures." Judgement comment: clearly states that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "All patients completed 24 months of follow‐up." Judgement comment: although the paper states "All patients completed 24 months of follow‐up", an exclusion criterion was that "Patients with surgical complications or incomplete follow‐up were excluded". Although "there were no eyes with intraoperative complications," it is unclear whether follow‐up was 100‐percent for all participants as this was an exclusion criterion. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant contributing to the analyses Exclusions after randomisation: none apparent Losses to follow‐up: none How missing data were handled: not reported; all participants were reported to complete the final study visit Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: diagnosed vitreoretinal pathologic features, including diabetic vitreous haemorrhage, macular hole, epiretinal membrane, or persisting macula oedema, and coexisting significant cataract; the need for combined surgery, namely pars plana vitrectomy, phaco‐emulsification, and IOL implantation; age > 50 years Exclusion criteria: pseudophakia on the non‐study eye; the need for silicone oil tamponade; optic atrophy Comparison of study groups at baseline: "Patients’ baseline demographic data were comparable between both IOL groups (p>0.05, t test)." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Intraoperative conditions for the surgeon, the functional outcome of the surgery (as related to BCVA, contrast sensitivity, colour vision and glare effects), complication rates (intraoperative and postoperative ) and vitreo‐retinal diagnoses, at seven days, one month and three months of follow‐up | |
| Identification | Sponsorship source: Funding sources: none Declaration of interest: none for all authors Country: Austria Setting: the Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser surgery, Department of Ophthalmology, Rudolf Foundation Clinic, Vienna, Austria Comments: Date study conducted: 14 October 2004‐March 2006 Trial registration number: ClinicalTrials.gov NCT00537992 Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Christiane I. Falkner‐Radler Institution: The Ludwig Boltzmann Institute of Retinology and Biomicroscopic Lasersurgery Department of Ophthalmology, Rudolf Foundation Clinic Email: christiane.falkner‐[email protected] Address: The Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser Surgery, Department of Ophthalmology Rudolf Foundation Clinic, Juchgasse 25, A‐1030, Vienna, Austria | |
| Notes | Interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients included were assigned randomly to receive 30 clear UV‐filter IOLs (clear IOL group) and 30 yellow‐ blue light–filter IOLs (yellow IOL group)." Judgement comment: not reported how allocation was administered. Assignment is described as "random" but without further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking; we assume that in the absence of reporting, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A masked statistical analysis for the questionnaire responses and the other main outcome parameters, namely mean differences between three‐month postoperative and baseline values for distance visual acuity (DVA), near visual acuity (NVA), contrast sensitivity, color vision, and glare effect for each IOL group was performed." Judgement comment: no information was provided in relation to the masking of outcome assessors (only the statistician); we assume that in the absence of reporting, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "No patient was lost to follow‐up, and all patients completed the three‐month postoperative follow‐up examination." Judgement comment: no participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to a protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group study, with no further details relating to the allocation of the interventions Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Preoperative and postoperative findings were obtained regarding colour discrimination (using the Farnsowrth panel D‐15 test) and contrast perception (Pelli‐Robson contrast / sensitivity test); the time point for follow‐up was not reported. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Austria Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information provided for review First author's name: B Hahsler Institution: not reported Email: not reported Address: not reported | |
| Notes | Conference abstract, in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge other sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with inter‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Functional acuity contrast test and contrast sensitivity; follow‐up period not reported | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Austria Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors contacted for more information about abstract; no additional information provided for review First author's name: B Hahsler Institution: not reported Email: not reported Address: not reported | |
| Notes | Conference abstract, in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information in abstract to assess other potential sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with bilateral implantation of the same intervention in both eyes, and data averaged between eyes for analyses Exclusions after randomisation: one participant was excluded due to an epi‐retinal membrane (the group assignment was not reported) Losses to follow‐up: of the 80 people enrolled, six were excluded from the analysis; four did not appear for a follow‐up examination because of scheduling conflicts, one refused the examination, and one had a clinically significant epiretinal membrane at the macula. Thus, 74 participants (92.5%) completed the examinations and remained in the analysis. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: admission for bilateral cataract surgery Exclusion criteria: pathology of the cornea, retina or optic nerve; a history of ocular surgery or inflammation; a pupillary diameter < 6.0 mm after mydriasis; eyes scheduled for extracapsular cataract extraction; eyes of patients with diabetes; patients who anticipated any difficulty in follow‐up. Comparison of study groups at baseline: "No statistically significant difference was found between the groups (at baseline) regarding age, the ratio of men to women, manifest spherical equivalent, keratometric cylinder or pupillary diameter." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Visual acuity, contrast visual acuity with and without a glare source under photopic (100 cd/m2) and mesopic (slightly higher luminance than typically used ‐ 5 cd/m2) conditions at two weeks and three months after surgery using the contrast sensitivity accurate tester The incidence of participants who noted cyanopsia at three months after surgery | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: "The authors have no proprietary interest in any of the materials described in this article" Country: Japan Setting: Hayashi Eye Hospital, 4‐7‐13 Hakataekimae, Hakata‐ Ku, Fukuoka 812, Japan Comments: Date study conducted: 3 November 2004‐20 April 2005 Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for the review Corresponding author's name: Ken Hayashi Institution: Hayashi Eye Hospital Email: hayaski‐[email protected] Address: Hayashi Eye Hospital, 4‐7‐13 Hakataekimae, Hakata‐ Ku, Fukuoka 812, Japan | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The clinical research coordinator generated a code using a random number table." Judgement comment: the randomisation sequence was generated using a random number table by the research co‐ordinator. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: method of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients and examiners were masked to the randomisation." Judgement comment: clearly stated that participants and personnel not aware of which treatment received |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients and examiners were masked to the randomisation. The surgeon, who was also the data analyst, did not participate in any of the examinations or in the data collection." Judgement comment: clearly stated that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of the 80 patients enrolled, six were excluded from the analysis; four did not appear for a follow up examination because of scheduling conflicts, one refused the examination, and one had a clinically significant epiretinal membrane in the macula. Thus, 74 patients (92.5%) completed the examinations and remained in the analysis." Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up) and relatively equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other source of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 48 eyes from 42 individuals Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Wavefront analysis by iTrace (Tracey technologies), and contrast sensitivity test at three months post‐surgery | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors contacted for more information about abstract; no additional information provided for review. First author's name: Ahn Hyunseok MD Institution: not reported Email: not reported Address: not reported | |
| Notes | AAO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient detail in abstract to judge whether other sources of bias are present |
| Methods | Study design: RCT Study grouping: parallel group, with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: participants with incomplete follow‐up were not included in the analyses. Losses to follow‐up: during the five‐year study period, five participants were lost to follow‐up; therefore, 25 participants were considered in the analyses How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with visually significant bilateral cataract and no history of colour vision deficiency were eligible for inclusion in the study. Exclusion criteria: ocular disease such as corneal opacity or irregularity, dry eye, amblyopia, anisometropia, glaucoma, retinal abnormalities; surgical complications; IOL tilt; previous or current use of medications known to cause colour‐vision deficiencies; incomplete follow‐up Comparison of study groups at baseline: paired‐eye trial, however participants who had surgical complications were excluded, and no details were provided about whether there were exclusions due to intraoperative complications. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | The primary outcome measures were contrast sensitivity, colour vision and macular findings at five years after surgery. | |
| Identification | Sponsorship source: Funding sources: no funding sources listed Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Country: Brazil Setting: Ophthalmology Department, University of Sao Paulo, Sao Paulo, Brazil Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors contacted not contacted; no additional information used for review Corresponding author's name: Marcony R. Santhiago, MD Institution: Cole Eye Institute, Cleveland Clinic Email: [email protected] Address: Cole Eye Institute Cleveland Clinic 1700 East 13th Street Apartment 15W, Cleveland, Ohio 44114, USA | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: described as “double‐masked” with no information on who was masked |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: described as “double‐masked” with no information on who was masked |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up), equal follow‐up in both groups (as paired‐eye study) and no obvious reason why loss to follow‐up should be related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other source of bias |
| Methods | Study design: RCT Study grouping: parallel group, with inter‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: 46 patients had cataract surgery; 27 patients met the prespecified inclusion criteria Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: 46 patients had cataract surgery under similar preoperative conditions using the same phacoemulsification technique with a Legacy 20000 machine and NeoSoniX system (Alcon Laboratories, Inc.) and were randomised. 27 patients met the following inclusion criteria: visual acuity better than 20/40 in both eyes at least 1 year after surgery, stable intraocular pressures (IOP) throughout the 1‐year interval, and no signs or symptoms of glaucoma. Exclusion criteria: not reported Comparison of study groups at baseline: paired‐eye study; no significant inter‐group differences. Participants potentially excluded due to the nature of the inclusion criteria, and details about such participants are not included. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Blue‐yellow perimetry values at one year of follow‐up | |
| Identification | Sponsorship source: Funding sources: not reported. Declaration of interest: no author had a financial or proprietary interest in any materials or methods mentioned. Country: Brazil Setting: cataract and glaucoma clinics of a medical school associated with a public hospital in Brazil Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for this review Corresponding author's name: Juliana Lopes Jardim, MD Institution: Hospital das Clinicas de Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil Email: Not reported Address: Juliana Lopes Jardim, MDAvenida Dr.Ene ́as de Carvalho Aguiar, 255, CEP: 05403‐000, São Paulo‐SP, Brazil | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The observers who conducted the postoperative visual evaluations did not have access to the randomization code or information about the surgical procedures." Judgement comment: clearly stated that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: 46 participants randomised, but only 27 included in analyses at one year |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 71 people and 98 eyes (but with no further details) Exclusions after randomisation: participants with intraoperative or postoperative complications and posterior capsule opacification were excluded, but no information provided in relation to whether participants were excluded on these grounds. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 2
Inclusion criteria: people from 55‐85 years of age who had clinically significant cataract Exclusion criteria: people with ocular pathology other than cataract, neurologic or other disease known to affect contrast sensitivity (e.g. high hyperopia (> +6.0 D), high myopia (> −6.0 D), keratometric cylinder greater than 1.5 D). people with intraoperative or postoperative complications and posterior capsule opacification. Comparison of study groups at baseline: there were no statistically significant differences between groups, with respect to age, mean preoperative refractive error and best‐corrected spectacle acuity, at baseline. However, participants with intraoperative of postoperative complications and posterior capsule opacification were excluded. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 2
| |
| Outcomes | BCVA, pupil size and contrast sensitivity under mesopic and photopic conditions (with and without glare), at six months of follow‐up | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Belgium or Switzerland (unclear which) Setting: eye hospital Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: we attempted to contact the study authors emailed on 1 September 2017 for information relating to the intra‐class correlation coefficient for the within‐person clustering of BCVA; we could not identify a contact email address for any of the authors, as this was not provided on this paper or identifiable from an extensive internet search. As a result we could not include these data in any meta‐analyses. Corresponding author's name: H. Kennis Institution: Department of Ophthalmology, University Hospital, Leuven Email: not reported Corresponding author's address: H. Kennis Dienst Oogziekten UZ Leuven Kapucijnenvoer 33B‐3000 Leuven | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not explicitly reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 52 people and 68 eyes (participants received a mixture of unilateral IOL implantation and bilateral IOL implantation) Exclusions after randomisation: participants with poor co‐operation, complicated cataracts and any negative events resulting from cataract surgery were excluded from the study; no details about these participants were provided. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL group 2
Inclusion criteria: people who underwent cataract surgery and IOL implantation with otherwise normal eye findings Exclusion criteria: people with diabetes mellitus, poor co‐operation, glaucoma (diagnosed by intraocular pressure, visual field exam, optic nerve morphology, and retinal nerve fibre layer findings), complicated cataracts and any negative events resulting from cataract surgery Comparison of study groups at baseline: there were no significant differences among the three IOL groups with respect to visual acuity and spherical equivalence in the preoperative and postoperative phases. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2
| |
| Outcomes | Frequency doubling technique ‐ Humphrey matrix pattern standard deviation and mean deviation at two months of follow‐up | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no potential conflict of interest relevant to this article was reported Country: Korea Setting: Department of Ophthalmology and Visual Science, The Catholic University of Korea College of Medicine, Seoul, Korea Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Choun‐Ki Joo, MD, PhD Institution: Department of Ophthalmology and Visual Science, The Catholic University of Korea College of Medicine, Seoul, Korea Email: [email protected] Corresponding author's address: Choun‐Ki Joo Department of Ophthalmology and Visual ScienceSeoul St. Mary’s Hospital 505 Banpo‐dong, Seocho‐gu, Seoul 137‐040, Korea | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not explicitly reported. "Any negative events resulting from cataract surgery were excluded from the study," so it was not an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: not reported Losses to follow‐up: 11 eyes were excluded because participants refused to receive SD‐OCT imaging or receive follow‐up examinations. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people who were undergoing phacoemulsification due to simple cataracts at the Department of Ophthalmology, Severance Hospital, Yonsei University College of Medicine, between February and September 2009. Exclusion criteria: conditions affecting retinal nerve fibre layer (RNFL) thickness measurements, such as diabetic retinopathy, glaucoma, degenerative or exudative retinopathy, optic nerve drusen, peripapillary atrophy and tilted disc syndrome; history of previous ocular surgery; vitreous opacity Comparison of study groups at baseline: "The participant characteristics are summarized in Table 1. There was no statistically significant difference between the two groups with respect to preoperative demographic findings." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Peripapillary retinal nerve fibre layer thickness, measured using a Cirrus SD‐OCT, before and eight weeks after cataract surgery | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper." Country: Korea Setting: Department of Ophthalmology, Severance Hospital, Yonsei University College of Medicine Comments: Date study conducted: February 2009‐September 2009 Trial registration number: not reported Contacting study investigators: study authors contacted on 20 July 2016 with regard to the content of Tables 1‐10 (which were not provided in the main text or as supplementary material). Tables provided by Dr Kim via email (20 July 2016); Dr Kim was contacted again by email on 1 August 2016 regarding Table 2, which should report BCVA data, but does not (we asked whether it was possible to obtain these data, but no response was received). Corresponding author's name: Chan Yun Kim, MD, PhD Institution: Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine Email: [email protected] Corresponding author's address: Institute of Vision Research, Department of Ophthalmology Yonsei University College of Medicine, 134 Shinchon‐dong, Seodaemun‐gu, Seoul, 120–752, Korea | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking. In the absence of reporting, assume patients and personnel were not masked |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. In the absence of reporting, assume outcome assessors were not masked |
| Incomplete outcome data (attrition bias) | High risk | Judgement comment: missing data for 11/50 participants (22%) and unequal follow‐up between groups (n=23 eyes in blue‐light filtering IOL group, n=16 in clear IOL group) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other source of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 15 participants (number of eyes not reported) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: Non‐amblyopic patients between 60 and 80 years, with "inconspicuous" colour recognition (Ishihara) Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Visual acuity and contrast sensitivity at six weeks postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Germany Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors contacted to obtain further details about the abstract; no additional information provided for review First author's name: J Kuchenbecker Institution: Universitäts‐Augenklinik Magdeburg Email: not reported Corresponding author's address: not reported | |
| Notes | Conference abstract, in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: study is described as open label |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: study is described as open label |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge other sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: one of the SN60 (blue‐light filtering) IOL participants was excluded early in the study as he refused to be operated on his other eye. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with age‐related cataracts requiring extraction, but an otherwise normal ocular examination. Exclusion criteria: people with other ocular pathologies, high hyperopia or myopia (6.00 D), or neurological diseases and patients using medications with a possible influence on contrast sensitivity or colour vision. Comparison of study groups at baseline: no significant differences, other than a difference in distribution by sex. A total of 10 participants were randomised to each group but one was dropped from the SN60 group (as they refused to be operated on the other eye). | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Distance BCVA (measured using a Snellen chart), contrast sensitivity (Pelli–Robson contrast sensitivity chart) and colour perception (Farnsworth–Munsell D‐15 panel test) at one, three and six months postoperatively The postoperative change in distance BCVA, considered as a dichotomous outcome, was interpreted from the reporting of the study results, "Preoperative BCVA in both groups ranged between 20/30 and 20/200. Postoperative BCVA after six months in the SN60 group was 20/20 or better in all eyes, except 1 (11%), which had a BCVA of 20/30. Postoperative BCVA in the SA60 group was 20/20 or better in all eyes." Intraoperative complications and postoperative complications at six months of follow‐up | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Australia Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Igal Leibovitch Institution: Department of Ophthalmology and Visual Sciences, Royal Adelaide Hospital Email: [email protected] Corresponding author's address: Dr Dinesh Selva Department of Ophthalmology and Visual Sciences Royal Adelaide Hospital North Terrace Adelaide 5000 South Australia, Australia | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomised to receive one of the two lenses by drawing a blank envelope for each patient out of a box of 20 envelopes. A note in each envelope stated whether an SA60 or SN60 lens would be implanted." Judgement comment: opaque envelopes used for allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "All patients were operated by a single surgeon (GP) and were unaware of the type of IOL implanted." Judgement comment: Participants are reported to be masked, but there is no mention of whether personnel were masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data is not explicitly described, but there appears to be 100% participant retention at six months |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, where both eyes were implanted with the same type of IOL and both eyes were used in the analyses (considered as independent samples) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: all eyes that received a test or control IOL and had at least one postoperative examination were included in the postoperative analyses. Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people had to be healthy adults older than 60 years who had bilateral age‐related cataracts. people had to be willing and able to wait at least 30 days (but no more than 60 days) between cataract extraction procedures and had to successfully pass the Ishihara colour test and Farnsworth‐Munsell D‐15 colour perception test preoperatively. Exclusion criteria: people with retinal abnormalities, glaucoma, diabetic retinopathy, and previous or current use of medications known to cause colour‐vision deficiencies. Comparison of study groups at baseline: there were no statistically significant differences between the test and control groups in terms of age, sex, or race (Table 1). | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | People were examined 1‐2 days, 7‐14 days, 30‐60 days, 120‐180 days, and 330‐420 days after the first procedure. Screening for IOL implantation in the fellow eye was performed at the 30‐ to 60‐day postoperative examination. Distance BCVA was determined at all postoperative evaluations using the standard Snellen chart. Contrast sensitivity was evaluated at the 30‐ to 60‐day and at the 120‐ to 180‐day postoperative examinations using the CSV‐ 1000E contrast sensitivity unit (Vector Vision) calibrated to assure 85 cd/m2 luminance, under photopic and mesopic conditions. Colour perception was measured at the 30‐ to 60‐day and at the 120‐ to 180‐day postoperative examinations, using the Farnsworth‐Munsell D‐15 colour perception test. Clinical observations of the operative eyes and the IOLs were recorded at each postoperative examination. The occurrence of hyphema, hypopyon, pupillary block, cystoid macular edema, infection or endophthalmitis, retinal detachment, and secondary surgical intervention was noted, as was the presence of IOL tilt, decentration, or dislocation. At all visits through 120 to 180 days, posterior capsule opacification was graded subjectively by slitlamp biomicroscopy as none, clinically nonsignificant, clinically significant, or clinically significant requiring neodymium: YAG (Nd:YAG) laser posterior capsulotomy, using pre‐established criteria. | |
| Identification | Sponsorship source: Funding sources: sponsored by Alcon Laboratories, Inc. Declaration of interest: Drs. Cionni, Lehmann, and Maxwell are consultants to Alcon. No author has a financial or proprietary interest in the materials and methods mentioned Country: USA Setting: multicentre USA clinical trial Comments: Date study conducted: September 2000‐December 2001 Trial registration number: not reported Contacting study investigators: study authors contacted on 1 September 2017 for details relating to the intra‐class correlation for the within‐person clustering, for the outcome measures of: BCVA, proportion of people with cystoid macular oedema at six months, intraoperative complications, and postoperative complications; no response was received after more than one month. First author's name: John Marshall, PhD Institution: Department of Ophthalmology GKT, The Rayne Institute, St. Thomas’ Hospital, London Email: [email protected] Corresponding author's address: John Marshall Department of Ophthalmology GKT The Rayne Institute, St. Thomas’ Hospital London SE1 7EH, England | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: study described as 'patient‐masked' only, and thus personnel were not masked |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: study described as 'patient‐masked' only |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: although 150 participants implanted with natural IOL and 147 with control (bilateral) only those eyes that had at least 1 postoperative examination were included in the analyses which dropped the numbers to 135 and 127 for BCVA and 109 and 102 for colour. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | High risk | Quote: "Philadelphia, Pennsylvania, USA, June 2002. Sponsored by Alcon Laboratories, Inc. Drs. Cionni, Lehmann, and Maxwell are consultants to Alcon. No author has a financial interest." Judgement comment: study was sponsored by Alcon Laboratories, and Drs Cionni, Lehmann and Maxwell were consultants to Alcon |
| Methods | Study design: RCT Study grouping: parallel group, with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL). Exclusions after randomisation: 51 patients were screened and enrolled in the study. Four participants were not treated as specified in the study protocol and were excluded. Thus, 47 participants were randomised to treatment Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL
Inclusion criteria: Bilateral cataract for which phacoemulsification and posterior IOL implantation were planned in both eyes. No prior ophthalmic surgical procedures. Age from 50‐80 years, inclusive. Bilateral potential visual acuity of 0.5 (20/40 Snellen) or better as estimated by the surgeon. No known colour‐vision deficiency. Normal colour‐vision test results on Ishihara plates. Surgery in both eyes performed by same surgeon within six weeks. Suitable for study participation according to the judgment of the clinical investigator Exclusion criteria: congenital ocular abnormalities. Inadequate visualisation of the fundus. IOL power calculation < +10.0 diopters (D) or > +30.00 D. Astigmatism > 2.5 D. intraoperative complications (e.g. capsule rupture, bleeding). Participation in another study at the same time or 14 days before enrolment in the study. People with a history of uveitis and current intraocular inflammation, uncontrollable glaucoma, proliferative diabetic retinopathy, or retinal detachment Comparison of study groups at baseline: the preoperative parameters for screened and enrolled participants were similar for the two IOL types, with no statistically significant differences in age; sex; UCVA; BCVA; IOP; corneal topography; photopic, mesopic, or scotopic pupil diameter; axial length; or endothelial cell density. It should be noted that participants with intraoperative complications were excluded, and no data were provided in relation to these potential exclusions, although the authors did state that "no serious ocular adverse events occurred during the study,” and "there were no pathologic findings on fundus examination in any eye at any follow‐up visit." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Uncorrected and BCVA, pupil size, contrast vision (Early Treatment Diabetic Retinopathy Study (ETDRS) chart), functional acuity contrast test (photopic, mesopic, mesopic with glare), and colour discrimination (Farnsworth‐Munsell 100‐hue test, photopic and mesopic) at six and 12 months postoperatively | |
| Identification | Sponsorship source: Funding sources: supported by Hoya, Frankfurt, Germany. Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: Germany Setting: five clinical ophthalmology centres in Germany Comments: Date study conducted: March 2005‐May 2007 Trial registration number: not reported Contacting study investigators: study authors contacted on 1 September 2017, regarding:
No response was received from the authors after more than one month, and as a result we could not incorporate these data in our meta‐analyses. First author's name: Ulrich Mester, MD Institution: Department of Ophthalmology Bundesknappschaft’s Hospital Email: sek‐[email protected] Corresponding author's address: Ulrich Mester Department of Ophthalmology Bundesknappschaft’s Hospital An der Klinik 10, G‐66280 Sulzbach Germany | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provide on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Fifty‐one patients were screened and enrolled in the study. Four patients were not treated as specified in the study protocol and were excluded. Thus, 47 patients were randomised to treatment arm 1‐1 (operation sequence: yellow IOL, clear" Judgement comment: missing data < 20%, intra‐individual comparative study, however no information is provided on four participants treated off protocol and excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
| Other bias | High risk | Quote: "Supported by Hoya, Frankfurt, Germany." Judgement comment: the study was funded by the IOL manufacturer. |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: Two IOLs in Group 3 (blue‐light filtering IOL group) were placed with one haptic in the capsular bag and one haptic outside the capsular bag; these two participants were also excluded from the final statistical analyses. Losses to follow‐up: one participant in Group 2 (one of the two non‐blue‐light filtering IOL groups) was lost to follow‐up and was not included in the final statistical analyses. How missing data were handled: it appears that missing data were excluded from the analysis. Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOL (Group 3)
Non‐blue‐light filtering IOL1 ‐ MA60AC (Group 1)
Non‐blue‐light filtering IOL2 ‐ SA60AT (Group 2)
Inclusion criteria: people ≥ 60 years, of either sex, of any race, and scheduled for cataract surgery; in good general and ocular health, willing to attend all postoperative visits, and expected to achieve postoperative visual acuities of 20/40 or better Exclusion criteria: people with a history of uveitis, uncontrolled diabetes, diabetic retinopathy, Sjogren syndrome, trauma to the operative eye, use of anti‐inflammatory medications for any reason, use of topical prostaglandin analogues for ocular hypertension or glaucoma, bleeding tendencies, congenital ocular abnormality, or a nonfunctioning fellow eye. people with intraoperative complications including capsule tears, significant anterior chamber hyphema, zonule rupture, or out‐of‐the‐bag IOL implantation. people at risk for intraoperative complications by virtue of pseudoexfoliation syndrome or poor pupil dilation (6.0 mm). people having multiple planned procedures (i.e. cataract and trabeculectomy or corneal transplantation), except those having concurrent relaxing keratotomy for astigmatism correction. Comparison of study groups at baseline: no statistically significant differences between groups in any parameter at baseline, but no data reported for those participants who appear to be excluded from the analyses (n = 1 participant from Group 2, and n = 2 participants from Group 3, but all of Group 2 were included in the demographic data). | |
| Interventions | Intervention characteristics Blue‐light filtering IOL (Group 3)
Non‐blue‐light filtering IOL1 ‐ MA60AC (Group 1)
Non‐blue‐light filtering IOL2 ‐ SA60AT (Group 2)
| |
| Outcomes | Postoperative evaluations were performed at one week, one month and three months. All visits included assessment of distance BCVA after manifest refraction, cells, flare, and adverse events. The primary objective of the study was to compare postoperative inflammation (presence of anterior chamber cells and flare) between the three IOL models. Anterior chamber cells were initially graded on a 5‐point scale (0‐4); however, because all eyes were graded 0 or 1, except for 1 eye that was graded 2, values were dichotomised into 2 categories: (1) cell or (2) no cell. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: France Setting: Service d’Ophtalmologie, Universite ́Paris Descartes Hospital Cochin, Paris, France Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Antoine P. Brezin, MD, PhD Institution: Universite ́ Paris Descartes, Assistance Publique Hopitaux de Paris, Hopital Cochin, Service d’Ophtalmologie Email: [email protected] Corresonding author's address: Antoine P. Brezin Universite ́ Paris Descartes, Assistance Publique Hopitaux de ParisHopital Cochin, Service d’Ophtalmologie 27 rue du Faubourg Saint‐Jacques 75014 Paris, France | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned in a 1:1:1 ratio to receive one of the IOL models according to a randomization list." Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "single‐center unmasked 3‐month study" Judgement comment: study is described as "unmasked" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "single‐center unmasked 3‐month study" Judgement comment: study is described as "unmasked" |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data < 20% (i.e. more than 80% follow‐up) and equal follow‐up in the intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: not reported Losses to follow‐up: of the 80 participants (80 eyes) enrolled, 76 (95%) completed the study. Four participants (5%) did not attend the final follow‐up visit. How missing data were handled: it appears that missing data were excluded from the analysis Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL group 1
Blue‐light filtering IOL group 2
Non‐blue‐light filtering IOL group 2
Inclusion criteria: people with no history of ocular surgery or ocular pathology, such as corneal disorders, uveitis, disorders of the vitreous body or retina, glaucoma, or amblyopia Exclusion criteria: people with known colour deficiencies or problems concentrating Comparison of study groups at baseline: there were no statistically significant differences between IOL groups in pre‐operative age, cataract stage, visual acuity, contrast sensitivity, or colour perception. However, not all of the baseline data were reported, as it excluded four participants who did not complete the study. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL 2
| |
| Outcomes | People were examined postoperatively at one week and eight weeks. Every visit included anterior and posterior segment evaluation, intraocular pressure, corrected distance visual acuity and corrected near visual acuity. Contrast sensitivity was measured with Pelli‐Robson charts and the 1000E contrast sensitivity unit (CSV‐1000E, Vector‐Vision) with a constant test luminance level of 85 candelas/ m2. Contrast sensitivity with glare was assessed using the halogen glare test on the contrast sensitivity unit. Corrected distance visual acuity and the Pelli‐Robson test were evaluated at three light intensities (1000 lux, 100 lux, and 10 lux) to simulate day‐light and twilight. Colour discrimination was tested with the Roth 28 Hue Test. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: Austria Setting: Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser‐Surgery, Department of Ophthalmology, Rudolf Foundation Clinic, Vienna, Austria Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Beatrix Neumaier‐Ammerer, MD Institution: Department of Ophthalmology, Rudolf Foundation Hospital, Ludwig Boltzmann Institute for Retinologie and Biomicroscopic Laser surgery Email: beatrix.neumaier‐[email protected] Corresponding author's address: Beatrix Neumaier‐Ammerer Department of Ophthalmology, Rudolf Foundation Hospital, Ludwig Boltzmann Institute for Retinologie and Biomicroscopic Lasersurgery Juchgasse 25, A‐1030 Vienna, Austria | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised (envelope method)" Judgement comment: insufficient information regarding "envelope method" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind study" Judgement comment: described as “double masked” with no information on who was masked |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "prospective randomised double‐blind study" Judgement comment: described as “double blind” with no information on who was masked |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of the 80 patients (80 eyes) enrolled, 76 (95%) completed the study." Judgement comment: high participant retention (95%) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 42 participants; it does not state whether the IOLs were unilaterally or bilaterally implanted Exclusions after randomisation: one participant from the AIOL group withdrew after Visit 1 (V1), two after Visit 2 (V2), and two after Visit 4 (V4) (n = 5 withdrawals in total). Three participants from the ANIOL group withdrew after V1, two after V2, and two after V4 (n = 7 withdrawals in total). The reasons for withdrawal were as follows: illness (non‐ocular); participant deceased; logistics of transport; and not interested in participating further Losses to follow‐up: Of the 42 participants recruited, 30 attended all study visits (one week before surgery, one week after surgery, and three, six, and 12 months after surgery: V1, V2, V3, V4, and V5, respectively) How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group (ANIOL group)
Non‐blue‐light filtering IOL group (AIOL group)
Inclusion criteria: people scheduled for cataract surgery at Waterford Regional Hospital Exclusion criteria: pre‐operative logMAR visual acuity of less than 0.5 (the minimum required for reliable measurement of MPOD) and those with any evidence of macular disease Comparison of study groups at baseline: groups were similar in age (P = 0.370) and sex distribution. The mean BMI was higher in the AIOL group compared with the ANIOL group (P = 0.017) | |
| Interventions | Intervention characteristics Blue‐light filtering IOL (ANIOL)
Non‐blue‐light filtering IOL (AIOL)
| |
| Outcomes | The spatial profile of MPOD (i.e. at 0.25°, 0.5°, 1.0°, and 1.75° eccentricity) was measured with customised heterochromatic flicker photometry (cHFP) one week before and one week after surgery, and at three, six, and 12 months after surgery. Serum concentrations of lutein and zeaxanthin were measured at each study visit. BCVA, reported as a "Visual Acuity Rating" at 12 months postoperatively. | |
| Identification | Sponsorship source: Supported in full by Alcon Laboratories Inc., Fort Worth, Texas. Disclosure: "J.M. Nolan, Alcon Laboratories Inc. (F); P. O’Reilly, Alcon Laboratories Inc. (F); J. Loughman, Alcon Laboratories Inc. (F); J. Stack, Alcon Laboratories Inc. (F); E. Loane, Alcon Laboratories Inc. (F); E. Connolly, Alcon Laboratories Inc. (F); S. Beatty, Alcon Laboratories Inc. (F) The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact." Country: Ireland Setting: Waterford Regional Hospital, Ireland Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information provided for review Authors name: John M. Nolan Institution: Macula Pigment Research Group, Waterford Institute of Technology, Cork Road, Waterford, Ireland Email: [email protected] Address: Macula Pigment Research Group, Waterford Institute of Technology, Cork Road, Waterford, Ireland | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The trial was conducted in a double‐blind, randomised, controlled fashion." Judgement comment: described as “double blind” with no information on who was masked |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: described as “double blind” with no information on who was masked |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Forty‐two patients were recruited. The patients were randomised to receive either the AIOL (n = 21) or the ANIOL (n = 21) implant as a lens replacement in their cataract surgery. Of the 42 patients recruited, 30 attended all study visits (one week before surgery, one week after surgery, and three, six, and 12 months after surgery: V1, V2, V3, V4, and V5, respectively). One patient from the AIOL group withdrew after V1, two after V2, and two after V4 (n = 5 withdrawals in total). Three patients from the ANIOL group withdrew after V1, two after V2, and two after V4 (n = 7 withdrawals in total). The reasons for withdrawal were as follows: patient illness (non‐ocular); patient deceased; logistics of transport; and not interested in participating further." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | High risk | Quote: "Supported in full by Alcon Laboratories Inc., Fort Worth, Texas... The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact." Judgement comment: industry funding (Alcon Laboratories), who manufactured the interventions |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: not reported Losses to follow‐up: 109 patients, from 120 randomised, completed the three‐month follow‐up. Of 120 participants (eyes), three were transferred to a distant destination, one developed cystoid macular edema, one became seriously ill, one died, two returned at the five‐month follow‐up, and three refused to return. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Non‐blue‐light filtering IOL group
Blue‐light filtering IOL group 1 (SN60AT)
Blue‐light filtering IOL group 2 (SN60WF)
Inclusion criteria: people aged 50‐70 years and scheduled for phacoemulsification for uncomplicated senile cataracts with in‐the‐bag implantation of an AcrySof IOL. Exclusion criteria: complicated cataract, coexisting ocular pathology, glaucoma, axial length greater than 25.0 mm, nondilating pupils, history of intraocular surgery, laser therapy, retinopathy, optic nerve or macular diseases, refusal or unable to maintain follow‐up, diabetes with or without retinopathy, preoperative and postoperative astigmatism greater than 1.5 diopters (D), residual posterior capsule plaque, postoperative best corrected visual acuity (BCVA) 20/25, and posterior capsule opacification. Eyes with intraoperative complications such as posterior capsule tear, zonular dialysis, or uveal manipulation. Comparison of study groups at baseline: there was no statistically significant difference in visual acuity, age, and sex ratio between the three groups. Only 109 of the 120 randomised participants are included in the analysis and reported in baseline characteristics. | |
| Interventions | Intervention characteristics Non‐blue‐light filtering IOL
Blue‐light filtering IOL 1
Blue‐light filtering IOL 2
| |
| Outcomes | The main outcome measure was the difference in contrast sensitivity between IOLs at each spatial frequency. Contrast sensitivity, measured using the CSV‐1000E contrast sensitivity chart test face (Vector Vision) at three, six, 12, and 18 cycles per degrees (cpd) under photopic conditions (85 cd/m2) and mesopic conditions (2.7 cd/m2) with 4.0 mm and 6.0 mm fixed central apertures, with and without glare, at three months postoperatively. The outcome measure was distance BCVA, measured at three months postoperatively. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: India Setting: ladevi Cataract IOL Research Centre, Ahmedabad, India Comments: Date study conducted: December 2005‐February 2006 Trial registration number: not reported Contacting study investigators: trials authors contacted on 14 August 2017 regarding contrast sensitivity unit of error (specifically, whether it was the SD or a different unit or error); no response was received to this email. As a result, we could not incorporate these data in a meta‐analysis. Corresponding author's name: Dr Abhay R Vasavada Institution: Iladevi Cataract Research Centre, Raghudeep Eye Clinic Email: [email protected] Corresponding author's address: Abhay R. Vasavada Iladevi Cataract IOL Research Centre Raghudeep Eye Clinic, Gurukul Road, Memnagar, Ahmedabad–380 052, India | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random numbers" Judgement comment: computer generated list used to assign interventions |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "triple‐masked trial (neither participant nor investigator responsible for patients was aware of IOL type)" Judgement comment: clearly stated that participants and personnel not aware of which treatment received |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: clearly stated that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The drop‐out was 11 of 120 patients (9.2%)." Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: eyes with intraoperative complications such as posterior capsule tear, zonular dialysis, or uveal manipulation that could lead to postoperative cystoid macular oedema were also excluded. However, none of the patients were excluded because of intraoperative manipulations. Losses to follow‐up: all participants maintained their follow‐up schedule, and none of the participants dropped out of the study. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people > 50 years of age with a congenital colour deficiency and senile bilateral uncomplicated cataracts with a visual acuity of > 20/200 on the Snellen visual acuity chart with all types of cataract: nuclear (type 1), cortical (types 2 and 3), posterior subcapsular (type 4), or mixed cataracts and all grades of cataract (in a nuclear sclerosis grading system from 1‐5) Exclusion criteria: people with mature cataracts, traumatic cataracts, glaucoma, history of intraocular surgery, laser therapy, retinopathy, or optic nerve or macular diseases; who refused to or were unable to maintain follow‐up; with diabetes with or without retinopathy; and those on medications such as chloroquine, digitalis, and indomethacin. Eyes with intraoperative complications such as posterior capsule tear, zonular dialysis, or uveal manipulation that could lead to postoperative cystoid macular oedema Comparison of study groups at baseline: paired‐eye study. All participants were moderate red–green anomalous trichromats. However, those randomised but excluded from the analysis were not adequately described. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | The main outcome measure was to detect an increase in the error scores on the Ishihara plates and an increase in the number of diametrical crossings in the circular diagram on the D‐15 test after implantation of AcrySof Natural IOL (blue‐light filtering IOL), at one month, three months and six months postoperatively. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in material or method mentioned. Country: India Setting: Iladevi Cataract IOL Research Centre, Raghudeep Eye Clinic Comments: Date study conducted: December 2002‐February 2004 Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author's name: Abhay R Vasavada, MS, FRCS Institution: Iladevi Cataract IOL Research Centre, Raghudeep Eye Clinic Corresponding author's mail: [email protected] Address: Abhay R Vasavada Iladevi Cataract IOL Research Centre Raghudeep Eye Clinic Gurukul Road, Memnager Ahmedabad 380 052, India | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Low risk | Quote: "the circulating nurse in the operation theater opened 1 of the 30 preprepared envelopes containing 2 options:" Judgement comment: random assignment with pre‐prepared envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "neither participant nor the investigator responsible for the patients knew the eye assigned." Judgement comment: clearly stated that participants and personnel not aware of which treatment received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐masked trial (neither participant nor the investigator responsible for the patients knew the eye assigned to the test IOL)." Judgement comment: although it states that the trial was "double‐masked" with respect to participants and investigators being masked, it is unclear whether outcome assessors were also masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: even though it states in exclusion criteria section "Eyes with intraocular complications... were also excluded" in the Results section it states "none of the patients was excluded because of intraocular manipulations." No participant dropout |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group trial involving a total of 120 eyes, with no further details provided Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL group
Blue‐light filtering IOL group 2
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL
| |
| Outcomes | Distance BCVA, contrast sensitivity (Pelli–Robson chart, Optec® 6500, performed under photopic and mesopic conditions, with and without glare) and wavefront abberation analysis (total root mean square and mean higher order abberations), at 30 and 90 days postoperatively. | |
| Identification | Sponsorship source: Funding sources: none Declaration of interest: none Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: K M Rocha Institution: not reported Email: not reported Address: not reported | |
| Notes | ARVO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in the absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in the absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information within abstract to judge other potential sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving a total of 60 people and 120 eyes, with no further details regarding how data were analysed Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL group 2
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Blue‐light filtering IOL 2
Non‐blue‐light filtering IOL
| |
| Outcomes | Total and high‐order wavefront aberrations and contrast sensitivity at 30 and 90 days postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review Corresponding author;s name: Karolline M Rocha, MD Institution: not reported Corresponding author's email: not reported Address: not reported | |
| Notes | AAO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information to judge other potential sources of bias from the abstract |
| Methods | Study design: RCT Study grouping: parallel group, involving 60 participants (120 eyes) with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: not reported Losses to follow‐up: one participant was lost at the 60‐day follow‐up visit because of unrelated health problems. How missing data were handled: wavefront measurement for a 5‐mm pupil diameter was not acquired in 13 eyes with either decentered IOLs, capsular constriction, or a small capsulorhexis. 35 eyes in AcrySof IQ group, 36 eyes in AcrySof SN60AT group, and 34 eyes in the Sensar AR40 group completed all follow‐up examinations. Although specifics of how missing data were handled is not described. Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL group
Blue‐light filtering IOL group 2
Inclusion criteria: people with visually significant bilateral senile cataracts, no other ocular diseases, corneal astigmatism less than 2.0 diopters (D), and potential acuity better than 0.2 logarithm of the minimum angle of resolution units (Guyton‐Minkowsky Potential Acuity Meter; MARCO Ophthalmic, Inc., Jacksonville, FL) Exclusion criteria: people with glaucoma, retinal pathologic features, corneal opacities or irregularities, dry eye, amblyopia, anisometropia, intraoperative complications, and decentration between pupil and IOL more than 0.4 mm, estimated by retroillumination and digital photos Comparison of study groups at baseline: there were no statistically significant differences in age, preoperative simulated mean keratometry, and axial length across study groups. Unclear whether baseline characteristics excludes some participants (15 eyes). It should also be noted that participants with intraoperative complications were excluded, and no data are provided in relation to these potential exclusions. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2
| |
| Outcomes | Participants were examined before surgery and at one, seven, 15, 30, and 90 days after surgery. The main outcome measures were spherical aberration and depth of focus (by means of distance‐corrected near and intermediate visual acuity) at 90 days after surgery. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: the authors have no financial interest in the technology described. Country: Brazil Setting: Department of Ophthalmology, Paulista School of Medicine, Federal University of São Paulo Comments: Date study conducted: February 2005‐October 2005 Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Karolinne Maia Rocha, MD Institution: Department of Ophthalmology, Paulista School of Medicine, Federal University of São Paulo, São Paulo, Brazil Email: [email protected] Corresponding author's address: Karolinne Maia Rocha Rua 3 de Maio, 130/124, São Paulo–SP, 04044‐020 Brazil | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up was related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: baseline characteristics exclude those potentially excluded post‐randomisation for various reasons including intraoperative and postoperative complications, and no details are provided about these potential participants. Losses to follow‐up: three participants were lost to follow‐up because of private reasons (n = 2) and non‐study‐related medical reasons (n = 1). How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people who were sequentially admitted for bilateral cataract phacoemulsification with implantation of an IOL between April 2007 and November 2007; aged ≥ 50 years who were likely to complete all study visits and had a potential postoperative corrected distance visual acuity (CDVA) of 0.30 or better (logMAR). Exclusion criteria: people with active or previous ocular disease of the cornea, iris, retina, or optic nerve with potential impact on the visual acuity; colour vision deficiency; intraocular inflammation (iritis or uveitis); monocular status; history of intraocular surgery; uncontrolled systemic disease; diabetes mellitus; neurologic disease; and unavailable for follow‐up. People with a preoperative cycloplegic pupil diameter of 5.0 mm or less and surgical or postoperative complications, including vitreous loss, capsule tears, or prolonged intraocular inflammation. Comparison of study groups at baseline: participants received one each of a orange (blue‐light filtering) and clear (UV‐filtering) IOL. Baseline characteristics exclude those potentially excluded postrandomisation for various reasons including intraoperative and postoperative complications. There were no statistically significant differences in uncorrected distance visual acuity, corrected distance visual acuity, or contrast sensitivity with or without glare between the two IOL groups. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Participants were evaluated at the time of enrolment (two days before surgery) and one month, three months, and six months after surgery for each eye. Primary outcome measures were corrected distance visual acuity under scotopic (1 candela (cd)/m2) and photopic (85 cd/m2) conditions, colour vision, and contrast sensitivity with and without glare. Uncorrected distance visual acuity (UDVA) and patient satisfaction were defined as secondary outcome measures. The UDVA was tested under photopic conditions only. The postoperative change in distance BCVA, considered as a dichotomous outcome, was interpreted from the reporting of the study results in Tables 2 and 3, which provides the range of BCVA values and indicates that there were no individuals with a loss of BCVA of at least three lines, in any of the study groups. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: Germany Setting: Department of Ophthalmology, Ruhr University, Bochum, Germany Comments: Date study conducted: April 2007‐November 2007 Trial registration number: not reported Contacting study investigators: study authors were emailed on 1 September 2017, for information regarding:
No response was received after at least one month of contacting the authors, and as a result these data were not able to be included in our meta‐analyses. First author's name: Ingo Schmack, MD Institution: Department of Ophthalmology, Ruhr University Email: ingo.schmack@kk‐bochum.de Corresponding author's address: Ingo Schmack Department of Ophthalmology, Ruhr University In der Schornau 23‐25, 44892 Bochum, German | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eye was randomised before IOL implantation using an envelope technique." Judgement comment: trial is described as "randomised" and used "envelope technique" to conceal allocation but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind randomised masked fashion." Judgement comment: described as “double blind” with no specific information on whether participants and personnel were masked |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The individual who was responsible for the follow‐up examinations and data acquisition was blinded and not aware of which IOL was implanted in the eye being evaluated." Judgement comment: clearly stated that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: reports the number (22 patients) who completed the study not how many were excluded post‐randomisation for "surgical or postoperative complications" |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: of the 31 participants (enrolled), one was excluded because of subjectively disturbing vitreous floaters and two because of previously undetected macular drusen Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with no history of corneal disorders, no abnormal pupil reaction, no sign of inflammation, no opacification of optic media apart from cataract, and no retinal disorders. Exclusion criteria: people with systemic disease or those having treatment known to affect colour perception. Based on specular microscopy, patients with evident signs of macular alteration or other ocular disease after surgery. Comparison of study groups at baseline: all participants had a similar cataract grade in both eyes. Baseline age was reported for 28 of the 31 participants. It should be noted that "patients with evident signs of macular alteration or other ocular disease after surgery were not included in the study", however "no intraoperative or postoperative complications or adverse events occurred." | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Distance BCVA, colour contrast sensitivity (measured using heterochromic flicker), central and peripheral tritan colour contrast sensitivities (measured using the Moorfields Vision System, CH Electronics) at three months postoperatively. The primary outcome measure was colour contrast sensitivity for short wavelengths of visible light at three months postoperatively. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author had a financial or proprietary interest in any material or method mentioned Country: Austria Setting: Medical University of Vienna, Department of Ophthalmology, Vienna, Austria Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Gerald Schmidinger, MD Institution: Department of Ophthalmology, Medical University of Vienna Email: [email protected] Corresponding author's address: Gerald Schmidinger Department of Ophthalmology, Medical University of Vienna Waehringer Guertel 18‐20, A‐1090 Vienna, Austria | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind study" Judgement comment: study is described as "double‐blind" without specific information on how participants and personnel were masked |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The investigator performing the test was blind to the type of IOL implanted." Judgement comment: clearly stated that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: missing data less than 20% (i.e., more than 80% follow‐up) and no obvious reason why loss to follow‐up should be related to outcome |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: in all, 108 participants were recruited (including 21 age‐matched "control" participants, who did not receive an IOL and were not included these analyses), of whom 80 completed the study. Of the 28 who did not, 26 voluntarily declined to attend for the second test appointment, one had uncontrolled glaucoma and one required intercurrent hospital admission for an exacerbation of chronic obstructive pulmonary disease. Losses to follow‐up: as described above How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: participants were voluntarily recruited from routine cataract assessment clinics after providing informed consent. Participants were included regardless of whether they were being assessed for first‐ or second‐eye cataract surgery, or as controls. Exclusion criteria: participants unable to read the N48 test display on the reaction time task, those diagnosed with Parkinson’s Disease or dementia, any intercurrent illness requiring hospital admission, any previous ophthalmic history involving retinal damage including proliferative diabetic retinopathy, diabetic macular oedema, exudative AMD, central retinal artery or vein occlusion, and retinal detachment surgery. Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Choice reaction time (CRT) and the Epworth Sleepiness Score (ESS), at three months postoperatively | |
| Identification | Sponsorship source: Funding sources: not commissioned Declaration of interest: no competing interests Country: Scotland Setting: St Johns Hospital, Livingston, United Kingdom Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Dr Conrad Schmoll Institution: Princess Alexandra Eye Pavilion Email: [email protected] Corresponding author's address: Conrad Schmoll Princess Alexandra Eye Pavilion 45 Chalmers Street, Edinburgh EH3 9HA, UK | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective randomised" Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants undergoing first‐eye cataract surgery were randomised to receive either a blue‐blocking IOL (Alcon Acrysof SN60WF) or a UV‐blocking IOL (AMO Tecnis ZCB00), and were masked as to which IOL type had been implanted. Study protocol dictated that participants undergoing second‐eye cataract surgery would receive an identical lens to their previous one. These patients had not been issued with information cards after their first‐eye surgery and were therefore unaware of which lens type they received." Judgement comment: participants were reported to be masked; although personnel were reported to be masked, this is not anticipated to significantly affect the study outcomes as most outcome measures were participant‐reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: unclear whether outcome assessors were masked |
| Incomplete outcome data (attrition bias) | High risk | Quote: "108 participants were recruited, of whom 80 completed the study. Of the 28 who did not, 26 voluntarily declined to attend for the second test appointment, one had uncontrolled glaucoma and one required intercurrent hospital admission for an exacerbation of chronic obstructive pulmonary disease." Judgement comment: overall follow‐up < 80%, and follow‐up in each group not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving a total of 120 eyes (and an unspecified number of participants) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1
Non‐blue‐light filtering IOL group
Blue‐light filtering IOL group 2
Inclusion criteria: not reported Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2
| |
| Outcomes | Near visual acuity, wave‐front analysis and pupil diameter at 60 days postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information provided for review First author's name: Eduardo S Soriano, MD Institution: not reported Email: not reported Corresponding author's address: not reported | |
| Notes | AAO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised prospective study" Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information provided within abstract to judge other potential sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 31 participants (although the number of eyes is not reported) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with cataract aged 40 to 80 years Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | The mean fluorescein transmittance in vitreous by vitreous fluorophotometry, the cystoid macular oedema by fluorescence angiography, and the thickness of fovea by optical coherence tomography at three months and 12 months postoperatively | |
| Identification | Sponsorship source: Funding sources: none Declaration of interest: none for all authors Country: not reported Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: T Ueda Institution: Ophthamology, Showa University, Tokyo, Japan Email: not reported Corresponding author's address: not reported | |
| Notes | ARVO conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information provided within abstract to judge other potential sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL
Inclusion criteria: 26 cataractous eyes of 26 patients scheduled for phacoemulsification and acrylic IOL implantation were enrolled in this study. The criteria used in this study were completely normal ophthalmic examination after pupil dilation, including visual acuity ≥ 20/30, intraocular pressure 21 mm Hg, no prior automated perimetry experience, and no family history of glaucoma Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Mean deviation and pattern standard deviation on frequency doubling perimetry (24‐2 threshold) at three months postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Japan Setting: Department of Ophthalmology, Nara Medical University Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Tetsuo Ueda, MD Institution: Department of Ophthalmology, Nara Medical University, Japan Email: tueda@naramed‐u.ac.jp Corresponding author's address: Tetsuo Ueda Department of Ophthalmology Nara Medical University, 840, Kashihara‐shi Nara, 634‐8522, Japan | |
| Notes | None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "IOL implanted intraoperatively was randomly selected from clear (VA60BB, HOYA) and yellow‐tinted lenses (YA60BB, HOYA), which only differed by color." Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 37 people (52 eyes) Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: in the photographic analysis, two eyes of one participant in the Acrysof Natural IOL group were excluded because of a very lightly pigmented fundus Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: white patients scheduled for phacoemulsification and IOL implantation Exclusion criteria: hereditary colour vision defects; medications that might affect colour vision, such as ethambutol; any medication for epilepsy; amiodarone; digitalis; anti‐inflammatory drugs; diabetes; any other ocular pathology except cataract Comparison of study groups at baseline: no apparent group differences, although this was not explicitly stated and sex of participants was not given | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Colour vision (measured using Standard pseudoisochromatic plates, part 2, (SPP2) (Ichikawa et al. 1983) and the FarnsworthMunsell 100‐hue test (FM 100) (Farnsworth 1957)) and visibility of the retinal nerve fibre layer, from retinal fundus photography at one to six months postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Finland Setting: Department of Ophthalmology, Turku University Hopsital, Turku, Finland Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Marja‐Liisa Vuori, MD Institution: Department of Ophthalmology Turku University Hospital, Kiinamyllynkatu 4–8, 20520 Turku, Finland Email: marja‐[email protected] Corresponding author's address: Marja‐Liisa Vuori Department of Ophthalmology Turku University Hospital Kiinamyllynkatu 4–820520 Turku, Finland | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes containing the code for the planned IOL type were used for randomization. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: described as “double blind” with no information on who was masked |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: described as “double blind” with no information on who was masked |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: results suggest no participants were lost to follow‐up; although this is not explicitly stated |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: sex distribution between study groups at baseline is not reported; the significance of this baseline imbalance is not clear |
| Methods | Study design: RCT Study grouping: parallel group, involving 29 people (58 eyes), where both eyes received the same type of IOL but no further details are provided in relation to the statistical approach Exclusions after randomisation: not reported Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: non‐amblyopic bilateral cataract patients aged 60‐80 years with inconspicuous (normal) colour recognition test (Ishihara) Exclusion criteria: not reported Comparison of study groups at baseline: not reported | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Visual acuity, contrast sensitivity and colour vision at six weeks and four and a half months postoperatively | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: Germany Setting: not reported Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: S Walter Institution: Universitäts‐Augenklinik Magdeburg, Germany Email: Not reported Corresponding author's address: not reported | |
| Notes | Conference abstract, in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: study described as open label |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment:study described as open label |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Unclear risk | Judgement comment: insufficient information within abstract to judge other potential sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: randomised participants were excluded from the baseline data on the basis of intraoperative complications and no details about these potential exclusions were provided. Losses to follow‐up: participants who were "unable to attend for follow‐up visits" (i.e., those with incomplete follow‐up) were excluded from the analyses. No details were provided in relation to those participants potentially excluded on these grounds. How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL 1 ‐ yellow
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2 ‐ photochromic
Inclusion criteria: this study enrolled consecutive eyes that had cataract surgery with IOL implantation from November 2008‐June 2009. The inclusion criteria were senile cataract, no previous ophthalmic surgery, a potential visual acuity of 0.5 or better, and no colour vision deficiency. Exclusion criteria: people with congenital ocular abnormalities, glaucoma, proliferative diabetic retinopathy, retinal detachment, inflammatory signs, IOL power calculation < +10.00 diopters (D) or > +30.00 D, astigmatism greater than 2.00 D, intraoperative complications (e.g., posterior capsule rupture, bleeding), abnormal pupil reaction, or unable to attend the follow‐up visits. Comparison of study groups at baseline: there were no significant differences in the demographic data reported for the three IOL groups. Randomised participants were excluded from the baseline data on the basis of intraoperative complications and no details about these potential exclusions were provided. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1 ‐ yellow
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2 ‐ photochromic
| |
| Outcomes | Postoperative visits were scheduled at one day, one week, and one and three months. Outcomes were the postoperative uncorrected distance visual acuity, contrast sensitivity (testing using an Optec 6500 device (Stereo Optical Co.) at spatial frequencies of 1.5, 3.0, 6.0, 12.0, and 18.0 cycles per degree (cpd) and under photopic (85 candelas (cd)/m2), mesopic (3 cd/m2), photopic with glare (135 lux), and mesopic with glare (28 lux) lighting conditions), contrast vision (under 400 lux, 30 lux and 5 lux at 100%, 25%, and 5% contrast), hue discrimination (using the Farnsworth‐Munsell (FM) 100‐hue test under outdoor daylight conditions and indoor mesopic conditions), and the Catquest‐9SF patient questionnaire (which includes subjective evaluation of glare, halo and colour‐vision perception), at three months postoperatively. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned Country: China Setting: Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University; Beijing Ophthalmology Visual Sciences Key Laboratory, Beijing, China Comments: Date study conducted: November 2008‐June 2009 Trial registration number: not reported Contacting study investigators: authors contacted on 14 August 2017 regarding the contrast sensitivity outcome unit of error (Figure 1); no response was received to this email and, as a result, we could not include these data in the meta‐analysis First author's name: Jun Wang, MD Institution: Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology Visual Sciences Key Laboratory Email: [email protected] Corresponding author's address: Jun Wang Beijing Tongren Eye Center, Beijing Tongren Hospital Capital Medical University, Beijing Ophthalmology Visual Sciences Key Laboratory, Number 2 Chongnei Street, Bejing, China 100730 | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Judgement comment: described as “double blind” with no information on whether participants and personnel were masked |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The physician who conducted the follow‐up examinations and collected the data was not aware which IOL had been implanted in the eye being evaluated." Judgement comment: clearly states that outcome assessors were masked |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported, and participants with intraoperative complications were excluded, without specifying which groups these individuals were assigned to |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 24 people (48 eyes), with paired‐eye comparison (i.e., one eye received a blue‐light filtering IOL and the fellow eye received a non‐blue‐light filtering IOL) Exclusions after randomisation: all included participants completed the scheduled vision tests and questionnaire Losses to follow‐up: none How missing data were handled: not applicable Reported power size calculation? yes | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL
Overall
Inclusion criteria: participants were recruited from a continuous cohort who presented with bilateral age‐related cataract in the outpatient centre of the Department of Ophthalmology, Hietzing Hospital, Vienna. Exclusion criteria: amblyopia; pseudoexfoliation syndrome; primary or secondary glaucoma; ocular hypertension; uveitis; diabetes; history of intraocular surgery; laser treatment; retinal pathology; other relevant ophthalmic diseases. People with medication known to potentially cause ophthalmologic side effects; with an expected postoperative visual acuity less than 1.0 (20/20); congenital colour vision anomaly; ocular surface disease; ametropia of more than 3 diopters; with nicotine or alcohol abuse Comparison of study groups at baseline: intra‐individual comparison (no group differences) | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Contrast acuity (measured at illumination levels of 500, 5, and 0.5 lux and contrast levels of 100%, 50%, 25%, 12.5%, and 6.25%), colour vision (assessed using the Lanthony desaturated D‐15 test, the Lanthony new colour test (Munsell chroma 2 and 4), and an anomaloscope), blue/yellow foveal threshold (using short‐wave automated perimetry) and the subjective visual impression of participants (evaluated using a questionnaire) at three months postoperatively The main outcome measures were contrast acuity, colour vision, and foveal threshold. | |
| Identification | Sponsorship source: Funding sources: "This study did not receive any financial support." Declaration of interest: "The authors have no proprietary interest in any of the materials or equipments mentioned in this study." Country: Austria Setting: Department of Ophthalmology, Hietzing Hospital, Vienna, Austria Comments: Date study conducted: surgery performed between December 2005 and February 2007 Trial registration number: clinicaltrials.gov NCT0061278 Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Matthias G. Wirtitsch Institution: Department of Ophthalmology, Hietzing Hospital Email: [email protected] Corresponding author's address: Department of Ophthalmology, Hietzing Hospital Wolkersbergenstrasse 1, 1130 Vienna, Austria | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The first eye was randomly assigned to receive a multipiece acrylic foldable IOL: either a Hoya AF‐1 (UY) YA‐60BB (both blue and UV light filter) or a Hoya AF‐1 (UV) VA‐60BB (only UV light filter) (both IOLs from Hoya Medical Europe, Frankfurt/ Main, Germany). The contralateral eye received the other IOL." Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients and examiner were masked for IOL assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Study testing was performed in 1 single test session 90 plus/minus 10 days after surgery of the second eye by the same experienced and masked examiner." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All included patients completed the scheduled vision tests and questionnaire." |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes listed in trials registry (clinicaltrials.gov NCT 00612781) are reported |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 92 people (120 eyes), but with no further details regarding the allocation of interventions Exclusions after randomisation: not reported. Participants could have been potentially excluded on the basis of intra‐ and postoperative complications, however no details were provided about any potential exclusions on these grounds. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1 (SN60WF)
Non‐blue‐light filtering IOL group (aspheric ZA)
Blue‐light filtering group 2 (SN60AT)
Blue‐light filtering IOL group 3 (Hoya Py60AD)
Inclusion criteria: consecutive eyes of patients who had cataract extraction and implantation of an acrylic IOL at Keio University Hospital between October 2007 and December 2008. People with significant senile cataract and a postoperative visual acuity better than 20/20 were eligible for inclusion in the study. Exclusion criteria: people with previous or coexisting ocular pathology and intraoperative or postoperative complications Comparison of study groups at baseline: the authors state "there were no significant differences between the groups in age, IOL power, or postoperative pupil diameters under photopic and mesopic conditions (P > 0.05, Mann‐Whitney U test)." However, participants could have been potentially excluded on the basis of intraoperative and postoperative complications. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2
Blue‐light filtering IOL 3
| |
| Outcomes | The main outcome was the postoperative higher‐order aberrations (HOAs) of the cornea and whole eye (measured under photopic and mesopic conditions), measured at one month postoperatively. Other outcomes were corrected distance visual acuity, and pupil diameter under photopic and mesopic conditions, measured at one month postoperatively. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: no author has a financial or proprietary interest in any material or method mentioned. Country: Japan Setting: Keio University Hospital, Tokyo, Japan Comments: Date study conducted: October 2007‐December 2008 Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Takefumi Yamaguchi, MD Institution: Keio University School of Medicine, Japan Email: [email protected] Corresponding author's address: Takefumi Yamaguchi Keio University School of Medicine Shinanomachi 35, Shinjuku‐ku, Tokyo, Japan. | |
| Notes | Data presented in this paper for the blue‐light filtering IOLs (SN60WF and SN60AT), and the non‐blue‐light filtering IOL(Tecnis ZA9003) appear the same as in the Yamaguchi 2011 paper. The data for the PY‐60 AD (Hoya) blue‐light filtering IOL appear different. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Exclusion criteria included previous or coexisting ocular pathology and intraoperative or postoperative complications." Judgement comment: whether incomplete outcome data is relevant is unclear as patients with intraoperative or postoperative complications were excluded |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, involving 92 people (120 eyes), but with no further details regarding the allocation of interventions Exclusions after randomisation: not reported. Participants could have been potentially excluded on the basis of having complications during cataract surgery or postoperatively. The authors do state that "no eyes had any postoperative complication", but no details were provided in relation to potential intraoperative complications. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group 1 (SN60WF)
Non‐blue‐light filtering IOL group
Blue‐light filtering IOL group 2 (SN60AT)
Blue‐light filtering IOL group 3 (PY60AD)
Inclusion criteria: people with significant senile cataract and postoperative visual acuity better than 20/20 were eligible for inclusion in the study. Exclusion criteria: people with previous or coexistent ocular pathology and complications during cataract surgery or postoperatively Comparison of study groups at baseline: the authors state that "there was no significant difference in the average IOL powers, age, and postoperative refraction, astigmatism, and pupil diameters under photopic and mesopic conditions between the three types of aspheric and one type of spherical IOLs (Kruskal–Wallis test, p > 0.05)" for those eligible to participate. However, those with intraoperative complications were excluded, and there is no details about these potential events/participants. Also, some participants received bilateral implants but there was no description of first eye versus second eye. | |
| Interventions | Intervention characteristics Blue‐light filtering IOL 1
Non‐blue‐light filtering IOL
Blue‐light filtering IOL 2
Blue‐light filtering IOL 3
| |
| Outcomes | Contrast sensitivity, higher‐order aberrations of the whole eye, and pupil diameter under photopic and mesopic conditions were measured one month postoperatively. Higher‐order aberrations were decomposed into Zernike coefficients, calculated according to individual pupil diameter. The correlation between higher‐order aberrations and contrast sensitivity was evaluated. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: the authors report no conflicts of interest in this work Country: Japan Setting: Keio University Hospital, Japan Comments: Date study conducted: October 2007‐December 2009 Trial registration number: not reported Contacting study investigators: study authors not contacted; no additional information used for review First author's name: Takefumi Yamaguchi Institution: Department of Ophthalmology, Keio University School of Medicine Email: [email protected] Corresonding author's address: Takefumi Yamaguchi Department of Ophthalmology Keio University School of Medicine Shinanomachi 35, Shinjuku, Tokyo, Japan | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: trial is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: trial is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Exclusion criteria were previous or coexistent ocular pathology and complications during cataract surgery or postoperatively." Judgement comment: completeness of outcome data is unclear as participants with complications during cataract surgery were potentially excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
| Methods | Study design: RCT Study grouping: parallel group, with one eye per participant randomised to one of the interventions Exclusions after randomisation: a total of 60 participants were originally randomised; the blue‐light filtering IOL group included 27 individuals (12 men, 15 women; as three patients were non‐compliant). No further details were provided. Losses to follow‐up: not reported How missing data were handled: not reported Reported power size calculation? no | |
| Participants | Baseline characteristics Blue‐light filtering IOL group
Non‐blue‐light filtering IOL group
Inclusion criteria: people with senile cataract Exclusion criteria: not reported Comparison of study groups at baseline: there was no significant difference in visual acuities (0.80–1.02) between the yellow UV and ordinary UV IOL groups (P > 0.05) (note: it was unclear if this was at baseline or postoperative) | |
| Interventions | Intervention characteristics Blue‐light filtering IOL
Non‐blue‐light filtering IOL
| |
| Outcomes | Visual acuity, spatial contrast sensitivity (Stereo Optical, Chicago, Illinois, U.S.A.), colour vision (FM‐100, Munsell, New Windsor, New York, U.S.A.), and subjective sensation at one week to six months postoperatively Participants were questioned about photophobia and cyanopsia. | |
| Identification | Sponsorship source: Funding sources: not reported Declaration of interest: not reported Country: China Setting: Tianjin Medical University Eye Center Comments: Date study conducted: not reported Trial registration number: not reported Contacting study investigators: authors contacted on 14 August 2017 regarding the contrast sensitivity outcome unit of error; no response was received to this email, and as a result we could not include these data in the meta‐analysis First author's name: Zhaoxu Yuan, MD, PhD Institution: Medical Research Building, Scottand White Hospital Email: [email protected] Corresponding author's address: Zhaoxu Yuan Medical Research Building, Scottand White Hospital 702 SW HK Dodgen Loop, Temple, TX, USA 76504 | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Study is described as “randomised” but with no further details |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Study is described as “randomised” but with no further details |
| Blinding of participants and personnel (performance bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) | High risk | Judgement comment: no information provided on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: follow‐up not reported, although three participants in the blue‐light blocking IOL group were reported to be "non‐compliant" and appear to have been excluded from the analyses |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry |
| Other bias | Low risk | Judgement comment: no other apparent sources of bias |
AAO: American Academy of Ophthalmology
AMD: age‐related macular degeneration
ARVO: Association for Research in Vision and Ophthalmology
BCVA: best‐corrected visual acuity
BMI: body mass index
D: dioptre
HRQoL: health‐related quality of life
IOL: intraocular lens
RCT: randomised controlled trial
UCVA: uncorrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a RCT | |
| Not a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Not a RCT | |
| Not a blue‐light filtering IOL | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Not a blue‐light filtering IOL | |
| Not a RCT | |
| Not a RCT | |
| Not a blue‐light filtering IOL | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Not a RCT | |
| Not a RCT | |
| Does not appear to be a RCT | |
| Not a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Does not appear to be a RCT | |
| Not a blue‐light filtering IOL | |
| Does not appear to be a RCT | |
| Not a RCT |
IOL: intraocular lens
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Awaiting translation |
| Participants | Awaiting translation |
| Interventions | Awaiting translation |
| Outcomes | Awaiting translation |
| Notes | Awaiting translation |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to source paper |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to source paper |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The CLOCK‐IOL colour (Cataract surgery and circadian biological rhythm among Japanese older people with cataract in Nara, Kansai Region: Influence of Intraocular Lens Implantation) study |
| Methods | Parallel group, open‐label, RCT |
| Participants | Patients diagnosed as having cataracts in Nara Medical University Hospital (Japan), according to the following inclusion and exclusion criteria Inclusion criteria
Exclusion criteria
|
| Interventions | Phacoemulsification with a small incision and implantation of an IOL. Participants will be randomly allocated to the clear or blue‐blocking IOL group in a ratio of 1:1. In the clear IOL group, a clear spherical IOL (SA60AT, Alcon, Fort Worth, USA) will be implanted. In the blue‐blocking, IOL group, a spherical blue‐blocking IOL (SN60AT) or an aspherical blue‐blocking IOL (SN60WF, Alcon, Fort Worth, USA) will be implanted in a randomly allocated 1:1 ratio. |
| Outcomes | Primary outcomes The primary outcomes are mortality and the incidence of CVD, cancer and AMD after surgery Secondary outcomes The secondary outcomes are:
|
| Starting date | As detailed in UMIN Clinical Trials Registry (UMIN ID: UMIN000014680), the recruitment status on 26 February 2018 was 'Open public recruitment', with the anticipated trial start date listed as 28 July 2014 |
| Contact information | Tomo Nishi Nara Medical University School of Medicine Department of Ophthalmology 840 Shijo‐cho, Kashiharashi, Nara, Japan, 634‐8521 Email: tomon@naramed‐u.ac.jp |
| Notes | None |
AMD: age‐related macular degeneration
BMI: body mass index
IOL: intraocular lens
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period. Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| Analysis 1.1  Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period.. | ||||
| 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period. Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period.. | ||||
| 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months) Show forest plot | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.14, 0.14] |
| Analysis 1.3  Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months). | ||||
| 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 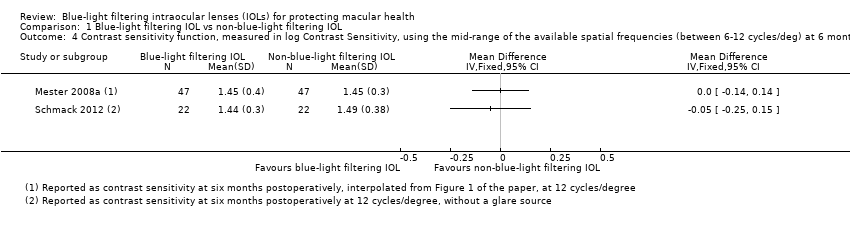 Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT). | ||||
| 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months) Show forest plot | 3 | 808 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [0.63, 7.68] |
| Analysis 1.5  Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months). | ||||

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.1 Change in distance best‐corrected visual acuity (BCVA), between baseline and 12 months (accepting measures for 6‐18 months' follow‐up. If change in distance BCVA not reported, we have utilised data reported at the end of the follow‐up period).

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period.

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.2 Effect on photopic contrast sensitivity function, measured in log Contrast Threshold (%) using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (with an acceptable follow‐up range of 3‐9 months).
![Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months) [logCT].](/es/cdsr/doi/10.1002/14651858.CD011977.pub2/media/CDSR/CD011977/image_n/nCD011977-AFig-FIG07.png)
Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months) [logCT].
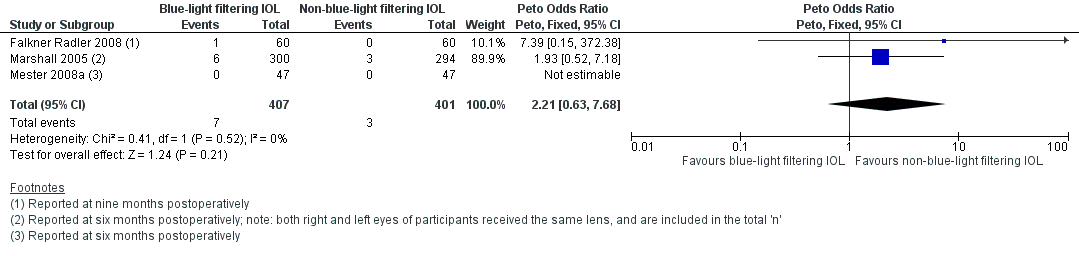
Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or optical coherence tomography or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months).

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period..

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period..

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months).

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT).
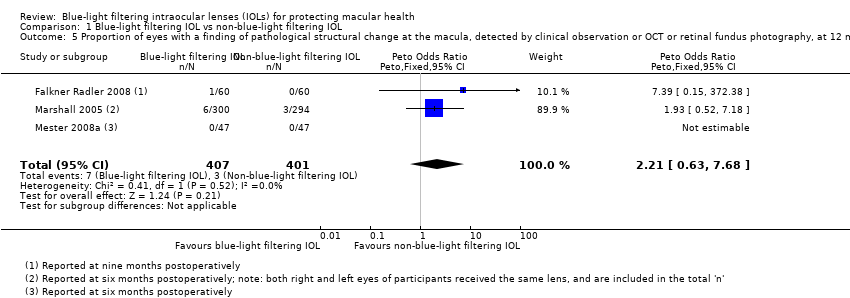
Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months).
| Blue‐light filtering IOL compared to non‐blue‐light filtering IOL for protecting macular health | ||||||
| Patient or population: adults undergoing cataract surgery with IOL implantation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐blue‐light filtering IOL | Risk with blue‐light filtering IOL | |||||
| Change in distance BCVA, between baseline and 12 months | Mean change in distance BCVA between baseline and 12 months was 0 logMAR | MD 0.01 logMAR lower | ‐ | 131 | ⊕⊕⊕⊝ | A lower BCVA (in logMAR) indicates a higher level of visual acuity. Studies in this analysis reported data at the end of the follow‐up period rather than change from baseline. |
| Distance BCVA, considered as a dichotomous outcome (being the proportion of eyes that experienced loss of 15 or more letters from baseline BCVA), at six months | See comments | Not estimable | 63 | ⊕⊝⊝⊝ | There were no eyes, in either intervention group that had a loss of 15 or more letters from baseline BCVA. | |
| Contrast sensitivity function, measured in log Contrast Sensitivity at six months | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Colour discrimination, measured as the proportion of eyes that had a measurable loss from baseline using Farnsworth‐Munsell 100‐hue colour test score under photopic conditions at six months | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Proportion of participants with adverse events with a probable causal link with the study interventions at six months follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Proportion of eyes that developed late‐stage AMD, being CNV and/or GA, at three years of follow‐up | See comments | Not estimable | 50 | ⊕⊝⊝⊝ | In the 1 trial (Kara Junior 2011) there were no eyes, in either intervention group that developed late‐stage AMD at five years of follow‐up. | |
| Proportion of eyes that developed any stage of AMD at 12 months | See comments | Not estimable | 144 | ⊕⊝⊝⊝ | In both studies, there were no eyes, in either intervention group that developed any stage of AMD over the nominated follow‐up period. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded for risk of bias (‐1). Data derive from two relatively small studies, with one (Caporossi 2009) judged to have a high risk of bias in both masking domains, and the other (Vuori 2006) having an unclear risk of bias in most domains. | ||||||
| Study | Blue‐light filtering IOL name(s)a (Manufacturer) | Non‐blue‐light filtering IOL name(s)a |
| YA‐60BB (Hoya) | AcrySof SA60AT (Alcon) or AcrySof MA60BM (Alcon) or VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) or PC4406 (Optech) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural (Alcon) | AcrySof MA60BM (Alcon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| Not reported | Not reported | |
| AcrySof IQ SN60WF (Alcon) | AMO ZCBOO (Abbott Medical Optics) | |
| AcrySof IQ SN60WF (Alcon) | AMO ZCBOO (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Sensar AR40e (Abbott Medical Optics) or Tecnis Z9000 (Abbott Medical Optics) or Sofport L161AO (Bausch & Lomb) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Sensar AR40e (Abbott Medical Optics) or Tecnis Z9000 (Abbott Medical Optics) | |
| AcrySof SB30AL (Alcon) | AcrySof SA30AL (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Tecnis Z9001 (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Tecnis Z9000 (Abbott Medical Optics) or Cee On Edge (Abbott Medical Optics) or Akreos AO (Bausch & Lomb) or Akreos Adapt (Bausch & Lomb) | |
| AcrySof Natural (Alcon) | AcrySof single‐piece (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | Akreos Fit (Bausch & Lomb) or Akreos AO (Bausch & Lomb) or AcrySof SA60AT as described in the methods (but appears to be inadvertently described as the MA60AC in the reporting of the results) | |
| AcrySof Natural (Alcon) or AF‐1 UY (Hoya) | AcrySof single‐piece (Alcon) or AF‐1 UV (Hoya) | |
| SN60 (Alcon) | SA60 (Alcon) | |
| YA (Hoya) or SN60 (Alcon) | VA (Hoya) or SA60 (Alcon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | Tecnis ZA9003 (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof MA30AC (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | Tecnis Z9000 (Pfizer) or Opti‐Edge (Abbott Medical Optics) | |
| AcrySof IQ SN60WF (Alcon) | OII Biovue3 (BioVue) or YA60BBR (Hoya) | |
| AcrySof IQ SN60WF (Alcon) | Tecnis Z9003 (Abbott Medical Optics) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof single‐piece SA60AT (Alcon) | |
| AcrySof Natural IOL SB30AL (Alcon) (the current marketed version of this lens is the SN60AT) | AcrySof SA30AL (Alcon) | |
| AF‐1 UY (Hoya) | AF‐1 UV (Hoya) | |
| AcrySof SN60AT (Alcon) | AcrySof MA60AC (Alcon) or AcrySof SA60AT (Alcon) | |
| AF1 UY (Hoya) or AcrySof SN60AT (Alcon) | AF1 UV (Hoya) or AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof SN60AT (Alcon) or AcrySof SN60WF (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof IQ (Alcon) or AcrySof Natural (Alcon) | Sensar (Abbott Medical Optics) | |
| AcrySof IQ (Alcon) or AcrySof Natural (Alcon) | Sensar (Abbott Medical Optics) | |
| AcrySof IQ (Alcon) or AcrySof SN60AT (Alcon) | Sensar AR40 (Allergan) | |
| Oculaid PC 440Y Orange Series (Ophtec BV) | Oculaid PC 430Y Elite Series (Ophtec BV) | |
| AF‐1 UY (Hoya) | AF‐1 UV (Hoya) | |
| AcrySof SN60WF (Alcon) | Tecnis ZCB (Abbott Medical Optics) | |
| AcrySof SN60AT (Alcon) or AcrySof SN60WF (Alcon) | Sensar (Abbott Medical Optics) | |
| ENV‐13 (Menicon) | ES‐13 (Menicon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AF‐1 YA‐60BB (Hoya) | AF‐1 UV‐60BB (Hoya) | |
| AY‐1 UY (Hoya) or Arium Matrix Model 4000 (Medennium) | MC611MI (HumanOptics) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof SN60WF (Alcon) or AcrySof SN60AT (Alcon) or Py60AD (Hoya) | Tecnis Z9003 (Abbott Medical Optics) | |
| AcrySof SN60WF (Alcon) or AcrySof SN60AT (Alcon) or Py60AD (Hoya) | Tecnis Z9003 (Abbott Medical Optics) | |
| Not reported (Hoya) | Not reported (not reported) | |
| aDetails of the interventions are provided as per the details available in the included studies. | ||
| Study | Study population: number of participants (number of eyes) | Number of intraoperative complication(s) | Details of intraoperative complication(s) |
| Blue‐light filtering IOLs: n = 65 (65); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 33 (33) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (60) Non‐blue‐light filtering IOL: n = 30 (60) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 75 (150); this group combined individuals assigned to 3 different non‐blue‐light filtering IOLs | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 39 (41) Non‐blue‐light filtering IOL: n = 18 (20) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Overall: n = 31 (31) | Not reported | One participant developed endophthalmitis and was removed from the trial, being replaced (after re‐randomisation) by another individual. There was one anterior capsular rim tear and one posterior capsule tear without vitreous loss. No details were provided in relation to which group(s) the adverse events occurred in. | |
| Blue‐light filtering IOL: n = 27 (27) Non‐blue‐light filtering IOL: n = 52 (77) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = ? (42) Non‐blue‐light filtering IOL: n = ? (26) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 9 (9) Non‐blue‐light filtering IOL: n = 10 (10) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 150 (300) Non‐blue‐light filtering IOL: n = 147 (294) | Blue‐light filtering IOL: n = 1 Non‐blue‐light filtering IOL: none | There was one case of lens dislocation during surgery in the blue‐light filtering IOL group, in a case in which a posterior capsule rupture had occurred during cataract extraction. | |
| Blue‐light filtering IOL: n = 19 (19) Non‐blue‐light filtering IOL: n = 40 (40); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: n = 2 Non‐blue‐light filtering IOL: none | In the blue‐light filtering IOL lens group "two IOLs were placed with 1 haptic in the capsular bag and 1 haptic outside the capsular bag; these 2 patients were also excluded from the final statistical analysis." | |
| Blue‐light filtering IOL: n = 73 (73); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 36 (36) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 31 (31) Non‐blue‐light filtering IOL: n = 31 (31) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 19 (25) Non‐blue‐light filtering IOL: n = 18 (27) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| IOL: intraocular lens | |||
| Study | Study population: number of participants (number of eyes) | Number of postoperative complication(s) | Details of postoperative complication(s) |
| Blue‐light filtering IOLs: n = 65 (65); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 33 (33) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at four weeks of follow‐up | |
| Blue‐light filtering IOL: n = 30 (60) Non‐blue‐light filtering IOL: n = 30 (60) | Blue‐light filtering IOL: n = 3 eyes Non‐blue‐light filtering IOL: n = 4 eyes | Although the study authors reported that "there were no postoperative complications", n = 3 eyes from the blue‐light filtering IOL group and n = 4 eyes from the non‐blue‐light filtering IOL group required Nd:YAG capsulotomy at six months of follow‐up. | |
| Blue‐light filtering IOL: n = 38 (38) Non‐blue‐light filtering IOL: n = 35 (35) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at six months of follow‐up. | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 75 (150); this group combined individuals assigned to three different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at two months of follow‐up | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs | Blue‐light filtering IOL: n = 1 for Nd:YAG capsulotomy Non‐blue‐light filtering IOL: n = 1 for Nd:YAG capsulotomy | This study reports the two‐year follow‐up data for a subset of participants from Caporossi 2007. The study authors reported that "patients who underwent a capsulotomy before two‐year follow‐up (two patients) were excluded as it was not possible to perform aberrometric analysis. Two Nd:YAG laser capsulotomies were required in two patients who had AcrySof SN60AT IOL (blue‐light filtering IOL) and Tecnis Z9000 IOL (non‐blue‐light filtering IOL) implantation." | |
| Blue‐light filtering IOL: n = 39 (41); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 18 (20) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at two months of follow‐up | |
| Blue‐light filtering IOL: n = 27 (27) Non‐blue‐light filtering IOL: n = 52 (77) | Blue‐light filtering IOL: none for glaucoma or Nd:YAG capsulotomy Non‐blue‐light filtering IOL: none for glaucoma or Nd:YAG capsulotomy | The study authors reported that "at two years after surgery, all lenses were well centered and there was no evidence of posterior capsule opacity or glaucoma." | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: n = 3 eyes Non‐blue‐light filtering IOL: n = 1 eye | Blue‐light filtering IOL: n = 1 postoperative iris capture, n = 1 spontaneously reabsorbed vitreous haemorrhage, n = 1 cystoid macular oedema at nine months after surgery Non‐blue‐light filtering IOL: n = 1 postoperative iris capture | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at eight weeks of follow‐up | |
| Blue‐light filtering IOL: n = 38 (38) Non‐blue‐light filtering IOL: n = 36 (36) (In both groups, data were obtained from both eyes and averaged) | Glare symptoms Blue‐light filtering IOL: n = 3 participants Non‐blue‐light filtering IOL: n = 2 participants Cyanopsia Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Postoperative complications (as measured by participant report of glare symptoms and cyanopsia) at three months after surgery One participant was reported to have a clinically significant epiretinal membrane in the macula (it is unclear which group the participant belonged to) | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | "Three patients required neodymium:YAG laser capsulotomy for posterior capsule opacification," however group allocations were not specified. | Three participants (although this was not distinguished by the IOL intervention) at five years of follow‐up | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at eight weeks of follow‐up | |
| Blue‐light filtering IOL: n = 9 (9) Non‐blue‐light filtering IOL: n = 10 (10) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at six months of follow‐up | |
| Blue‐light filtering IOL: n = 150 (300) Non‐blue‐light filtering IOL: n = 147 (294) | Blue‐light filtering IOL: n = 12 Non‐blue‐light filtering IOL: n = 6 | At six months of follow‐up, in the blue‐light filtering IOL group, six eyes developed cystoid macula oedema and six eyes required secondary surgical intervention; none of the occurrences were considered IOL‐related. At six months of follow‐up, in the non‐blue‐light filtering IOL group, three eyes developed cystoid macula oedema and three eyes required secondary surgical intervention; none of the occurrences were considered IOL‐related. The study authors stated that "no Nd:YAG capsulotomy was performed in the first‐eye subgroup in either the test or control groups throughout the study period." | |
| Blue‐light filtering IOL: n = 19 (19) Non‐blue‐light filtering IOL: n = 40 (40); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at three months of follow‐up | |
| Blue‐light filtering IOL: n = 31 (31) Non‐blue‐light filtering IOL: n = 31 (31) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at three months of follow‐up | |
| Blue‐light filtering IOL: n = 80 (80); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 38 (38) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at 3 months of follow‐up | |
| Blue‐light filtering IOL: n = 19 (25) Non‐blue‐light filtering IOL: n = 18 (27) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | The study authors stated that "in the biomicroscopic examination (at one month postoperatively) the posterior capsule appeared clear in all test eyes." | |
| Blue‐light filtering IOL: n = ? (120); this group combined individuals assigned to 3 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = ? (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Reported at one month of follow‐up | |
| IOL: intraocular lens | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period. Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period. Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months) Show forest plot | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.14, 0.14] |
| 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months) Show forest plot | 3 | 808 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [0.63, 7.68] |

