Transkutana električna stimulacija živca (TENS) za neuropatsku bol kod odraslih
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 30 participants with postherpetic neuralgia, divided into 2 groups initially TENS (n = 16) and sham (n = 14). Each group further subdivided by concurrent dose of pregabalin. TENS group (pregabalin 300 mg, n = 9; pregabalin 600 mg, n = 7). Sham group (pregabalin 300 mg, n = 8; pregabalin 600 mg, n = 6). Baseline participant characteristics presented by gender not group. Age (mean ± SD): men 65 ± 8.6 years; women 64 ± 8.2 years. Pain duration: men 15.6 ± 8.8 months; women: 14.9 ± 8.6 months. Formal neuropathic pain assessment: no. Sites of pain: left hemithorax: men 9, women 10; right hemithorax: men 3, women 4; leg: men 4, women 2; arm/forearm: men 4, women 4. Concomitant treatment: all participants received pregabalin (300 mg or 600 mg) over initial 8 days' treatment until a pain intensity VAS of ≤ 60 mm was achieved. Following this, participants were randomised to TENS or sham. TENS/sham treatment continued for 4 weeks following randomisation. All participants continued with pregabalin treatment during the TENS/sham phase. | |
| Interventions | TENS group: TENS 100 Hz (inconsistent description in text, later described as 50 Hz), 125 µs. Intensity: "Clear non‐painful paraesthesia." Sham TENS group: as per active TENS but no current passed through electrodes. Sham credibility assessment: no. Location: electrodes placed around site of pain. Frequency of treatment: daily for 4 weeks. Duration: 30 minutes per session. Clinic administered. | |
| Outcomes | Daily pain intensity. 0‐10 cm VAS. Outcomes measured daily pretreatment and post‐treatment. VAS comparisons presented between baseline (day of randomisation to VAS group), week 3 and final VAS (post‐treatment completion ‐ week 4). Did not report adverse events. | |
| Notes | There may be mistakes in text of the article. VAS comparisons presented at 'week 3' and 'final' (week 4). It may be 'week 3' comparison is in fact 'week 4'. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Unclear risk for blinding of participants (TENS vs sham, attempted to manage participant expectations of sensation but no detail on whether TENS device appeared 'live' or not). Personnel high risk as the same care provider applied both active and sham treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | TENS vs active sham but see comments above for blinding of participants. |
| Incomplete outcome data (attrition bias) | Low risk | No participant dropout after TENS group randomisation. No details regarding dropout during drug titration phase. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | No obvious exclusions and dropouts data described. |
| Selective reporting (reporting bias) | Unclear risk | Inconsistencies in data presentation. VAS pain data presented in text for week 3 post‐randomisation while data in tables presented for final (week 4) VAS |
| Other bias | Unclear risk | Baseline characteristics presented by gender not group characteristics. |
| Size of study | High risk | TENS group: n = 16; sham TENS group: n = 14. |
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 52 participants with spinal cord injury. 4 dropouts, 2 per group. TENS: 17 men, 7 women; sham TENS 15 men, 9 women. Age (mean ± SD): TENS 35 ± 9 years; sham TENS 33.6 ± 8.5 years. Time since spinal cord injury (mean ± SD): TENS 7 ± 4.1 months; sham TENS 6.8 ± 3.1 months. Formal neuropathic pain assessment: no. Sites of pain: mixed. Concomitant treatment: no details supplied. | |
| Interventions | TENS group: TENS 2 Hz, 200 ms. Intensity: 50 mA. No description of perceived sensation. Sham TENS group: as per active TENS but no current passed through electrodes. Sham credibility assessment: no. Location: electrodes placed on region with pain. Frequency of treatment: 3 times per week for 12 weeks. Duration: 20 minutes per session. Clinic administered. | |
| Outcomes | Current pain intensity. 0‐10 cm VAS. Outcomes measured at baseline (pretreatment) and immediately post‐treatment at 12 weeks. Study did not report adverse events. | |
| Notes | No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Unclear risk for blinding of participants (sham control but no attempt to manage participant expectations of sensation and no detail on whether TENS device appeared 'live' or not). Personnel high risk as the same care provider applied both active and sham treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | TENS vs active sham but see comments above for blinding of participants. |
| Incomplete outcome data (attrition bias) | Low risk | Approximately 4% dropout balanced between groups. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | No obvious exclusions and dropout data adequately described. |
| Selective reporting (reporting bias) | Low risk | All outcomes adequately reported |
| Other bias | Low risk | Baseline characteristics comparable, outcome assessment times equal. |
| Size of study | High risk | n = 24 per group. |
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 236 participants divided into TENS group: 45 men, 72 women, sham TENS group: 43 men, 76 women, Neuropathic (radicular pain) subgroup n = 139. Of this neuropathic group, VAS pain intensity data provided by authors for radicular pain at baseline and post‐treatment for 122 participants (TENS group n = 64, sham TENS group n = 58). At 3 months, 38% dropout with TENS group n = 43, sham TENS group n = 32. Age (mean ± SD): TENS group 52.0 ± 13 years for whole group. No data reported for neuropathic subgroup; sham TENS group 53.4 ± 12.9 years for whole group. No data reported for neuropathic subgroup. Unable to determine duration of pain for neuropathic subgroup. Formal neuropathic pain assessment: clinical assessment and DN4 ≥ 4. Sites of pain: lower limb (radicular pain subgroup). Concomitant treatment: no details supplied for neuropathic subgroup. | |
| Interventions | TENS group: TENS mixed, 80‐100 alternated with 2 Hz, 200 ms. Intensity: alternating low intensity paraesthesia with high intensity perceived sensation including muscle twitches. Sham TENS group: as per active TENS but no current passed through electrodes. Sham credibility assessment: no. Location: 2 electrodes placed in low back area and 2 electrodes on radicular region. Frequency of treatment: 4 treatment sessions per day for 3 months. Duration: 1 hour per session. Self‐administered. | |
| Outcomes | Primary outcome: RDQ. Secondary outcomes: pain and quality of life (SF‐36). Neuropathic subgroup outcomes reported as Pain reduction (3 months) and RDQ (6 weeks). No separate SF‐36 reported for neuropathic subgroup. Pain recorded on 0‐10 cm VAS. Pain intensity data at baseline and post‐treatment supplied by authors for neuropathic group, specifically for the radicular pain component. VAS scored as weekly mean measures. Outcomes measured at baseline (pretreatment) and immediately post‐treatment at 12 weeks. Minor skin irritation in 14 participants. | |
| Notes | Funding sources acknowledged and no conflict noted. Authors contacted with request for detailed data on pain intensity outcome measures for neuropathic subgroup and kindly provided these data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated stratified randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blind (TENS vs sham, attempts made to manage participant expectations of sensation and the TENS device appeared 'live') and treatment self‐administered. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants blinded, sham vs active TENS. |
| Incomplete outcome data (attrition bias) | High risk | At 3 months, 47 participants were missing from the original baseline data for participants with radicular pain. This represents a 38.5% dropout. |
| Incomplete outcome data (participant exclusion from analysis) | Unclear risk | No detail provided with respect to missing data and participant exclusion from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Low risk for total study. Unable to assess for neuropathic subgroup and lack of SF‐36 data for neuropathic subgroup. |
| Other bias | Low risk | Baseline characteristics for total study well described. |
| Size of study | Unclear risk | Neuropathic subgroup: TENS group: n = 71; sham TENS group: n = 68. |
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 20 participants with carpal tunnel syndrome. TENS group: 5 women, 5 men; laser group: 5 women, 5 men. Age (mean ± SD): TENS group: 56.8 ± 12 years; laser group: 57.3 ± 12.9 years. Duration of pain: no detail supplied. Formal neuropathic pain assessment: nerve conduction study. Sites of pain: hand. Concomitant treatment: no details supplied. | |
| Interventions | TENS group: TENS 100 Hz, 80 ms. Intensity: "below muscle contraction," no details on perceived sensation. Location: electrodes placed on carpal ligament and course of median nerve. Frequency of treatment: daily for 3 weeks, 15 sessions in total. Duration: 30 minutes per session. Clinic administered. Laser group: 250 J/cm2 25 W. Probe size 1 cm2. Location: 10 cm length along course of median nerve in wrist area. Frequency of treatment: daily for 3 weeks, 15 sessions in total. Duration: 100 seconds per session. Clinic administered. | |
| Outcomes | Pain intensity: no further detail. 0‐10 cm VAS. Outcomes measured at baseline (pretreatment) and post‐treatment at 3 weeks. Study did not report adverse events. | |
| Notes | No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer aided sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details supplied. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both groups received an 'active' treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants received active treatment in both groups. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | No obvious exclusions from analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Low risk | Baseline characteristics between groups adequately tested and described. |
| Size of study | High risk | n = 10 per group. |
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 33 participants with spinal cord injury. No participant dropout reported. TENS 4 men, 13 women; sham TENS 11 men, 5 women. Age (mean ± SD): TENS group: 38.18 ± 9.86 years; sham TENS group: 34.81 ± 10.91 years. Mean duration of pain (range): 19.1 (1‐170) months for whole sample. No further data supplied. Formal neuropathic pain assessment: LANSS > 12. Sites of pain: mixed; cervical and 'back', thigh, knee and foot. Concomitant treatment: amitriptyline 10 mg both groups. | |
| Interventions | TENS group: TENS 4 Hz, 200 µs. Intensity: 50 mA. No description of perceived sensation. Sham TENS group: as per active TENS but no current passed through electrodes. Sham credibility assessment: no. Location: electrodes placed around region with pain. Frequency of treatment: 1 application per day for 10 days. Duration: 30 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity mean of morning, noon, evening and night VAS scores. 0‐10 cm VAS. Outcomes measured at baseline (pretreatment) on day 1 and 1 day following treatment cessation (day 12). Study reported adverse events and none occurred. | |
| Notes | Baseline testing between group for difference in pain location, duration were reported as not being significantly different but no data provided. No description of baseline comparison for LANSS score. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate participant group allocation. |
| Allocation concealment (selection bias) | High risk | Alternate participant group allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Low risk for blinding of participants (sham controlled study and no sensation reported from either active or sham device given participants had spinal cord injury). Personnel high risk as the same care provider applied both active and sham treatments. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants blinded, sham vs active TENS. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout of participants. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | No obvious exclusion from analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes adequately reported. |
| Other bias | Low risk | Baseline testing reported albeit without data presented for all tests. |
| Size of study | High risk | TENS group: n = 17; sham TENS group: n = 16. |
| Study characteristics | ||
| Methods | Randomised parallel design. | |
| Participants | 29 participants with postherpetic neuralgia. TENS group (n = 16), drugs group (n = 13). No detail on gender across groups. n = 10 dropouts in TENS group and n = 7 dropout in drugs group. No baseline characteristics supplied for either group. Formal neuropathic pain assessment: no. Sites of pain: no details. Concomitant treatment: no details. | |
| Interventions | TENS group: no detail supplied for TENS application parameters or participant perceived intensity. Location: 'Electrodes placed over the surface of the affected dermatome.' Frequency of treatment: 1 TENS treatment session per week for 4 weeks then 1 treatment applied every second week for 3 weeks. Duration: 15 minutes per session. Clinic administered. Drug group: carbamazepine plus clomipramine. No further detail supplied on dosage. Duration of treatment: 8 weeks. | |
| Outcomes | Pain intensity at each visit. 0‐10 cm VAS. No detail whether mean, current or maximal pain recorded at each visit. Outcomes measured at baseline (pretreatment) day 0 then at weeks 2, 4, 6 and 8. Study did not report adverse events. | |
| Notes | Inconsistencies in text with respect to treatment protocol and duration. Data analysed on per protocol basis. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail supplied. |
| Allocation concealment (selection bias) | Unclear risk | No detail supplied. |
| Blinding of participants and personnel (performance bias) | High risk | Given discrepancy in treatment types and application. |
| Blinding of outcome assessment (detection bias) | High risk | As above. |
| Incomplete outcome data (attrition bias) | High risk | Approximately 60% dropout. |
| Incomplete outcome data (participant exclusion from analysis) | High risk | Per protocol analysis. |
| Selective reporting (reporting bias) | High risk | No variance in reported TENS data. Follow‐up data un‐interpretable. |
| Other bias | High risk | No baseline characteristics described. |
| Size of study | High risk | TENS group: n = 16; drug group: n = 13. |
| Study characteristics | ||
| Methods | 3 phase cross‐over study. | |
| Participants | 64 participants with lumbar radicular pain. 34 women and 30 men. No dropouts reported over entire study. Participants randomised to 3 treatment sequences 1: sham, PENS, TENS; 2: PENS, TENS, sham; and 3: TENS, sham, PENS. Age (mean ± SD): 43 ± 19 years (of the whole sample). Duration of pain (mean ± SD): 21 ± 9 months. Formal neuropathic pain assessment: pain radiating below knee, positive straight leg raise testing. Radiological evidence of L5‐S1 nerve root compression. Sites of pain: low back /leg, radicular pain. Concomitant treatment: non‐opioid analgesia. | |
| Interventions | Treatment sequence 1: sham, PENS, TENS. Treatment sequence2: PENS, TENS, sham. Treatment sequence3: TENS, sham, PENS. TENS treatment: TENS 4 Hz, 100 ms. Intensity: maximum tolerated amplitude without producing muscle contraction. Location: 4 electrodes placed on posterior lower limb. PENS treatment: 4 Hz, 100 ms. Intensity: highest tolerable sensation without muscle contraction. Location: 10 × 32G acupuncture needles inserted into posterior lower limb. Sham PENS treatment: as per active PENS but no current passed through electrodes. Sham credibility assessment: no. Frequency of treatment: 3 applications per week for 3 weeks. 1 week washout between treatment modalities. Duration: 30 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity recorded at each visit and 24 hours after last treatment of each modality. Score reflected pain intensity during previous 24 hours. SF‐36 completed at baseline and 24 hours after last treatment session of each modality. NSAID use reported as change within modality. 0‐10 cm VAS for pain. Study did not report adverse events. | |
| Notes | SF‐36 and NSAID use appears to have been taken at initial baseline and then 24 hours following each treatment modality completion. No apparent testing for carry‐over effects on outcomes. Similar sham PENS was an invasive procedure compared to TENS. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details supplied. |
| Allocation concealment (selection bias) | Low risk | Cross‐over design. |
| Blinding of participants and personnel (performance bias) | High risk | Invasive vs non‐invasive treatment modalities. |
| Blinding of outcome assessment (detection bias) | High risk | Invasive vs non‐invasive treatment modalities. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data or dropouts not reported over the multiple treatment contacts. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | Not applicable. |
| Selective reporting (reporting bias) | High risk | SF‐36 data not adequately reported or tested. |
| Other bias | Unclear risk | No formal assessment of carry‐over effects but data appeared very similar at baseline. |
| Size of study | Unclear risk | n = 64. |
| Study characteristics | ||
| Methods | RCT, parallel. | |
| Participants | 75 participants with carpal tunnel syndrome equally to 3 treatment groups. 12 people dropped out during/follow‐up approximately evenly across groups. Splint group, 15 women, 7 men; TENS group 13 women, 7 men; IFT group 15 women, 6 men. Age (mean ± SD): splint group: 35.4 ± 4.2; TENS group: 34.2 ± 5.2; IFT group: 34.9 ± 4.8 years. Mean duration of pain: splint group: 12.4 ± 6.2; TENS group: 13.5.2 ± 6.6; IFT group: 13.0 ± 6.0 months. Formal neuropathic pain assessment: positive nerve conduction studies. Sites of pain: hand. Concomitant treatment: paracetamol as required daily. | |
| Interventions | Splint group: wrist‐hand resting splint at night for 3 weeks. TENS group: TENS 100 Hz, 80 ms. Intensity: no description of perceived sensation. IFT group: 4000 Hz with base 20 Hz. Intensity: no description of perceived sensation. Location: electrodes for both modalities placed around palmar aspect of hand/wrist/thenar area. Frequency of treatment: 5 times per week for 3 weeks. Duration: 20 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity: mean levels of pain in previous week. 0‐10 cm VAS. Outcomes measured at baseline and 3 weeks after completion of treatment (6 weeks after randomisation). 2 participants in TENS group reported mild tenderness at application site. | |
| Notes | No conflicts of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential admission into study. |
| Allocation concealment (selection bias) | High risk | Sequential allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Participant blinding was unclear if comparing TENS to IFT but high when comparing TENS to splint therapy. Personnel high risk as the same care provider applied both TENS and IFT treatments. |
| Blinding of outcome assessment (detection bias) | High risk | Participant blinding was unclear if comparing TENS to IFT but high when comparing TENS to splint therapy. Personnel high risk as the same care provider applied both TENS and IFT treatments. |
| Incomplete outcome data (attrition bias) | High risk | Participants lost to follow‐up specifically excluded. |
| Incomplete outcome data (participant exclusion from analysis) | High risk | Participants excluded if they failed to take part in the treatment regimen. |
| Selective reporting (reporting bias) | Low risk | Stated outcomes adequately reported. |
| Other bias | Unclear risk | Baseline characteristics tested and reported. |
| Size of study | High risk | n = 75 randomised across 3 treatment groups. |
| Study characteristics | ||
| Methods | RCT, parallel. | |
| Participants | 65 participants with diabetic neuropathy to 2 treatment groups, TENS and PRF sympathectomy. Overall, 10 participants (15%) described as having dropped out, however, sample sizes for both groups were stated as n = 30 (29 women, 31 men). Unable to accurately state gender composition of each group. Age (mean ± SD): TENS group: 56.63 ± 5.86 years; PRF sympathectomy group: 56.76 ± 6.94 years. Mean duration of diabetes: TENS group: 12.56 ± 2.96; PRF sympathectomy group: 13.32 ± 3.91. Formal neuropathic pain assessment: no ‐ diagnosed by neurologist. Sites of pain: lower limb. Concomitant treatment: pregabalin 300‐600 mg. | |
| Interventions | TENS group: TENS 80 Hz, appears to be 200 µs. Intensity: 'two to three times sensory threshold." Location: electrodes placed around shin and ankle. Frequency of treatment: 10 treatment sessions delivered on alternate days. Duration: 20 minutes per session. Clinic administered. PRF sympathectomy group: PRF sympathectomy delivered as one‐off invasive intervention. | |
| Outcomes | Pain intensity: mean levels of pain in previous week. 0‐10 cm NRS. Outcomes measured at baseline, 1 week, 1 month and 3 months following cessation of treatment (either one‐off PRF sympathectomy or 10 sessions of TENS on alternate days). Hence outcomes between groups were measured at differing time points postrandomisation. "Skin irritation reported in a few TENS group subjects." | |
| Notes | Supported by university funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No detail supplied. |
| Blinding of participants and personnel (performance bias) | High risk | Clearly different treatments and 1 invasive. |
| Blinding of outcome assessment (detection bias) | High risk | Impossible to blind given the protocol. |
| Incomplete outcome data (attrition bias) | Unclear risk | Discrepancies in dropout and indicated analysis. |
| Incomplete outcome data (participant exclusion from analysis) | Unclear risk | Analysis not fully described and inconsistencies in dropout description. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Differences in time postrandomisation outcome measurement between groups. |
| Size of study | High risk | Reported as TENS group: n = 30; PRF sympathectomy group: n = 30. |
| Study characteristics | ||
| Methods | Randomised cross‐over design. | |
| Participants | 26 participants with spinal cord injury to 2 treatment groups: 1. VI followed by TENS; 2. TENS followed by VI. n = 12 per group (2 participants dropped out). Total sample: 6 women, 18 men. Age (mean ± SD): 32.33 ± 12.97 years. Mean pain duration: 12.46 ± 17.83 months. Formal neuropathic pain assessment: ≥ 4 on DN4. Sites of pain: at or below level of spinal cord injury. Concomitant treatment: pregabalin 300‐600 mg. | |
| Interventions | TENS treatment: TENS 80 Hz, 180 µs. Intensity: perceptible but not uncomfortable. Location: electrodes placed bilateral spinal region above level of injury. Frequency of treatment: 5 days per week for 2 weeks. Duration: 30 minutes per session. VI treatment: 20 minutes of VI treadmill walking. Frequency of treatment: 5 days per week for 2 weeks. Duration: 15 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity: mean, maximal and minimal pain intensity levels. Brief pain inventory measured pretreatment and post‐treatment. Pain 0‐10 cm VAS. Outcomes measured at baseline, pretreatment and post‐treatment each treatment session/treatment modality. Study reported adverse events and none occurred. | |
| Notes | No carry‐over tests reported. No baseline comparisons between groups reported. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Cross‐over. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both active non‐invasive treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) | Low risk | Dropout from study described and minimal. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | Appears adequate. |
| Selective reporting (reporting bias) | Low risk | Adequately reported. |
| Other bias | Unclear risk | No formal assessment of carry‐over effects but data appeared very similar at baseline. |
| Size of study | High risk | n = 12 per group. |
| Study characteristics | ||
| Methods | RCT, parallel design. | |
| Participants | 75 participants with cervical radicular pain. No participant dropout reported. Randomised into 3 groups: joint mobilisation, TENS and isometric exercises. No details supplied on individual group size or gender composition. Whole sample 48% women, 52% men. Between‐group baseline tests for age, body mass and pain duration reported as "homogenous;' no formal statistical testing. Age (mean ± SD): Group A: 36.33 ± 9.4 years; Group B: 37.25 ± 9 years; Group C: 39.33 ± 8.6 years. Mean duration of pain: no data supplied. Formal neuropathic pain assessment: no. Sites of pain: cervical spine and unilateral upper limb pain. Concomitant treatment: heat packs applied to the cervical spine area. | |
| Interventions | Joint mobilisation group: cervical spine lateral flexion joint mobilisation, 10 sessions on alternate days over 3 weeks. TENS group: TENS 100 Hz, 50 µs. Intensity: no detail supplied, 10 sessions on alternate days over 3 weeks, 30 minute per session. Electrodes placed at cervical spinal segment and distal dermatomal area. Exercise group: isometric neck exercises: isometric flexion, lateral flexion, rotation and extension. 6‐8 seconds per contraction. 5 repetitions for each muscle group. No details on intensity of contraction. 10 sessions on alternate days over 3 weeks. All treatments administered/supervised in clinic. | |
| Outcomes | Pain intensity. No details on pain intensity instructions with respect to current pain, mean pain, etc. 0‐10 cm VAS. Outcomes measured at baseline (pretreatment) week 3 and week 6 (3 weeks post‐treatment finished). Study did not report adverse events. | |
| Notes | Week 3 VAS results were reported as reduction from baseline. Unable to extract baseline data. Week 6 data not reported in text. No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details supplied. |
| Allocation concealment (selection bias) | Unclear risk | No details supplied. |
| Blinding of participants and personnel (performance bias) | Unclear risk | 2 active non‐invasive treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above in terms of active treatments. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details supplied. |
| Incomplete outcome data (participant exclusion from analysis) | Unclear risk | No details supplied. |
| Selective reporting (reporting bias) | High risk | Key baseline data and week 6 data not supplied. |
| Other bias | High risk | Baseline group characteristic testing not described. Age and pain duration at baseline described as homogenous. |
| Size of study | High risk | Unknown sample size per group. Whole group: n = 75. |
| Study characteristics | ||
| Methods | Randomised parallel design. | |
| Participants | 26 participants with postherpetic neuralgia to 2 treatment groups: TENS group (n = 13) and ACU group (n = 10). At 6 months, 13 dropouts in TENS group and 9 dropouts in ACU group. Total sample = 13 women, 10 men. Age (median (range)): 73 (57‐85) years. Mean pain duration: 3 months to > 9 years. Formal neuropathic pain assessment: no. Sites of pain: mixed. Concomitant treatment: no details supplied. | |
| Interventions | TENS group: TENS 100 Hz, 200 µs. Intensity: amplitude increased until 'a fairly strong sensation' was perceived. Location: electrodes placed either side of painful area. Frequency of treatment: 3 clinic administered 30 minute treatments in first week. Then TENS unit loaned for home use for 5 weeks. No information regarding frequency of use given for this period. ACU group: 2 treatment session per week for 6 weeks. Body and auricular stimulation. Steel needles stimulated with current at 5‐60 Hz. Duration: no details supplied. Clinic administered. | |
| Outcomes | Pain intensity, visual stepwise scale, 10 steps. Measured at intake, 6 weeks, 9 weeks and 6 months. No details supplied as to parameters of pain rating (current pain, mean pain, etc.). Study did not report adverse events. | |
| Notes | No formal statistical tests employed. At 9 weeks, study had 7 participants left in study (73% dropout). Private funding body acknowledged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details supplied. |
| Allocation concealment (selection bias) | Unclear risk | No details supplied. |
| Blinding of participants and personnel (performance bias) | High risk | TENS vs invasive treatment. |
| Blinding of outcome assessment (detection bias) | High risk | Impossible due to treatments being compared. |
| Incomplete outcome data (attrition bias) | High risk | > 70% dropout at 9 weeks. |
| Incomplete outcome data (participant exclusion from analysis) | High risk | No final statistical tests performed but appears a per protocol approach. |
| Selective reporting (reporting bias) | High risk | No data supplied for outcomes. |
| Other bias | Unclear risk | No baseline data supplied. |
| Size of study | High risk | TENS group: n = 10; ACU group: n = 13. |
| Study characteristics | ||
| Methods | Randomised parallel design. | |
| Participants | 60 participants with chronic DPN were randomised to 3 treatment groups: TENS group n = 20, exercise group n = 20, pharmacological group n = 20. In the total sample, there were 32 women and 28 men. Age (mean ± SD): TENS group: 51.6 ± 4.75 years; exercise group: 51.7 ± 4.44 years; pharmacological group: 51.95 ± 4.38. Mean duration of DPN: TENS group: 12.05 ± 3.17; exercise group: 12.15 ± 0.38; pharmacological: 12.3 ± 3.38 (unit of measurement not stated). Formal neuropathic pain assessment: no, diagnosed clinically. Sites of pain: lower limb. Concomitant treatment: all groups continued with "regular pharmacological therapy." There was no description of this for TENS and exercise group in either drugs or dosage. However, the pharmacological group (regular therapy) was described as consisting of "nerve growth stimulant; vitamin B complex and oral hypoglycaemic drugs or insulin." No further details or comparisons made between groups in this area. | |
| Interventions | TENS group: TENS 15 Hz, 250 µs. Intensity: increased until "strong rhythmic muscle contractions" observed. Location: 2 electrodes placed bilaterally on lower aspect of medial tibial condyle and superior to medial malleolus. Frequency of treatment: 3 days per week for 8 weeks. Duration: 30 minutes per session. TENS treatment clinic administered. Exercise group: aerobic exercise on stationary bicycle. Intensity: following warm‐up, participants exercised at 50‐70% of maximal heart rate. Frequency of treatment: 3 days per week for 8 weeks. Duration: 50 minutes per session (5 minutes' warm‐up, 40 minutes' exercise, 5 minutes' cool down). Pharmacological group: "regular therapy." No further information supplied. | |
| Outcomes | Pain intensity recorded pretreatment and post‐treatment on a 0‐10 VAS. No detail supplied with respect to parameter measured with VAS (e.g. mean pain, minimal pain, maximal pain, etc.). Nerve conduction studies of medial plantar sensory nerve performed pretreatment and post‐treatment. Study did not report adverse events. | |
| Notes | Data not supplied for concomitant drug treatment. No data supplied for baseline or post‐treatment pain intensity scores. Paper stated Kruskal‐Wallis testing was used to assess between‐group differences in pain intensity scores post‐treatment; however, this analysis was not reported. All significant pain intensity findings are based on within‐group analysis and no detail on output of these tests supplied. Pain intensity only presented in descriptive form; percentage change from baseline. Have contacted authors regarding pain intensity data. No conflict of interest reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information supplied. |
| Allocation concealment (selection bias) | Unclear risk | No information supplied. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both interventions were active treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Self‐reported VAS pain intensity data. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information supplied. |
| Incomplete outcome data (participant exclusion from analysis) | Unclear risk | No information supplied. |
| Selective reporting (reporting bias) | High risk | No data on primary outcome of study. No data on concomitant drug treatment. |
| Other bias | Unclear risk | No baseline comparison on pain intensity scores. |
| Size of study | High risk | n = 20 per group. |
| Study characteristics | ||
| Methods | RCT, parallel. | |
| Participants | 26 participants with phantom limb pain to 2 groups. TENS group: 11 men, 2 women, 1 dropout therefore n = 12; mirror group: 12 men, 1 female, n = 13. Age (mean ± SD): TENS group: 36.38 ± 9.55 years; mirror group: 42.62 ± 10.69 years. Amputations: TENS group: 3 upper and 10 lower limb amputations; mirror group: 4 upper and 9 lower limb amputations. Onset of phantom limb pain from date of surgery: TENS group: 13 ± 1.6 days; mirror group: 13 ± 1.4 days. Formal neuropathic pain assessment: no. Sites of pain: upper and lower limb. Concomitant treatment: no detail supplied. | |
| Interventions | TENS group: no TENS frequency details supplied. Intensity: "strong but comfortable" without visible muscle contraction. Location: electrodes placed at site of pain on contralateral limb. Frequency of treatment: 1 session per day for 4 days. Duration: 20 minutes per session. Clinic administered. Mirror group: intact limb movements performed with mirror. Frequency: 1 session per day for 4 days. Duration: 20 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity: no details supplied as to parameters of pain rating (current pain, mean pain, etc.). 0‐10 cm VAS. Outcomes measured at baseline and 4 days later. Study did not report adverse events. | |
| Notes | Funding from higher education institution acknowledged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both interventions active treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Both interventions active treatments. |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant dropout adequately described. |
| Incomplete outcome data (participant exclusion from analysis) | Low risk | Dropout minimal. All participants analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Adequate description and testing of baseline characteristics. |
| Size of study | High risk | TENS group: n = 12; mirror group: n = 13. |
| Study characteristics | ||
| Methods | RCT, parallel. | |
| Participants | 25 participants with spinal cord injury. 4 participants dropped out. No details on group allocation given. TENS group: 10 men, 1 woman; sham TENS: 9 men, 1 woman. Age (mean ± SD): TENS group: 31.72 ± 7.7 years; sham TENS group: 28.9 ± 6.1 years. Duration of pain (mean (range)): 12.7 (0.5‐14) months for whole sample. No further data supplied. Formal neuropathic pain assessment: LANSS > 12; mean (range) score 15.95 (13‐20). Sites of pain: mixed. Concomitant treatment: gabapentin started day 1 and increased in 300 mg increments daily to basic dose of 900 mg/day by day 3. | |
| Interventions | TENS group: TENS 4 Hz, 200 ms. Intensity: 50 mA. No description of perceived sensation. Sham TENS group: as per active TENS but no current passed through electrodes. Sham credibility assessment: no. Location: electrodes proximal and distal to region with pain. Frequency of treatment: 1 application per day for 10 days. Duration: 30 minutes per session. Clinic administered. | |
| Outcomes | Pain intensity mean of morning and evening. Mean of these two scores at day 0 and day 10 used in analysis. 0‐10 cm VAS. Outcomes measured at baseline (pretreatment) on day 0 and day 10 of the study. Study reported adverse events and none occurred. | |
| Notes | No conflict of interest stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details supplied. |
| Allocation concealment (selection bias) | Unclear risk | No details supplied. |
| Blinding of participants and personnel (performance bias) | High risk | Unclear risk for blinding of participants (TENS vs sham but no attempt to manage participant expectations of sensation and no detail on whether TENS device appeared 'live' or not). Personnel high risk as the same care provider applied active and sham treatments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | TENS vs active sham but see comments above for blinding of participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | 16% dropout rate. No information given with regards to group allocation. |
| Incomplete outcome data (participant exclusion from analysis) | Unclear risk | No obvious exclusion from analysis; however, dropout rate not fully described with respect to group allocation. |
| Selective reporting (reporting bias) | Low risk | All outcomes adequately reported. |
| Other bias | Low risk | Baseline testing reported albeit without data presented for all tests. |
| Size of study | High risk | TENS group: n = 11; sham TENS group: n = 10. |
μs: microseconds; ACU: electroacupuncture; DN4: Douleur Neuropathique 4; DPN: diabetic peripheral neuropathy; IFT: interferential therapy; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs; n: sample size; NRS: numerical rating scale; NSAID: non‐steroidal anti‐inflammatory drug; PENS: percutaneous electrical nerve stimulation; PRF: pulsed radiofrequency; RCT: randomised controlled trial; RDQ: Roland‐Morris Disability Questionnaire; SD: standard deviation; SF‐36: 36‐item Short Form; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale; VI: visual illusion.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not defined neuropathic pain. | |
| Not a standard TENS unit application. Unable to contact authors. | |
| Not randomised/quasi‐randomised trial. | |
| Not randomised/quasi‐randomised trial. | |
| Outcome measure not pain intensity. | |
| Pain intensity scoring in response to stimulus evoked pain. Stimulus applied by researcher. | |
| Outcome measure a VAS composite of pain and sensory complaints. | |
| All participants received perceptual TENS. | |
| Outcome measure not Pain intensity. | |
| Outcome measure a VAS composite of pain and sensory symptoms. | |
| Not defined neuropathic pain. | |
| TENS applied below perceptual level. | |
| Not clearly randomised trial. | |
| Not a standard TENS device. | |
| Outcome measured < 24 hours post‐treatment. | |
| Outcome measure not pain intensity. VAS was a composite of pain intensity, paraesthesia and sleep disturbance. Outcome measure not self‐reported. | |
| Outcome measure not pain intensity. VAS was a composite of pain intensity, paraesthesia and sleep disturbance. Outcome measure not self‐reported. | |
| Outcome measured < 24 hours post‐treatment. | |
| Not defined neuropathic pain. | |
| Outcome measure not pain intensity. Not defined neuropathic pain participants. | |
| All participants received TENS. | |
| No pain intensity follow‐up data. Unable to extract potential neuropathic participant data. | |
| Outcome measure not pain intensity. VAS was a composite measure of pain and non‐pain symptoms. | |
| Not all participants had pain as a symptom. Outcome measure encompassed non‐pain symptoms. | |
| Not defined neuropathic pain. | |
| Not defined neuropathic pain. | |
| Outcome measured < 24 hours post‐treatment. | |
| Not defined neuropathic pain condition in study. | |
| Not defined neuropathic pain condition in study. | |
| Not randomised/quasi‐randomised trial. | |
| All participants received TENS. |
TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale.
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Not available. |
| Participants | Not available. |
| Interventions | Not available. |
| Outcomes | Not available. |
| Notes | Unable to contact study authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Attempted contact with author. No reply. |
| Methods | RCT, parallel |
| Participants | Randomised n = 139 with 'senile radical sciatica' randomised to electroacupuncture (n = 70) or TENS (n = 69) treatments. Awaiting translation. No further details. |
| Interventions | Awaiting translation. |
| Outcomes | |
| Notes |
n: number of participants; TENS: transcutaneous electrical nerve stimulation.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain intensity Show forest plot | 5 | 207 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| Analysis 1.1  Comparison 1: TENS versus sham TENS, Outcome 1: Pain intensity | ||||
| 1.2 Pain intensity sensitivity analysis (Celik 2013 removed) Show forest plot | 4 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.87, ‐1.02] |
| Analysis 1.2  Comparison 1: TENS versus sham TENS, Outcome 2: Pain intensity sensitivity analysis (Celik 2013 removed) | ||||
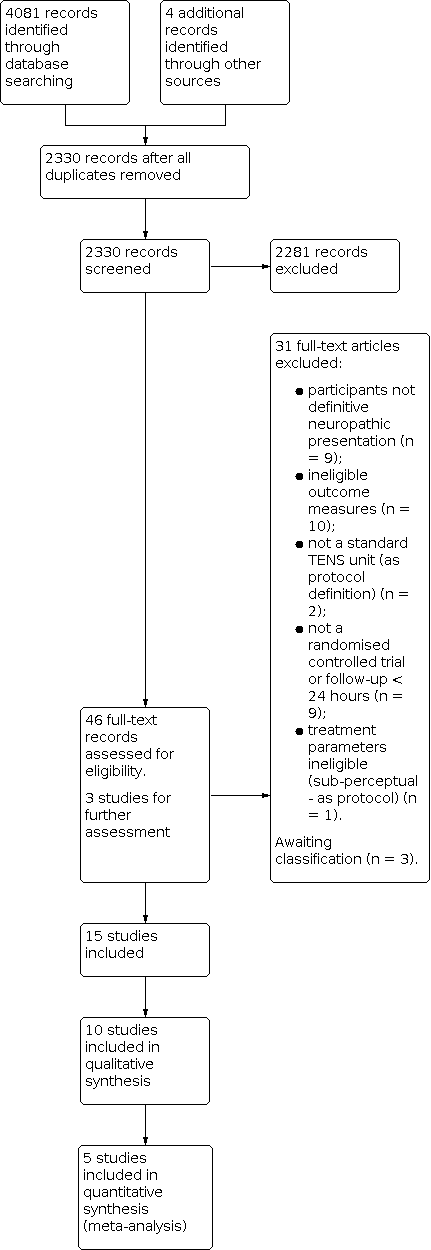
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.1 Pain intensity.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.2 Pain intensity sensitivity analysis (Celik 2013 removed).

Comparison 1: TENS versus sham TENS, Outcome 1: Pain intensity

Comparison 1: TENS versus sham TENS, Outcome 2: Pain intensity sensitivity analysis (Celik 2013 removed)
| TENS versus sham TENS for neuropathic pain in adults | ||||
| Patient or population: adults with neuropathic pain Settings: secondary care Intervention/comparison: TENS vs sham TENS Outcome: Pain intensity (VAS) | ||||
| Outcomes | Effect estimate (95% CI) | No of participants | Quality of the evidence | Comments |
|---|---|---|---|---|
| Post‐intervention pain intensity (VAS 0‐10) | Favoured TENS. Mean difference ‐1.58 (95% CI ‐2.08 to ‐1.09) | 207 (5) | ⊕⊝⊝⊝ Very lowa | Downgraded 3 levels due to multiple sources of potential bias, small number and size of studies. |
| Health related quality of life | No data | ‐ | ‐ | ‐ |
| Participant global impression of change | No data | ‐ | ‐ | ‐ |
| Analgesic medication use | Not estimable | ‐ | ‐ | ‐ |
| Incidence/nature of adverse events | Not estimable | ‐ | ‐ | ‐ |
| CI: confidence interval; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded twice for limitations of studies and once for imprecision. | ||||
| Study, comparison (admitted sample size) | Group baseline pain intensity VAS/NRS | Neuropathic condition | Reported mean duration | Diagnostic criteria | Hz and pulse width | Electrode location | Intensity | Duration, frequency and site of administration |
|---|---|---|---|---|---|---|---|---|
| TENS vs sham TENS (30) | P300 + TENS: 4 ± 0.93 P600 + TENS: 3.8 0.95 P300 + sham TENS: 4.1 ± 1.19 P600 + sham TENS: 3.2 ± 0.81 | Postherpetic neuralgia | 15.25 ± 8.7 months | No formal or clinical neuropathic diagnostic criteria | 100 Hz (later described in text as 50 Hz) 125 µs | "Around site of pain" | "Clear non‐painful paraesthesia". Titrated to maintain strength of perception | 30 minutes daily for 4 weeks Clinic administration |
| TENS vs sham TENS (52) | TENS: 5.17 ± 2.34 Sham TENS: 5.56 ± 2.07 | Spinal cord injury | 6.9 ± 3.6 months (since spinal cord injury) | No formal or clinical neuropathic diagnostic criteria | 2 Hz 200 ms | Placed "on region with pain" | 50 mA. No description of perceived sensation | 20 minutes 3 × weekly for 12 weeks Clinic administration |
| TENS vs sham TENS (122) | TENS: 6.15 ± 2.24 Sham TENS: 5.91 ± 2.12 | Lumbar radicular pain (subgroup data supplied by authors) | Not reported | Clinical assessment | Mixed: 80‐100 Hz alternated with 2 Hz 200 ms | Placed on low back and radicular region of pain | Low intensity paraesthesia alternated with high intensity (muscle twitches) | 1 hour. 4 × daily for 3 months Self‐administered at home |
| TENS vs laser? (20) | TENS: 6 ± 0.8 Laser?: 6.6 ± 1.1 | Carpal tunnel syndrome | Not reported | Nerve conduction study | 100 Hz 80 ms | Over carpal ligament and median nerve | "Below muscle contraction" | 30 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs sham TENS (33) | TENS: 5.79 ± 2.17 Sham TENS: 5.64 ± 1.81 | Spinal cord injury | 19.1 months | LANSSa > 12 | 4 Hz 200 µs | Placed "on region with pain" | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| TENS vs drug treatment (29) | TENS: 27.0 Drug: 59.0 (0‐100) | Postherpetic neuralgia | No details | No formal or clinical neuropathic diagnostic criteria | No details | "Placed on affected dermatome" | No detail | 15 minutes 1 × weekly for 4 weeks then 1 × fortnightly for 3 weeks |
| TENS vs PENS (64) | TENS: 7.0 ± 1.9 PENS: 7.2 ± 1.8 Sham PENS: 6.6 ± 1.9 | Lumbar radicular pain | 21 ± 9 months | Clinical assessment. Radiological assessment of nerve root compression | 4 Hz 100 ms | Placed on posterior lower limb | "Highest tolerable sensation" without muscle twitch | 30 minutes 3 × weekly for 3 weeks Clinic administration |
| TENS vs IFT (75) | TENS: 8.06 ± 0.55 IFT: 8.25 ± 0.4 Splint: 8.31 ± 0.6 | Carpal tunnel syndrome | 13.3 ± 6.3 months | Nerve conduction study | 100 Hz 80 ms | Placed on "palmar aspect of hand/wrist" | No details | 20 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs PRF sympathectomy (65) | TENS: 6.10 PRF sympathectomy: 6.46 (NRS) | Peripheral diabetic neuropathy | 12.9 ± 3 years (since diabetes onset) | Clinical diagnosis | 80 Hz 200 µs | "Around shin and ankle" | "two to three times sensory threshold" | 20 minutes 10 treatment sessions on alternate days Clinic administration |
| TENS vs visual illusion (26) | TENS: 5.33 ± 1.20 Visual illusion: 5.33 ± 1.37 | Spinal cord injury | 12.4 ± 17.8 months | ≥ 4 on DN4 | 80 Hz 180 µs | Bilaterally around spine above level of injury | "perceptible but comfortable" | 30 minutes 5 × weekly for 2 weeks Clinic administration |
| TENS vs cervical spine mobilisation (75) | Not stated | Cervical radicular pain (75) | No details | No formal or clinical neuropathic diagnostic criteria | 100 Hz 50 µs | Placed at 'cervical spinal segment and distal dermatome | No details | 30 minutes 10 sessions on alternate days over 3 weeks Clinic administration |
| TENS vs acupuncture (26) | Not stated | Postherpetic neuralgia | "3 months to 9 years" | No formal or clinical neuropathic diagnostic criteria | 100 Hz 200 µs | "Either side of painful area" | "Fairly strong sensation" | 3 × 30 minute clinic sessions week 1. Then home use for 5 weeks. No detail on home use frequency/duration |
| TENS vs exercise (60) | Not stated | Peripheral diabetic neuropathy | 12.2 ± 2.3 years (since onset of neuropathy ) | No formal or clinical neuropathic diagnostic criteria | 15 Hz 250 µs | Lower leg/ankle | "Strong rhythmic muscle contractions" | 30 minutes 3 × weekly for 8 weeks Clinic administration |
| TENS vs mirror therapy | TENS: 5.00 ± 1.63 Mirror: 5.46 ± 1.67 | Phantom limb pain | 13 ± 1.5 days (since onset of phantom limb pain) | No formal or clinical neuropathic diagnostic criteria | No details | Site of pain contralateral limb | "Strong but comfortable" | 20 minutes 1 × daily for 4 days Clinic administration |
| TENS vs sham TENS (25) | TENS: 8.09 ± 0.97 Sham TENS: 8.05 ± 1.05 | Spinal cord injury | 12.7 months | LANSS > 12 | 4 Hz 200 ms | Proximal and distal to pain region | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| DN4: Douleur Neuropathique 4; IFT: interferential therapy; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs pain scale; NRS: numerical rating scale; P300: pregabalin 300 mg; P600: pregabalin 600 mg; PENS: percutaneous electrical nerve stimulation; PRF: pulsed radiofrequency; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain intensity Show forest plot | 5 | 207 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| 1.2 Pain intensity sensitivity analysis (Celik 2013 removed) Show forest plot | 4 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.87, ‐1.02] |

