Transkutane elektrische Nervenstimulation (TENS) zur Behandlung von Erwachsenen mit neuropathischen Schmerzen
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MESH DESCRIPTOR Transcutaneous Electric Nerve Stimulation EXPLODE ALL TREES
#2 ("TENS" or "TNS" or "ENS" or "TES"):TI,AB,KY
#3 (("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation")):TI,AB,KY
#4 (("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*")):TI,AB,KY
#5 (("electric nerve therap*" or "electrical nerve therap*" or electroanalgesi*)):TI,AB,KY
#6 ( ("transcutaneous electric stimulation" or "transcutaneous electrical stimulation")):TI,AB,KY
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 MESH DESCRIPTOR PAIN EXPLODE ALL TREES
#9 MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES
#10 MESH DESCRIPTOR SOMATOSENSORY DISORDERS EXPLODE ALL TREES
#11 (((pain* or discomfor*) adj10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*))):TI,AB,KY
#12 (((neur* or nerv*) adj6 (compress* or damag*))):TI,AB,KY
#13 #8 OR #9 OR #10 OR #11 OR #12
#14 #7 AND #13
MEDLINE
1 exp Transcutaneous Electric Nerve Stimulation/
2 ("TENS" or "TNS" or "ENS").ti.
3 ("TENS" or "TNS" or "ENS").ab.
4 ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp.
5 ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp.
6 ("electric$ nerve therap$" or electroanalgesi$).mp.
7 transcutaneous electric$ stimulation.mp.
8 TES.ti,ab.
9 or/1‐8
10 exp PAIN/
11 exp PERIPHERAL NERVOUS SYSTEM DISORDERS/
12 exp SOMATOSENSORY DISORDERS/
13 ((pain* or discomfor*) adj10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).tw.
14 ((neur* or nerv*) adj6 (compress* or damag*)).tw.
15 10 or 11 or 12 or 13 or 14
16 9 and 15
17 randomized controlled trial.pt.
18 controlled clinical trial.pt.
19 randomized.ab.
20 placebo.ab.
21 drug therapy.fs.
22 randomly.ab.
23 trial.ab.
24 groups.ab.
25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26 exp animals/ not humans.sh.
27 25 not 26
28 16 and 27
Embase
1. exp Transcutaneous Electric Nerve Stimulation/
2. ("TENS" or "TNS" or "ENS").ti.
3. ("TENS" or "TNS" or "ENS").ab.
4. ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation").tw.
5. ("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").tw.
6. ("electric nerve therap$" or "electrical nerve therap$" or electroanalgesi$).tw.
7. ("transcutaneous electric stimulation" or "transcutaneous electrical stimulation").tw.
8. TES.ti,ab.
9. or/1‐8
10. exp PAIN/
11. exp PERIPHERAL NEUROPATHY/
12. exp SOMATOSENSORY DISORDERS/
13. ((pain* or discomfor*) adj10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).tw.
14. ((neur* or nerv*) adj6 (compress* or damag*)).tw.
15. 10 or 11 or 12 or 13 or 14
16. 9 and 15
17. random$.tw.
18. factorial$.tw.
19. crossover$.tw.
20. cross over$.tw.
21. cross‐over$.tw.
22. placebo$.tw.
23. (doubl$ adj blind$).tw.
24. (singl$ adj blind$).tw.
25. assign$.tw.
26. allocat$.tw.
27. volunteer$.tw.
28. Crossover Procedure/
29. double‐blind procedure.tw.
30. Randomized Controlled Trial/
31. Single Blind Procedure/
32. or/17‐31
33. (animal/ or nonhuman/) not human/
34. 32 not 33
35. 16 and 34
36. limit 35 to embase
PsycINFO
1. exp Transcutaneous Electric Nerve Stimulation/
2. ("TENS" or "TNS" or "ENS").ti.
3. ("TENS" or "TNS" or "ENS").ab.
4. ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation").tw.
5. ("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").tw.
6. ("electric nerve therap$" or "electrical nerve therap$" or electroanalgesi$).tw.
7. ("transcutaneous electric stimulation" or "transcutaneous electrical stimulation").tw.
8. TES.ti,ab.
9. or/1‐8
10. exp PAIN/
11. exp PERIPHERAL NEUROPATHY/
12. exp SOMATOSENSORY DISORDERS/
13. ((pain* or discomfor*) adj10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).tw.
14. ((neur* or nerv*) adj6 (compress* or damag*)).tw.
15. 10 or 11 or 12 or 13 or 14
16. 9 and 15
17. clinical trials/
18. (randomis* or randomiz*).tw.
19. (random$ adj3 (allocat$ or assign$)).tw.
20. ((clinic$ or control$) adj trial$).tw.
21. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
22. (crossover$ or "cross over$").tw.
23. random sampling/
24. Experiment Controls/
25. Placebo/
26. placebo$.tw.
27. exp program evaluation/
28. treatment effectiveness evaluation/
29. ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw.
30. or/17‐29
31. 16 and 30
AMED
1. exp Transcutaneous Electric Nerve Stimulation/
2. ("TENS" or "TNS" or "ENS").ti.
3. ("TENS" or "TNS" or "ENS").ab.
4. ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation").tw.
5. ("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").tw.
6. ("electric nerve therap$" or "electrical nerve therap$" or electroanalgesi$).tw.
7. ("transcutaneous electric stimulation" or "transcutaneous electrical stimulation").tw.
8. TES.ti,ab.
9. or/1‐8
10. exp PAIN/
11. exp PERIPHERAL Nervous system disease/
12. ((pain* or discomfor*) adj10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).tw.
13. ((neur* or nerv*) adj6 (compress* or damag*)).tw.
14. 10 or 11 or 12 or 13
15. 9 and 14
16. randomized controlled trials/
17. randomized controlled trial.pt.
18. controlled clinical trial.pt.
19. placebo.ab.
20. random*.ti,ab.
21. trial.ti,ab.
22. groups.ab.
23. 16 or 17 or 18 or 19 or 20 or 21 or 22
24. 15 and 23
CINAHL
S26 S16 AND S25
S25 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24
S24 (allocat* random*)
S23 (MH "Quantitative Studies")
S22 (MH "Placebos")
S21 placebo*
S20 (random* allocat*)
S19 (MH "Random Assignment")
S18 (Randomi?ed control* trial*)
S17 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S16 S9 AND S15
S15 S10 OR S11 OR S12 OR S13 OR S14
S14 ((neur* or nerv*) N6 (compress* or damag*)).
S13 ((pain* or discomfor*) N10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).
S12 (MH "Somatosensory Disorders+")
S11 (MH "Peripheral Nervous System Diseases+")
S10 (MH "Pain+")
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
S8 TES
S7 ("transcutaneous electric stimulation" or "transcutaneous electrical stimulation").
S6 ("electric nerve therap*" or "electrical nerve therap*" or electroanalgesi*)
S5 "electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").
S4 ("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation")
S3 ("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").
S2 ("TENS" or "TNS" or "ENS").
S1 (MH "Transcutaneous Electric Nerve Stimulation")
Web of Science
#17 #16 AND #10
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#16 #15 AND #14
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#15 TOPIC: (human*)
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#14 #13 OR #12 OR #11
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#13 TOPIC: (((((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)))))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#12 TOPIC: ((((controlled clinical trial OR controlled trial OR clinical trial OR placebo))))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#11 TOPIC: ((((randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial))))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#10 #9 AND #6
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#9 #8 OR #7
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#8 TOPIC: (((neur* or nerv*) Near/6 (compress* or damag*)).)
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#7 TOPIC: (((pain* or discomfor*) near/10 (central or complex or rheumat* or muscl* or nerv* or neuralgia* or neuropath*)).)
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#6 #5 OR #4 OR #3 OR #2 OR #1
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#5 TOPIC: (("transcutaneous electric stimulation" or "transcutaneous electrical stimulation"))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#4 TOPIC: (("electric nerve therap*" or "electrical nerve therap*" or electroanalgesi*))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#3 TOPIC: (("transcutaneous electric nerve stimulation" or "transcutaneous electrical nerve stimulation" or "transcutaneous nerve stimulation"))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#2 TOPIC: (("electric nerve stimulation" or "electrical nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").)
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
#1 TOPIC: (("TENS" or "TNS" or "ENS" or "TES"))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
LILACS
TENS or TNS or ENS or transcutaneous or TES or nerve stimulation or electrostimulat$ [Words] and pain$ or discomfor$ or compress$ or damag$ [Words] and random$ or trial$ or crossover$ or blind$ or placebo$ [Words]
Appendix 2. Included study methodology description
Pooled studies
Barbarisi 2010 (n = 30) used a two arm parallel design in participants with post‐herpetic neuralgia PHN). All participants undertook an initial eight day programme of pregabalin drug treatment at varying doses with the aim of reducing all participants baseline visual analogue scale (VAS) pain intensity scores to 60 mm or less on a 0 to 100 mm VAS scale. There was no information with regard to how many participants were initially enrolled in the drug titration phase. Following this, 30 drug treatment responders were randomised to either transcutaneous electrical nerve stimulation (TENS) or sham TENS applied for 30 minutes per day (clinic administered) for four weeks. Baseline pain intensity post drug titration phase was compared to final pain intensity scores at four weeks. VAS scores of pain intensity appeared to reflect 'current' pain intensity. Analysis of participants was subdivided according to the concomitant dose of pregabalin taken during the study. The comparison was: pregabalin 300 mg plus TENS versus pregabalin 300 mg plus sham TENS, pregabalin 600 mg plus TENS versus pregabalin 600 mg plus sham TENS.
Bi 2015 randomised 52 participants with spinal cord injury into TENS versus sham TENS groups. Pain intensity was assessed (on a 0 to 10 VAS) at baseline and then immediately upon cessation of 12 weeks of treatment. The VAS reflected current pain intensity at time of measurement. Participants were treated three times per week for 12 weeks and the TENS/sham TENS was administered in the treating clinic. Celik 2013 carried out a similar sized study in 33 participants with spinal cord injury randomised to TENS or sham TENS. Daily treatment of 30 minute duration was administered in the clinic. Pain intensity VAS scores (on a 0 to 10 VAS) were recorded morning, noon, evening and night pretreatment and post‐treatment; day one of the protocol consisted of these four VAS assessments. Participants then had 10 days of treatment intervention. Day 12 of the protocol consisted of assessing the pain intensity with the same four VAS measures used at day one. Means of the four measures obtained at day one and day 12 were calculated and used in the final analysis. It is worth noting that both groups were also taking amitriptyline 10 mg as a concomitant treatment in this study. Vitalii 2014 used a similar methodology with participants who had spinal cord injury. Participants were randomised to TENS or sham TENS groups and then received 30 minute clinic administered treatment daily for 10 days. This study employed concomitant treatment with gabapentin 900 mg. Pain intensity (0 to 10 VAS) scores were a mean of morning and evening reporting. Data were reported as 'day zero' baseline and post‐treatment 'day 10' scores.
Buchmuller 2012 randomised 236 participants with chronic low back pain into two groups receiving either TENS or sham TENS. As a subgroup within this sample, 139 participants were classified as having a neuropathic component to their condition. This classification was made on the basis of clinical assessment. The primary outcome of this study was functional change assessed via the Roland‐Morris Disability Questionnaire (RDQ). Secondary outcomes included dichotomising participants according to pain intensity changes (50% decrease in on a 0 to 10 VAS classed as criteria for improvement) from baseline to post‐treatment. While the paper reported the data in this dichotomised method, following contact the authors were able to provide pain intensity data for participants from the neuropathic group and specifically for those participants with a radicular pain component. These pain intensity data were used when pooling data. Radicular pain was assessed in 122 participants at baseline and then reassessed at three months. In the active TENS group at baseline there were data for 64 participants while in the sham TENS group baseline data were available for 58 participants. Following completion of treatment and with dropout there were data for 43 participants in the active TENS group and 32 participants in the sham TENS group. The TENS/sham TENS units were supplied to the participant for home administration. Participants were instructed to compete four TENS session per day with each session lasting one hour.
Narrative review single studies
Casale 2013 compared laser with TENS for pain intensity (on a 0 to 10 VAS) and paraesthesia in 20 participants with carpal tunnel syndrome. Treatments were applied five times per week for five weeks. Treatment duration was 30 minutes for TENS. Treatment duration for laser application was unclear. There was no information given with respect to the pain intensity VAS measure (mean pain, peak pain, etc.) and timing of assessment was only described as being "evaluated before and after treatment."
Gerson 1977 compared pharmacological treatment (carbamazepine plus clomipramine) versus TENS in 29 participants with postherpetic neuralgia. There were no reported parameters around TENS application beyond stating the duration of treatment was 15 minutes per session. It appears the TENS group initially received four treatments on a weekly basis followed by three TENS sessions at fortnightly intervals (seven TENS sessions in total). This equated to a 10 week treatment period; however, the drug treatment group was reported as being eight weeks in duration and outcomes are reported at eight weeks. Pain intensity (on a 0 to 100 mm VAS) was assessed at initially weekly then fortnightly intervals via a VAS; however, it was not stated whether this was current pain, mean pain or maximal pain.
Ghoname 1999 in a one‐arm randomised cross‐over study compared percutaneous electrical nerve stimulation (PENS) versus TENS versus sham PENS in participants with lumbar radicular pain. However, the sham treatment was invasive, involving insertion of "acupuncture like needles" into the involved area. We considered this to be very problematic as a sham intervention and therefore only considered the TENS versus PENS comparison. The main comparison involved a non‐invasive intervention (TENS) being compared against an inherently invasive procedure (PENS), therefore this study rated high risk across the key domains of participant/personnel bias. Sixty‐four participants were randomised to three different treatment sequences 1. sham PENS, PENS, TENS; 2. PENS, TENS, sham PENS; 3. TENS, sham PENS, PENS. Each treatment phase lasted three weeks with a one week washout break between. Participants received three treatment sessions per week (clinic administered) of 30 minutes' duration. Pain intensity data (0 to 10 VAS) were reported and analysed during treatment and at 24 hours post treatment phase completion.
One three arm study compared TENS, interferential (IFT) and resting splints in participants with carpal tunnel syndrome (Koca 2014). This study randomised 75 participants to one of three treatment groups. Pain intensity was assessed (on a 0 to 10 VAS) as a mean of the previous week's pain at baseline and three weeks after completion of treatment. The splint group were instructed to use resting wrist‐hand night splints during the intervention period. The TENS and IFT therapies were delivered in the clinic five times per week for 20 minutes each session.
Nabi 2015 investigated TENS versus pulsed radiofrequency (PRF) sympathectomy in 65 participants with painful peripheral diabetic neuropathy of the lower limb. Participants were randomised to either PRF sympathectomy or TENS interventions and both groups received concomitant treatment with pregabalin 300 mg/day to 600 mg/day. The main comparison involved a non‐invasive intervention (TENS) being compared against an inherently invasive procedure (PRF sympathectomy), therefore this study rated high risk across the key domains of participant/personnel bias. Participants assigned to the PRF sympathectomy group initially underwent a sympathetic blockade with local anaesthetic. Participants who reported a 50% reduction in pain then progressed to PRF sympathectomy. There were no data on how many participants underwent the initial local anaesthetic procedure or how many of this group went on to full PRF sympathectomy. Participants in the both groups had pain intensity (0‐10 numerical rating scale (NRS)) assessed four times before the procedure (PRF sympathectomy or TENS treatment to completion) and then at one week, one month and three months after completion of the procedure. It was unclear if NRS scores at baseline were a mean of the four preintervention assessments or whether the NRS elicited at each assessment represented current pain, maximal pain or mean pain. The PRF sympathectomy intervention was a one‐off single day procedure whereas the TENS was delivered as 10 × 20 minute sessions delivered on alternate days. Given the NRS assessments were completed at fixed times post 'procedure' and completion of the TENS treatment was regarded as a procedure, there was an imbalance in outcome assessment timing postrandomisation for the two groups (TENS assessments approximately three weeks later than PRF sympathectomy).
One study investigated TENS versus visual illusion in participants with neuropathic pain following spinal cord injury (Őzkul 2015). This two‐arm randomised cross‐over study allocated 24 participants to groups and received the following intervention sequences: Group one (12 participants) received visual illusion then TENS and Group two (12 participants) received TENS followed by visual illusion. Treatments were delivered five times per week over two weeks followed by one week washout between treatments. TENS sessions lasted 30 minutes while virtual illusion sessions lasted 15 minutes. This study was rated overall unclear in terms of bias and was not allocated high risk of bias in any domain. Pain intensity data (0 to 10 VAS) was reported at baseline and immediately upon completion of treatment (two weeks). Group mean pain intensity data were presented across the combined groups preintervention and postintervention. Carry‐over testing prior to initiation of second sequence treatment was not reported. Pain intensity reported as present pain (immediately upon cessation of treatment), mean (timeframe not described), minimal and maximal at baseline and post‐treatment.
Prabhakar 2011 investigated TENS versus cervical mobilisation versus isometric exercises in participants with cervical radiculopathy. This randomised parallel design allocated 75 participants to one of these three interventions. The number of participants per group was not described. All participants initially received hot pack therapy and treatment interventions were applied on alternate days for 10 sessions over three weeks. TENS sessions lasted approximately 30 minutes. There were no details on duration of treatment in the mobilisation or isometric exercise group. Pain intensity (VAS not specified) was assessed at baseline then at three weeks (completion of treatment) and six weeks (three weeks after treatment completed). The parameters of the VAS pain intensity measure (e.g. mean, minimal, maximal pain) were not described.
One study investigated acupuncture (ACU, 10 participants) and TENS (13 participants) in people with postherpetic neuralgia (Rutgers 1988). All treatment interventions lasted six weeks. The ACU group were treated twice per week for six weeks with body and auricular acupuncture while the TENS group received 3 × 30 minute TENS sessions in the first week (clinic administered) and were then instructed to apply TENS themselves at home for the next five weeks. There were no details on TENS duration, dosage or treatment parameters for the home treatment component of the intervention. Pain intensity was assessed via a 10‐point stepwise scale. There was no further detail provided for this scale. Pain intensity was assessed at baseline, six weeks, nine weeks and six months postrandomisation. This study was rated overall at high risk of bias with the key domains blinding of participants and personnel, incomplete outcome data and selective reporting of outcomes being rated high.
Serry 2015 investigated TENS versus exercise in 60 participants with diabetic peripheral neuropathy randomised to TENS, exercise or regular pharmacological therapy groups (20 per group). TENS and exercise groups received treatment three times per week for eight weeks. TENS sessions lasted 30 minutes, aerobic exercise sessions lasted in total 50 minutes. All treatments were applied under supervision. Additionally, participants in the TENS and exercise groups continued with concomitant treatment of their regular pharmacological therapy. This study was rated at overall high risk of bias with particular risk in the 'selective reporting of outcome' domain. In this study, pain intensity was assessed at baseline and post‐treatment on a 0 to 10 VAS although it was unclear what aspect of the pain experience was assessed (e.g. mean, minimal, maximal pain, etc.).
We include one study investigating TENS versus mirror therapy in participants with phantom limb pain (Tilak 2016). In this study, 26 participants (88% men) were randomised to either TENS (n = 13) or mirror (n = 13) intervention groups. Pain intensity was assessed with a 0 to 10 VAS and a 'Universal Pain Score' (participants selects from a range of hand‐drawn faces depicting pain expressions which face most closely matches their experience). It was unclear what aspect of the pain experience was assessed (e.g. mean, minimal, maximal pain, etc.). Treatments were applied daily for four days. Each treatment session lasted 20 minutes. Baseline demographics and site of amputation were well described and no significant differences in age, duration of phantom limb pain or pain intensity was found. Overall, this study was rated unclear on risk of bias with the only domain assessed as high being sample size.
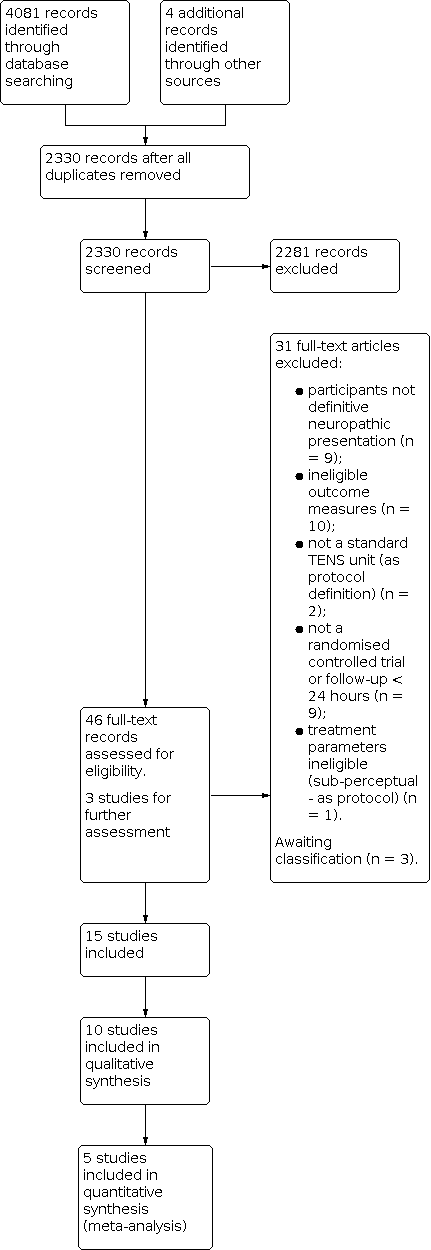
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.1 Pain intensity.

Forest plot of comparison: 1 TENS versus sham TENS, outcome: 1.2 Pain intensity sensitivity analysis (Celik 2013 removed).

Comparison 1: TENS versus sham TENS, Outcome 1: Pain intensity

Comparison 1: TENS versus sham TENS, Outcome 2: Pain intensity sensitivity analysis (Celik 2013 removed)
| TENS versus sham TENS for neuropathic pain in adults | ||||
| Patient or population: adults with neuropathic pain Settings: secondary care Intervention/comparison: TENS vs sham TENS Outcome: Pain intensity (VAS) | ||||
| Outcomes | Effect estimate (95% CI) | No of participants | Quality of the evidence | Comments |
|---|---|---|---|---|
| Post‐intervention pain intensity (VAS 0‐10) | Favoured TENS. Mean difference ‐1.58 (95% CI ‐2.08 to ‐1.09) | 207 (5) | ⊕⊝⊝⊝ Very lowa | Downgraded 3 levels due to multiple sources of potential bias, small number and size of studies. |
| Health related quality of life | No data | ‐ | ‐ | ‐ |
| Participant global impression of change | No data | ‐ | ‐ | ‐ |
| Analgesic medication use | Not estimable | ‐ | ‐ | ‐ |
| Incidence/nature of adverse events | Not estimable | ‐ | ‐ | ‐ |
| CI: confidence interval; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded twice for limitations of studies and once for imprecision. | ||||
| Study, comparison (admitted sample size) | Group baseline pain intensity VAS/NRS | Neuropathic condition | Reported mean duration | Diagnostic criteria | Hz and pulse width | Electrode location | Intensity | Duration, frequency and site of administration |
|---|---|---|---|---|---|---|---|---|
| TENS vs sham TENS (30) | P300 + TENS: 4 ± 0.93 P600 + TENS: 3.8 0.95 P300 + sham TENS: 4.1 ± 1.19 P600 + sham TENS: 3.2 ± 0.81 | Postherpetic neuralgia | 15.25 ± 8.7 months | No formal or clinical neuropathic diagnostic criteria | 100 Hz (later described in text as 50 Hz) 125 µs | "Around site of pain" | "Clear non‐painful paraesthesia". Titrated to maintain strength of perception | 30 minutes daily for 4 weeks Clinic administration |
| TENS vs sham TENS (52) | TENS: 5.17 ± 2.34 Sham TENS: 5.56 ± 2.07 | Spinal cord injury | 6.9 ± 3.6 months (since spinal cord injury) | No formal or clinical neuropathic diagnostic criteria | 2 Hz 200 ms | Placed "on region with pain" | 50 mA. No description of perceived sensation | 20 minutes 3 × weekly for 12 weeks Clinic administration |
| TENS vs sham TENS (122) | TENS: 6.15 ± 2.24 Sham TENS: 5.91 ± 2.12 | Lumbar radicular pain (subgroup data supplied by authors) | Not reported | Clinical assessment | Mixed: 80‐100 Hz alternated with 2 Hz 200 ms | Placed on low back and radicular region of pain | Low intensity paraesthesia alternated with high intensity (muscle twitches) | 1 hour. 4 × daily for 3 months Self‐administered at home |
| TENS vs laser? (20) | TENS: 6 ± 0.8 Laser?: 6.6 ± 1.1 | Carpal tunnel syndrome | Not reported | Nerve conduction study | 100 Hz 80 ms | Over carpal ligament and median nerve | "Below muscle contraction" | 30 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs sham TENS (33) | TENS: 5.79 ± 2.17 Sham TENS: 5.64 ± 1.81 | Spinal cord injury | 19.1 months | LANSSa > 12 | 4 Hz 200 µs | Placed "on region with pain" | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| TENS vs drug treatment (29) | TENS: 27.0 Drug: 59.0 (0‐100) | Postherpetic neuralgia | No details | No formal or clinical neuropathic diagnostic criteria | No details | "Placed on affected dermatome" | No detail | 15 minutes 1 × weekly for 4 weeks then 1 × fortnightly for 3 weeks |
| TENS vs PENS (64) | TENS: 7.0 ± 1.9 PENS: 7.2 ± 1.8 Sham PENS: 6.6 ± 1.9 | Lumbar radicular pain | 21 ± 9 months | Clinical assessment. Radiological assessment of nerve root compression | 4 Hz 100 ms | Placed on posterior lower limb | "Highest tolerable sensation" without muscle twitch | 30 minutes 3 × weekly for 3 weeks Clinic administration |
| TENS vs IFT (75) | TENS: 8.06 ± 0.55 IFT: 8.25 ± 0.4 Splint: 8.31 ± 0.6 | Carpal tunnel syndrome | 13.3 ± 6.3 months | Nerve conduction study | 100 Hz 80 ms | Placed on "palmar aspect of hand/wrist" | No details | 20 minutes 5 × weekly for 3 weeks Clinic administration |
| TENS vs PRF sympathectomy (65) | TENS: 6.10 PRF sympathectomy: 6.46 (NRS) | Peripheral diabetic neuropathy | 12.9 ± 3 years (since diabetes onset) | Clinical diagnosis | 80 Hz 200 µs | "Around shin and ankle" | "two to three times sensory threshold" | 20 minutes 10 treatment sessions on alternate days Clinic administration |
| TENS vs visual illusion (26) | TENS: 5.33 ± 1.20 Visual illusion: 5.33 ± 1.37 | Spinal cord injury | 12.4 ± 17.8 months | ≥ 4 on DN4 | 80 Hz 180 µs | Bilaterally around spine above level of injury | "perceptible but comfortable" | 30 minutes 5 × weekly for 2 weeks Clinic administration |
| TENS vs cervical spine mobilisation (75) | Not stated | Cervical radicular pain (75) | No details | No formal or clinical neuropathic diagnostic criteria | 100 Hz 50 µs | Placed at 'cervical spinal segment and distal dermatome | No details | 30 minutes 10 sessions on alternate days over 3 weeks Clinic administration |
| TENS vs acupuncture (26) | Not stated | Postherpetic neuralgia | "3 months to 9 years" | No formal or clinical neuropathic diagnostic criteria | 100 Hz 200 µs | "Either side of painful area" | "Fairly strong sensation" | 3 × 30 minute clinic sessions week 1. Then home use for 5 weeks. No detail on home use frequency/duration |
| TENS vs exercise (60) | Not stated | Peripheral diabetic neuropathy | 12.2 ± 2.3 years (since onset of neuropathy ) | No formal or clinical neuropathic diagnostic criteria | 15 Hz 250 µs | Lower leg/ankle | "Strong rhythmic muscle contractions" | 30 minutes 3 × weekly for 8 weeks Clinic administration |
| TENS vs mirror therapy | TENS: 5.00 ± 1.63 Mirror: 5.46 ± 1.67 | Phantom limb pain | 13 ± 1.5 days (since onset of phantom limb pain) | No formal or clinical neuropathic diagnostic criteria | No details | Site of pain contralateral limb | "Strong but comfortable" | 20 minutes 1 × daily for 4 days Clinic administration |
| TENS vs sham TENS (25) | TENS: 8.09 ± 0.97 Sham TENS: 8.05 ± 1.05 | Spinal cord injury | 12.7 months | LANSS > 12 | 4 Hz 200 ms | Proximal and distal to pain region | 50 mA. No description of perceived sensation | 30 minutes 1 × daily for 10 days Clinic administration |
| DN4: Douleur Neuropathique 4; IFT: interferential therapy; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs pain scale; NRS: numerical rating scale; P300: pregabalin 300 mg; P600: pregabalin 600 mg; PENS: percutaneous electrical nerve stimulation; PRF: pulsed radiofrequency; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain intensity Show forest plot | 5 | 207 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| 1.2 Pain intensity sensitivity analysis (Celik 2013 removed) Show forest plot | 4 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.87, ‐1.02] |

