Neuroestimulación eléctrica transcutánea (TENS) para el dolor neuropático en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011976.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
WG: led the design of the review as primary author, implemented the search strategy with the Pain, Palliative and Supportive Care group's Trials Search Co‐ordinator, applied eligibility criteria, assessed studies, extracted and analysed data, led the write up and updating of the review.
BMW: closely informed the design, applied eligibility criteria, assessed studies, extracted and aided in analysis of data, assisted the writing and will aid future updating of the review.
NEO: closely informed the design, acted as third review author when assessing eligibility criteria and during assessment of studies, assisted in analysis of data, assisted the writing and will aid future updating of the review.
Sources of support
Internal sources
-
The University of Notre Dame, Australia
-
Brunel University London, UK
External sources
-
No sources of support supplied
Declarations of interest
WG: none known.
BMW: none known.
NEO: none known.
All review authors are qualified physiotherapists and involved in the professional training of physiotherapists.
Acknowledgements
The protocol followed the agreed template for neuropathic pain, which was developed in collaboration with the Cochrane Musculoskeletal Group and Cochrane Neuromuscular Diseases Group. The editorial process was managed by the Cochrane Pain, Palliative and Supportive Care Review Group. The authors would like to thank the following people: Bita Mesgarpour, Jo‐Aine Hang and Andrea Wand for generous help with translation; Mark Rockett, Peter Cole and Juliana Ester Martin Lopez for peer review; Joanne Abbott for expertise and help with devising and running literature searches; and Anna Erskine for her patience and ongoing editorial assistance. Many thanks to Andrea Buchmuller and team for kindly supplying additional data on their study.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 14 | Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults | Review | William Gibson, Benedict M Wand, Neil E O'Connell | |
| 2015 Nov 26 | Transcutaneous Electrical Nerve Stimulation (TENS) for neuropathic pain in adults | Protocol | William Gibson, Benedict M Wand, Neil E O'Connell | |
Differences between protocol and review
The protocol of this review contained an error that was overlooked in the review process. In the review, we made the following statement in the 'Assessment of Heterogeneity' section: "We will attempt to deal with clinical heterogeneity by combining studies that examine similar conditions because placebo response rates with the same outcome can vary between conditions, as can the treatment specific effects."
Following this in the 'Data Synthesis' section we made this statement: "We will pool data from studies of neuropathic pain regardless of the specific diagnosis. We will pool data for adverse events across conditions."
These are conflicting and incompatible. This was done in error and has now been corrected.
The protocol of this review outlined the criteria involved in grading the quality of evidence according to the GRADE approach. However, we did not explicitly mention that individual criteria may be double downgraded if there were sufficient reasons to do so. In this review, we downgraded twice on "Limitations of studies" due to sample sizes and multiple high risk of bias issues across at least four of the five studies included in the pooled analysis.
In the protocol of this review, we stated that we planned to investigate the following comparisons: TENS versus sham TENS, TENS versus usual care, TENS versus no treatment and TENS in addition to usual care versus usual care alone. We were only able to perform a quantitative synthesis for the comparison of TENS versus sham TENS. No studies investigated TENS versus no treatment and TENS in addition to usual care versus usual care alone. The studies investigating TENS versus usual care employed a wide range of comparative treatments which precluded pooling of data. For the sake of completeness of the evidence, we therefore included a series of individual narrative reviews of studies investigating TENS versus these other active treatments.
Notes
2018
In the initial published version of this review the language used to describe the primary outcome (pain intensity) was ambiguous. For all our comparisons we focused on the between group absolute post intervention pain scores, rather than “change in pain intensity” from baseline, or the between group difference in change in pain intensity from baseline. We have edited the results to more accurately reflect this.
March 2019
A restricted search in March 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Assessed for updating in 2021
In February 2021 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Please note that Neil O'Connell is the PaPaS Co‐ordinating Editor and he was not involved in the editorial assessment or decisions when considering this review for updating; we thank the Editors Professors Christopher Eccleston and Andrew Moore for their input.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
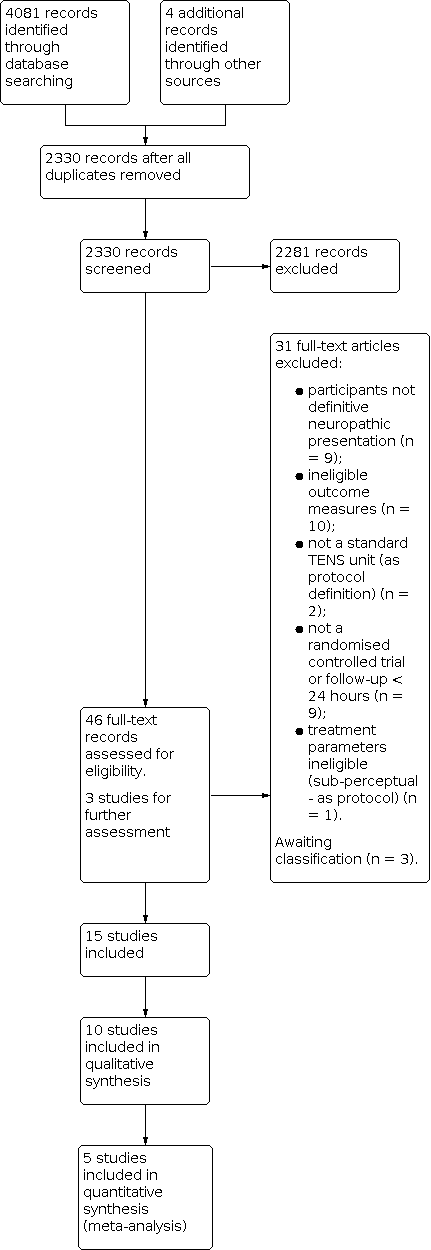
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.1 Pain intensity.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.2 Pain intensity sensitivity analysis (Celik 2013 removed).

Comparison 1: TENS versus sham TENS, Outcome 1: Pain intensity

Comparison 1: TENS versus sham TENS, Outcome 2: Pain intensity sensitivity analysis (Celik 2013 removed)
| TENS versus sham TENS for neuropathic pain in adults | ||||
| Patient or population: adults with neuropathic pain Settings: secondary care Intervention/comparison: TENS vs sham TENS Outcome: Pain intensity (VAS) | ||||
| Outcomes | Effect estimate (95% CI) | No of participants | Quality of the evidence | Comments |
|---|---|---|---|---|
| Post‐intervention pain intensity (VAS 0‐10) | Favoured TENS. Mean difference ‐1.58 (95% CI ‐2.08 to ‐1.09) | 207 (5) | ⊕⊝⊝⊝ Very lowa | Downgraded 3 levels due to multiple sources of potential bias, small number and size of studies. |
| Health related quality of life | No data | ‐ | ‐ | ‐ |
| Participant global impression of change | No data | ‐ | ‐ | ‐ |
| Analgesic medication use | Not estimable | ‐ | ‐ | ‐ |
| Incidence/nature of adverse events | Not estimable | ‐ | ‐ | ‐ |
| CI: confidence interval; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded twice for limitations of studies and once for imprecision. | ||||
| Study, comparison (admitted sample size) | Group baseline pain intensity VAS/NRS | Neuropathic condition | Reported mean duration | Diagnostic criteria | Hz and pulse width | Electrode location | Intensity | Duration, frequency and site of administration |
|---|---|---|---|---|---|---|---|---|
| TENS vs sham TENS (30) | P300 + TENS: 4 ± 0.93 P600 + TENS: 3.8 0.95 P300 + sham TENS: 4.1 ± 1.19 P600 + sham TENS: 3.2 ± 0.81 | Postherpetic neuralgia | 15.25 ± 8.7 months | No formal or clinical neuropathic diagnostic criteria | 100 Hz (later described in text as 50 Hz) 125 µs | "Around site of pain" | "Clear non‐painful paraesthesia". Titrated to maintain strength of perception | 30 minutes daily for 4 weeks Clinic administration |
| TENS vs sham TENS (52) | TENS: 5.17 ± 2.34 Sham TENS: 5.56 ± 2.07 | Spinal cord injury | 6.9 ± 3.6 months (since spinal cord injury) | No formal or clinical neuropathic diagnostic criteria | 2 Hz 200 ms | Placed "on region with pain" | 50 mA. No description of perceived sensation | 20 minutes 3 × weekly for 12 weeks Clinic administration |
| TENS vs sham TENS (122) | TENS: 6.15 ± 2.24 Sham TENS: 5.91 ± 2.12 | Lumbar radicular pain (subgroup data supplied by authors) | Not reported | Clinical assessment | Mixed: 80‐100 Hz alternated with 2 Hz 200 ms | Placed on low back and radicular region of pain | Low intensity paraesthesia alternated with high intensity (muscle twitches) | 1 hour. 4 × daily for 3 months Self‐administered at home |
| TENS vs laser? (20) | TENS: 6 ± 0.8 Laser?: 6.6 ± 1.1 | Carpal tunnel syndrome | Not reported | Nerve conduction study | 100 Hz 80 ms | Over carpal ligament and median nerve | "Below muscle contraction" | 30 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs sham TENS (33) | TENS: 5.79 ± 2.17 Sham TENS: 5.64 ± 1.81 | Spinal cord injury | 19.1 months | LANSSa > 12 | 4 Hz 200 µs | Placed "on region with pain" | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| TENS vs drug treatment (29) | TENS: 27.0 Drug: 59.0 (0‐100) | Postherpetic neuralgia | No details | No formal or clinical neuropathic diagnostic criteria | No details | "Placed on affected dermatome" | No detail | 15 minutes 1 × weekly for 4 weeks then 1 × fortnightly for 3 weeks |
| TENS vs PENS (64) | TENS: 7.0 ± 1.9 PENS: 7.2 ± 1.8 Sham PENS: 6.6 ± 1.9 | Lumbar radicular pain | 21 ± 9 months | Clinical assessment. Radiological assessment of nerve root compression | 4 Hz 100 ms | Placed on posterior lower limb | "Highest tolerable sensation" without muscle twitch | 30 minutes 3 × weekly for 3 weeks Clinic administration |
| TENS vs IFT (75) | TENS: 8.06 ± 0.55 IFT: 8.25 ± 0.4 Splint: 8.31 ± 0.6 | Carpal tunnel syndrome | 13.3 ± 6.3 months | Nerve conduction study | 100 Hz 80 ms | Placed on "palmar aspect of hand/wrist" | No details | 20 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs PRF sympathectomy (65) | TENS: 6.10 PRF sympathectomy: 6.46 (NRS) | Peripheral diabetic neuropathy | 12.9 ± 3 years (since diabetes onset) | Clinical diagnosis | 80 Hz 200 µs | "Around shin and ankle" | "two to three times sensory threshold" | 20 minutes 10 treatment sessions on alternate days Clinic administration |
| TENS vs visual illusion (26) | TENS: 5.33 ± 1.20 Visual illusion: 5.33 ± 1.37 | Spinal cord injury | 12.4 ± 17.8 months | ≥ 4 on DN4 | 80 Hz 180 µs | Bilaterally around spine above level of injury | "perceptible but comfortable" | 30 minutes 5 × weekly for 2 weeks Clinic administration |
| TENS vs cervical spine mobilisation (75) | Not stated | Cervical radicular pain (75) | No details | No formal or clinical neuropathic diagnostic criteria | 100 Hz 50 µs | Placed at 'cervical spinal segment and distal dermatome | No details | 30 minutes 10 sessions on alternate days over 3 weeks Clinic administration |
| TENS vs acupuncture (26) | Not stated | Postherpetic neuralgia | "3 months to 9 years" | No formal or clinical neuropathic diagnostic criteria | 100 Hz 200 µs | "Either side of painful area" | "Fairly strong sensation" | 3 × 30 minute clinic sessions week 1. Then home use for 5 weeks. No detail on home use frequency/duration |
| TENS vs exercise (60) | Not stated | Peripheral diabetic neuropathy | 12.2 ± 2.3 years (since onset of neuropathy ) | No formal or clinical neuropathic diagnostic criteria | 15 Hz 250 µs | Lower leg/ankle | "Strong rhythmic muscle contractions" | 30 minutes 3 × weekly for 8 weeks Clinic administration |
| TENS vs mirror therapy | TENS: 5.00 ± 1.63 Mirror: 5.46 ± 1.67 | Phantom limb pain | 13 ± 1.5 days (since onset of phantom limb pain) | No formal or clinical neuropathic diagnostic criteria | No details | Site of pain contralateral limb | "Strong but comfortable" | 20 minutes 1 × daily for 4 days Clinic administration |
| TENS vs sham TENS (25) | TENS: 8.09 ± 0.97 Sham TENS: 8.05 ± 1.05 | Spinal cord injury | 12.7 months | LANSS > 12 | 4 Hz 200 ms | Proximal and distal to pain region | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| DN4: Douleur Neuropathique 4; IFT: interferential therapy; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs pain scale; NRS: numerical rating scale; P300: pregabalin 300 mg; P600: pregabalin 600 mg; PENS: percutaneous electrical nerve stimulation; PRF: pulsed radiofrequency; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain intensity Show forest plot | 5 | 207 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| 1.2 Pain intensity sensitivity analysis (Celik 2013 removed) Show forest plot | 4 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.87, ‐1.02] |

