Местное лечение при блефарокератоконъюнктивите у детей
Appendices
Appendix 1. Meibomian gland dysfunction
An international workshop defined meibomian gland dysfunction (MGD) in adults as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease” (Nichols 2011). MGD is one cause of posterior blepharitis, an inflammatory change of the posterior lid margin. MGD may initially be asymptomatic; as it progresses, lid margin signs such as the expressibility and quality of meibomian gland secretions and telangiectasia of the lid margin may develop, which is then called posterior blepharitis (Nelson 2011; Nichols 2011; Tomlinson 2011).
Located within the tarsal plate of the eyelids, the meibomian glands produce and secrete lipids and proteins that spread onto the tear film, making it more stable and resistant to evaporation (Nichols 2011). Most commonly, MGD is caused by obstruction of the opening of the gland onto the posterior lid margin; this obstruction is often caused by thickened secretions and keratinised cellular debris (Nichols 2011). Age, gender, hormonal factors and medication can all contribute to the viscosity and quality of meibomian gland secretions and may be involved in the obstruction of the meibomian gland opening (Nichols 2011).
With MGD, the tear film becomes unstable and hyperosmolar, and evaporates more quickly, leading to signs and symptoms of dry eye syndrome (Nichols 2011; Suzuki 2011). In human corneal epithelial cells in vitro, hyperosmolarity increases the expression and production of proinflammatory cytokines and chemokines such as interleukins 81, 21, and matrix metalloproteinase inhibitors 1, 9 and 13, in a process mediated by a group of key signalling molecules called mitogen‐activated protein kinases (Li 2006; Luo 2004).
The altered microenvironment associated with MGD may also allow increased bacterial growth on the lid margin (Nichols 2011).
Appendix 2. CENTRAL search strategy
#1 blepharokeratoconjunctivitis or blepharokeratitis or blepharoconjunctivitis or BKC
Appendix 3. MEDLINE (OvidSP) search strategy
(blepharokeratoconjunctivitis or blepharokeratitis or blepharoconjunctivitis or BKC).tw.
Appendix 4. EMBASE (OvidSP) search strategy
(blepharokeratoconjunctivitis or blepharokeratitis or blepharoconjunctivitis or BKC).tw.
Appendix 5. ISRCTN search strategy
blepharokeratoconjunctivitis OR blepharokeratitis OR blepharoconjunctivitis OR BKC
Appendix 6. ClinicalTrials.gov search strategy
blepharokeratoconjunctivitis OR blepharokeratitis OR blepharoconjunctivitis OR BKC
Appendix 7. WHO ICTRP search strategy
blepharokeratoconjunctivitis OR blepharokeratitis OR blepharoconjunctivitis OR BKC
Appendix 8. Data extraction form
| Review author | ||||||
| Study ID | ||||||
| Dates when study was conducted | If unavailable, comment 'dates not available' | |||||
| Funding source(s) | ||||||
| Declarations of interest by researchers | ||||||
| Methods | Study design
Eyes
| |||||
| Risk of bias | Selection bias | |||||
| Participants | Country | |||||
| Interventions | Intervention 1 = active intervention 1 | |||||
| Outcomes (as defined in study) Please specify which | Primary outcome
Secondary outcomes
| |||||
| Primary outcome Improvement in symptoms | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % with improvement of symptoms | Total number of participants | % with improvement of symptoms | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % with complete success | Total number of participants | % with complete success | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % with partial success | Total number of participants | % with partial success | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 3 | ||||
| Time point | Total number of participants | Mean | Standard deviation* | Total number of participants | Mean | Standard deviation* |
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % with adverse events | Total number of participants | % with adverse events | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % with adverse events | Total number of participants | % with adverse events | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | % of issued medication used | Total number of participants | % of issued medication used | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | Total amount of co‐medication used (specify) | Total number of participants | Total amount of co‐medication used (specify) | ||
| 3 months | ||||||
| Secondary outcome | Intervention 1 | Intervention 2 | ||||
| Time point | Total number of participants | Treatment cost, utility data | Total number of participants | Treatment cost, utility data | ||
| 3 months | ||||||
Appendix 9. Proposed outline for 'Summary of findings' table
| Treatment A versus treatment B for children with blepharokeratoconjunctivitis (BKC) | ||||||
| Population: children aged zero to 16 years with a clinical diagnosis of BKC Settings: hospital clinics, ophthalmologists’ offices/clinics Intervention: topical antibiotics, anti‐inflammatories, immunosuppressants and immunomodulators, lubricants Comparison: placebo or other active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intervention A | Intervention B | |||||
| Percentage of children who experience an improvement in symptoms, reported by the child or by their parents/carers, preferably measured by a validated tool, at three months (± one month) after start of treatment | ||||||
| Percentage of children with elimination of all clinical signs of ocular surface inflammation ('complete success'), preferably measured by a composite grading system | ||||||
| Percentage of children with improvement of clinical signs of ocular surface inflammation ('partial success'), preferably measured by a composite grading system | ||||||
| Change from baseline in best corrected visual acuity in affected eye(s) in logMAR measured with an ETDRS chart at 4 m, or, in younger children, with a Keeler crowded logMAR chart at 3 m | ||||||
| Percentage of participants suffering from uncontrolled or poorly controlled disease progression due to treatment failure | ||||||
| Percentage of participants suffering from adverse effects of medication | ||||||
| Adherence to treatment, as a percentage of (study medication issued minus residual study medication returned at end of trial)/(study medication issued) | ||||||
| Total amount of topical steroids and topical immunosuppressants used during the duration of the trial | ||||||
| Cost‐effectiveness or cost‐utility of treatments | ||||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the intervention group and the relative effect of the intervention (and its 95% CI). Abbreviations: BKC: blepharokeratoconjunctivitis; CI: confidence interval; RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation. GRADE Working Group grades of evidence (see explanations). | ||||||
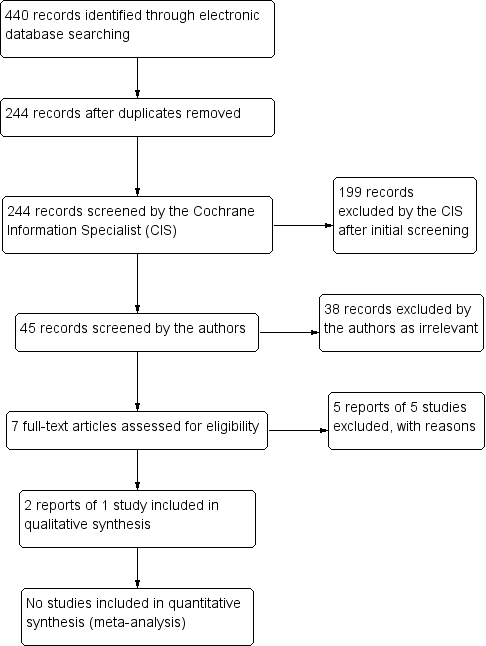
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Topical treatments compared with control for blepharokeratoconjunctivitis in children | ||||||
| Patient or population: children with blepharokeratoconjunctivitis Settings: eye clinic Intervention: topical treatments (antibiotics and/or steroids) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (vehicle) | topical treatments (antibiotics/steroids) | |||||
| Improvement in symptoms, reported by the child or by their parents/carers, preferably measured by a validated tool, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Elimination of all clinical signs of ocular surface inflammation ('complete success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Improvement of clinical signs of ocular surface inflammation ('partial success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (10 | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Change from baseline in best corrected visual acuity in affected eye(s) in logMAR measured with an ETDRS chart at 4 m, or, in younger children, with a Keeler crowded logMAR chart at 3 m, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Limited data in a form that could not be extracted; not statistically significant differences between groups. |
| Uncontrolled or poorly controlled disease progression due to treatment failure, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects of medication, at any time during treatment | Ocular adverse events
Non‐ocular adverse events
| 137 (1) | ⊕⊝⊝⊝ | |||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some data on blepharoconjunctivitis grade reported on trials registry but we were unable to estimate a measure of effect for this outcome. 2 Data were not fully reported and we were unable to estimate a measure of effect for change in visual acuity. 3 Very low certainty due to very low numbers of events. | ||||||
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| Case series | 7 | 6 to 14 years | 6 months | Lid hygiene | Amoxicillin/ clavulanate | Chloramphenicol (PF) drops, chloramphenicol ointment to lids, prednisolone 0.5% (PF) | Eyelid condition, corneal epitheliopathy, stromal defects | Improvement of symptoms | None | None | |
| Case series | 3 | 30 months to 8 years | Variable | Lid hygiene | Azithromycin | Loteprednol 0.2%, CSA 0.05%, | Chalazia, keratitis, corneal ulcer/scar, phlyctenule, MGD | Improvement of itching | None | None | |
| Case series | 16 | 4 to 16 years | Variable | Lid hygiene | Erythromycin (1 participant only) | Azithromycin 1.5%, CSA 2% | Bulbar conjunctival hyperaemia, conjunctival phlycten, corneal inflammation, blepharitis grade | Ocular redness | None | Ocular irritation (redness, burning, stinging) | |
| Case series | 8 | 3.5 to 13 years | 8.3 months | Lid hygiene | Erythromycin suspension 450 mg divided into 3 doses | Prednisolone 0.5% (PF); hydrocortisone acetate 1% ointment nocte | Bulbar conjunctival redness, inferior superficial corneal vascularisation, punctate corneal epithelial staining, inferior subepithelial vascularisation and infiltrate, conjunctival phlyctenules, corneal phlyctenules, circumferential pannus, corneal scar | Red eyes, photophobia, itching, discharge | Corneal scarring and thinning | Stomach disturbance, diarrhoea | |
| Case series | 615 | 7 months to 16 years | Not reported | Lid hygiene | Erythromycin | Topical steroids and antibiotics (not specified) | Outcomes not reported (presenting signs only) | Outcomes not reported (presenting symptoms only) | None | None | |
| Case series | 10 | 6 to 27 years | 4.4 years | Lid hygiene | Azathioprine, mycophenolate mofetil, prednisolone | Steroids (not specified) | Disease remission/ control of inflammation | None | Corneal perforation | none | |
| Case series | 29 | 2 to 12 years | 5.4 months | Warm compresses | Erythromycin, doxycyclin | Prednisolone 1%, dexamethasone 0.1%, antibiotic, fluorometholone, loteprednol etabonate 0.5% | Eyelid inflammation, superficial punctate keratitis, corneal vascularisation, corneal infiltrates, phlyctenules, corneal scarring | None | Amblyopia | Gastrointestinal distress, mouth ulcers (unrelated) | |
| Case series | 27 | 7 months to 15.9 years | 2.3 years | Warm compresses, lid hygiene | Erythromycin, doxycyclin, flaxseed oil | chloramphenicol, ciprofloxacin, gentamicin, prednisolone 1% or 0.5%, fluorometholone 0.1% | Visual acuity, astigmatism | Discomfort, photophobia | Amblyopia | Vaginal candidiasis | |
| Case series | 5 | 4 to 9 years | Not specified | Erythromycin | Lid hyperaemia and swelling, corneal infiltrates | None | None | None | |||
| Case series | 114 | Mean 9.3 years (± 4.2) | 26.4 months | Lid hygiene | Flaxseed oil, erythromycin | Lubricants (hyaluronate, methylcellulose), erythromycin, ciprofloxacin, steroids (dexamethasone 0.1% (PF), loteprednol 0.5%, fluorometholone 0.1%), CSA 0.05% | Visual acuity | None | None reported | None reported | |
| Case series | 51 | Mean 10.2 years (± 3.6) | 58.9 months | Warm compresses, lid hygiene | Antibiotics (erythromycin, amoxicillin/ clavulanate, doxycycline), steroids | Steroids (dexamethasone 1%, prednisolone 0.12 to 1%, fluorometholone 0.1%) , antibiotics (fucidic acid, levofloxacin, tobramycin), CSA 0.5% | Visual acuity | Redness, tearing, blurred vision, pain, irritation, photophobia, white spot, swelling, discharge, itching, rubbing | Corneal perforation | Raised intraocular pressure, cataract, gastrointestinal disturbance | |
| Case series | 44 | 1 to 14 years | 7 years | Lid hygiene | Erythromycin | Chloramphenicol, steroids | Reduction of clinical signs | Redness, watering, itching, grittiness, discharge, photophobia, pain | None reported | None reported | |
| Abbreviations: CSA: ciclosporin | |||||||||||
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| RCT | 137 | 0 to 6 years | 15 days | None | None | Loteprednol 0.5% , tobramycin 0.3% | Visual acuity | None | None | Eye pain, conjunctivitis, eye discharge, and eye inflammation | |
| RCT | Total 417; 19 children | Not specified | 15 days | None | None | Azithromycin 1%, dexamethasone 0.1% | Complete bacterial eradication from conjunctiva and eyelids, complete resolution of clinical signs | Complete resolution of symptoms | None | Eye disorder, reduced visual acuity, punctate keratitis, blurred vision, conjunctival oedema, discharge, lid oedema, irritation, pain, itching | |
| RCT | Total 71; number of children not specified | 10 to 86 years | 14 to 15 days | None | None | Gentamycin 0.3%, betamethasone 0.1% | Ocular sign score, specific ocular inflammatory signs, bacterial eradication | None | None | Conjunctival hyperaemia | |
| Abbreviations: N: number of participants | |||||||||||
| Follow‐up | Loteprednol Etabonate and Tobramycin | Loteprednol Etabonate | Tobramycin | Vehicle |
| Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | |
| Day 3 | ‐7.32 (3.27) 34 | ‐7.74 (3.90) 34 | ‐5.94 (4.00) 32 | ‐6.58 (3.46) 31 |
| Day 7 | ‐11.03 (3.20) 34 | ‐10.94 (4.69) 34 | ‐9.90 (3.80) 30 | ‐10.03 (4.63) 30 |
| Day 15 | ‐11.41 (3.29) 34 | ‐11.23 (3.98) 35 | ‐10.68 (4.71) 34 | ‐10.30 (5.19) 33 |
| Loteprednol/tobramycin | Loteprednol | Tobramycin | Vehicle | |
| Ocular AEs | 1/34 (eye pain) | 4/35 (eye pain, conjunctivitis, eye discharge, eye inflammation) | 0/34 | 0/33 |
| Non‐Ocular AEs | 2/34 3 AEs (gastroenteritis, pyrexia, bronchiolitis) | 6/35 9 AEs (ear infection, lip swelling, vomiting, URI, varicella, cough, phyarngolaryngeal pain, rash) | 6/34 9 AEs (ear infection, otitis media acute, diarrhea, pyrexia, bronchioltis, URI, nasophayngitis, respirator distress, dermatitis (diaper) ) | 5/33 7 AEs (ear pain, pyrexia, urticaria, bronchioloitis, URI, tonsilitis, dehydration) |
| Abbreviations: AEs: adverse events | ||||

