Astragalus por vía oral (Huang qi) para la prevención de los episodios frecuentes de infección respiratoria aguda en los niños
Resumen
Antecedentes
Las infecciones respiratorias agudas (IRA) son frecuentes en los niños y pueden comprometer tanto las vías respiratorias superiores como las inferiores. Muchos niños presentan episodios de IRA frecuente o infecciones respiratorias recurrentes (IRR) en los primeros años de vida, lo que implica un desafío para los pediatras, los médicos de atención primaria, los padres y los ciudadores de los niños.
En China, el Astragalus (Huang qi), solo o en combinación con otras hierbas, es usado por los profesionales de la Medicina Tradicional China (MTC) en forma de un extracto líquido, para reducir el riesgo de IRA; se cree que estimula el sistema inmunológico. Una mejor comprensión de los mecanismos terapéuticos del Astragalus puede aclarar cuestiones sobre la prevención de la IRA y en consecuencia reducir el uso de antibióticos.
Objetivos
Evaluar la efectividad y la seguridad del Astragalus por vía oral para prevenir los episodios frecuentes de infecciones respiratorias agudas (IRA) en los niños en el ámbito de la comunidad.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, número 12, 2015), MEDLINE (Ovid) (1946 hasta 31 diciembre 2015), Embase (Elsevier) (1974 hasta 31 diciembre 2015), AMED (Ovid) (1985 hasta 31 diciembre 2015), Chinese National Knowledge Infrastructure (CNKI) (1979 hasta 31 diciembre 2015) y en la Chinese Scientific Journals full text database (CQVIP) (1989 hasta 31 diciembre 2015), China Biology Medicine disc (CBM 1976 hasta 31 diciembre 2015) y en la Wanfang Data Knowledge Service Platform (WanFang) (1998 hasta 31 diciembre 2015).
Criterios de selección
Se incluyeron ensayos controlados aleatorios (ECA) que comparaban el Astragalus oral como una única preparación herbaria china con un placebo para prevenir los episodios frecuentes de IRA en los niños.
Obtención y análisis de los datos
Se utilizaron los los procedimientos metodológicos Cochrane estándar para esta revisión. Se evaluaron los resultados de la búsqueda para identificar estudios relevantes. Se planificó extraer datos utilizando formularios estandarizados. Los desacuerdos se resolvían mediante debate. El riesgo de sesgo se evaluaba con la herramienta Cochrane de "Riesgo de sesgo". Se planificó usar la diferencia de medias (DM) o la diferencia de medias estandarizada (DME) para los datos continuos, y el cociente de riesgos (CR) o el odds ratio (OR) para analizar los datos dicotómicos, ambos con intervalos de confianza (IC) del 95%.
Resultados principales
Se identificaron 6080 registros: 3352 de bases de datos en inglés, 2724 de bases de datos chinas y cuatro de otras fuentes. Después del cribado inicial y la eliminación de los duplicados, se obtuvieron 120 documentos de texto completo para la evaluación. De éstos, 21 no eran ECA; 55 no cumplían los criterios de inclusión porque: los participantes tenían más de 14 años de edad; la definición no estaba incluida para los episodios recurrentes ni frecuentes; la preparación de Astragalus no era una intervención; la preparación de Astragalus se incluía en la fórmula pero no era el único agente; la preparación de Astragalus no fue administrada por vía oral; o el Astragalus se usó para el tratamiento en lugar de la prevención de la IRA. Cuarenta y cuatro estudios adicionales fueron excluidos porque no eran controlados con placebo, aunque cumplían otros criterios de inclusión.
Ningún ECA cumplió los criterios de inclusión.
Conclusiones de los autores
No se encontraron pruebas suficientes para permitir una evaluación de la efectividad y la seguridad del Astragalus oral como una única intervención para prevenir las IRA frecuentes en niños de hasta 14 años de edad.
PICO
Resumen en términos sencillos
¿Puede prevenir el Astragalus por vía oral (Huang qi) las infecciones respiratorias agudas frecuentes en los niños?
Pregunta de la revisión
Se evaluaron las pruebas de efectos beneficiosos y perjudiciales del Astragalus oral usado solo para prevenir los episodios frecuentes de infecciones respiratorias agudas (IRA) en los niños de hasta 14 años de edad, comparados con un tratamiento falso. Las IRA incluyen: resfriados, faringitis, laringitis, gripe, bronquitis y neumonía.
Antecedentes
Se utilizan muchos tratamientos para prevenir las IRA, especialmente en los niños. El Astragalus, es un tratamiento herbario de uso y disponibilidad amplia, que se ha utilizado por miles de años en China para ayudar a prevenir las IRA. Se piensa que refuerza la inmunidad. Casi uno de cada cinco niños presentan IRA frecuentes, y el tratamiento cubre hasta un 75% de todas las prescripciones de antibióticos para niños. Dado que la mayoría de las IRA son causadas por virus, los antibióticos no son efectivos para tratar estas enfermedades.
Fecha de la búsqueda
Se hicieron búsquedas en la literatura hasta el 31 diciembre 2015.
Características de los estudios
Se identificaron 6080 registros potencialmente relevantes. Después de retirar los registros duplicados y los que no cumplían con los criterios de inclusión, se obtuvieron 120 estudios de texto completo. Se evaluaron cuidadosamente estos estudios para su posible inclusión. Se excluyeron la mayoría de los estudios porque: presentaron criterios de diagnóstico poco claros; la preparación de Astragalus se usó con otros agentes; o el Astragalus no se comparó con un tratamiento falso. Ningún estudio cumplió con los criterios de inclusión y por lo tanto, no se pudo analizar ningún resultado.
Fuentes de financiación de los estudios
No se pudieron evaluar las fuentes de financiamiento de los estudios.
Resultados clave
No se encontraron estudios que compararan el uso de Astragalus oral solo con un tratamiento falso para prevenir los episodios frecuentes de IRA en niños de hasta 14 años de edad. Se necesitan estudios bien diseñados, realizados e informados que investiguen este tema para permitir la evaluación en el futuro.
Calidad de la evidencia
No se pudo evaluar la calidad de las pruebas.
Authors' conclusions
Background
Description of the condition
Acute respiratory tract infection (ARTI) is a frequent childhood illness that can involve both upper and lower airways (WHO 1998). ARTIs include common cold, influenza, otitis media, sinusitis, tonsillitis, laryngitis, pharyngitis, bronchitis and pneumonia (CPSCMA 1999). Symptoms include nasal congestion and discharge, sneezing, sore throat, cough, sputum production, shortness of breath, chest pain and fever. Managing ARTIs is a continuing challenge for paediatricians and primary care physicians (CPSCMA 1999; Heikkinen 2003).
On average, children aged under two years experience about six common colds each year (Kvaerner 2000; Leder 2003; Monto 1993; Monto 2002), and healthy children under six years of age may experience up to five respiratory tract infections annually (Griffin 2004). A cohort study observed the history of respiratory infections among children living in urban areas in Germany, and indicated that the mean cumulative number of ARTIs in the first 12 years of life was 21.90 ± 9.00 episodes (Grüber 2008). In high‐income countries, up to 25% of children aged up to one year, and 18% of children between the ages of one and four years, experience frequent ARTI episodes or RRTIs (Bellanti 1997); these are characterised as recurrent airway inflammation caused by infectious agents (Martino 2007).
In China, the definition of 'frequency' is based on age and disease type in accordance with Chinese guidelines (CPSCMA 2008; Table 1). This definition was developed by the respiratory group of the Paediatrics Committee Chinese Medical Association in 1988 (Hu 1988) and updated in 2007 (CPSCMA 2008). All criteria emphasise infection frequency and requires the absence of underlying pathological conditions such as primary or secondary immunodeficiency, cystic fibrosis, airway malformations or immotile‐cilia syndrome.
| Age (years) | Recurrent URTI (episodes per year) | Recurrent LRTI (episodes per year) | |
| Recurrent tracheitis or bronchitis | Recurrent pneumonia | ||
| Birth to 2 years of age | 7 | 3 | 2 |
| 2 to 5 years of age (> 2 years and < 5 years) | 6 | 2 | 2 |
| 5 to 14 years of age (> 5 years and < 14 years) | 5 | 2 | 2 |
Abbreviations: LRTI ‐ lower respiratory tract infection; URTI ‐ upper respiratory tract infection
Although factors influencing the aetiology of ARTIs are not always readily identifiable, viral agents are typically responsible for infection. Over 200 different viruses have been associated with ARTIs (Mäkelä 1998). Consequently, vaccination is theoretically an effective way to prevent some ARTIs in children; however, the high frequency of genetic exchange among different strains or subtypes, and high rates of nucleotide substitution, increase the difficulty of developing vaccines that remain persistently effective (Brichacek 1996; O'Brien 1995).
Description of the intervention
There are a number of non‐specific preventative strategies for children with frequent ARTI episodes: general hygiene methods (Martino 2007); physical interventions (Jefferson 2009); administration of vitamin A and zinc (Kartasurya 2012), vitamin C (Hemilä 2013), or vitamin D supplements (Jolliffe 2013); probiotics (Hao 2015); homeopathic medicines (Steinsbekk 2005a; Steinsbekk 2005b); and immunostimulants (Del‐Rio‐Navarro 2012).
Traditional Chinese Medicine (TCM) follows a theoretical and methodological pathway, that differs from Western medicine, to assess cause, diagnosis and treatment. TCM may give new insight into preventive measures for children with frequent ARTI episodes. A main TCM treatment principle is to strengthen the Zheng Qi (a concept of the body's ability to self‐regulate, pathogen resistance and self‐recovery) and eliminate Evil Qi (pathogens). This emphasises the importance of immune system functioning of patients and assumes that strengthening patients' immune systems might prevent and control infections. Certain herbs have been found to affect the distribution and expression of cytokines and their receptors in the immune system (Patwardhan 2005). Astragalus (Huang qi), a herb widely used to strengthen Qi, is widely available in supermarkets in China. For many centuries, Astragalus, alone or in combination with other herbs, has been used by TCM practitioners in the form of a water extract to prevent respiratory infections and to correct a condition called 'Qi deficiency', which typically includes symptoms such as: feelings of weakness, fatigue, apathy, poor appetite and vulnerability to respiratory infections (WHO 2007). In daily clinical TCM practice, there are different routes of administration, such as injection, self‐made oral water extraction, oral liquid and oral granules. The dosage or equivalent of raw Astragalus varies from 10 g/day to 20 g/day and is adjusted according to age. In this review, we focused on all kinds of oral Astragalus preparations, however, we did not include parenteral preparations due to their limited use in children for ARTIs.
How the intervention might work
A study has suggested that Astragalus confers some immune‐stimulating effects, including promoting white blood cell production, accelerating peripheral blood mononuclear cells and cytokine proliferation (Wang 2002). Hou 1981 found that healthy people who received oral Astragalus (8 g per day) for two months experienced significant improvement in the interferon‐inducing ability of blood cells compared with control group participants. Two months after therapy ceased, the interferon‐inducing ability remained significantly higher in the Astragalus group. In another study, Astragalus extract was prescribed to healthy adults for 20 consecutive days and increases were observed in immune parameters such as immunoglobulin (Ig) M, IgE and cyclic adenosine monophosphate (IBMSCAMS 1979). Nie 2009b demonstrated that Astragalus could decrease soluble interleukin‐2 receptor (sIL‐2R) and interleukin‐8 levels, while increasing IgA, IgM and IgG levels in patients experiencing recurrent upper respiratory tract infections (URTIs). Based on these findings, Astragalus may have a biological basis for use in preventing ARTIs in children.
Why it is important to do this review
ARTIs in children impose significant social and financial burdens, particularly when parental time off work is considered, in addition to healthcare costs (Fendrick 2003; Hollinghurst 2008). A third of children in the USA and UK visit primary care physicians for ARTIs or related symptoms each year (Fendrick 2003; Hay 2005). Up to 75% of all antibiotics prescribed for children are for ARTIs, despite their mainly viral aetiology (Doan 2012). Overuse of antibiotics for children in primary care can induce bacterial resistance (Costelloe 2010), which may result in medicalisation of illness whereby patients are more likely to consult when similar symptoms recur – creating a cycle that exacerbates inappropriate use of antibiotics (Andrews 2012). Despite being mostly self‐limiting illnesses, ARTIs remain a common cause of hospitalisation for children in high‐income countries, and a major cause of death in low‐income countries (Culebras 2013). Consequently, there are many social, financial and health reasons for preventing ARTI. Complementary and alternative medicine interventions may provide novel and efficient solutions.
Until the early nineteenth century in China, only TCM was practiced, and consequently it played an important role in healthcare. The popularity of TCM practice is increasing outside of China. According to recent national survey data, 60% to 75% of populations in Singapore, Japan, Taiwan and Korea consult TCM practitioners at least once per year (Cheung 2011). The USA is now the second largest consumer of TCM products (after China) where expenditure in 2010 was USD 7.6 billion (Cheung 2011). This indicates that interest in the potential of herbal treatments is widespread. The potential impact on public health is unknown, but could be substantial, given that herbal products can be purchased at pharmacies, supermarkets and health food stores.
Astragalus is a herb, extensively used both in clinical practice and as a food supplement in daily life in China. Although it is widely used to prevent ARTI in children who experience frequent episodes, no definitive conclusions about its effectiveness have been determined. Safety is an important concern, especially in the context of medications for children. Allergic reactions to Astragalus injections have been reported, and the safety of oral Astragalus preparation remains unclear (Fu 2009; Hu 2008; Xiong 2002).
Six Cochrane Reviews relate to herbal medicine and ARTI (Del‐Rio‐Navarro 2012; Huang 2012b; Jiang 2012; Jiang 2013; Liu 2012; Wu 2007); however, none focus on oral Astragalus for ARTI in children. Four of these reviews focused on the therapeutic effect of Astragalus rather than its prophylactic potential, or in reducing ARTI incidence (Huang 2012b; Jiang 2012; Liu 2012; Wu 2007). Del‐Rio‐Navarro 2012 focused on preventing respiratory tract infection in children, but the intervention was not related to Astragalus.
Our aim was to assess the effectiveness and safety of Astragalus for preventing frequent ARTIs in children in community settings, where Astragalus preparations were used as sole oral agents. We planned to assess ARTI frequency during the study period as the primary outcome.
Objectives
To assess the effectiveness and safety of oral Astragalus for preventing frequent episodes of acute respiratory tract infections (ARTIs) in children in community settings.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared oral Astragalus prescription as a sole Chinese herbal preparation, with placebo, to prevent frequent episodes of acute respiratory tract infection (ARTI) in children. We did not include trials in which Astragalus was one of multiple components in Traditional Chinese Medicine (TCM), such as Yupingfeng, BuZhongYiQiTang, etc.
Types of participants
We included children aged up to 14 years (without chronic disease) who experienced frequent ARTIs and were in community settings.
Frequency was defined in accordance with the current Chinese guideline (CPSCMA 2008; Table 1). We excluded trials that included participants who had asthma, asthmatic bronchitis, allergy and atopy, recurrent spastic laryngitis, tuberculosis, idiopathic pulmonary haemosiderosis, bronchiolitis obliterans with organising pneumonia, eosinophilic pneumonia, sequoiosis or idiopathic interstitial pneumonia.
Types of interventions
-
Astragalus preparation as the sole agent, administered orally. We excluded formulas with multiple herbs even if they included Astragalus.
-
Astragalus preparation prescribed alone or as an adjunct for at least four weeks. Adjunctive treatment was only relevant if Astragalus could be isolated as the intervention: concurrent treatment was permitted, such as additional symptomatic treatment, but this needed to follow guidelines, and needed to be the same in both intervention and control groups.
-
Control group participants received placebo. We excluded trials comparing other active interventions.
-
No limit on the duration of treatment or follow‐up.
Types of outcome measures
Primary outcomes
The primary outcome was acute respiratory tract infection (ARTI) frequency in children during the study period.
The diagnostic criteria for ARTI were in accordance with the Chinese Paediatrics Antibiotics Application Guideline of Acute Respiratory Tract Infection (CPSCMA 1999). We accepted a broad definition of ARTI, using different specific diagnoses, such as the common cold, influenza, sinusitis, tonsillitis, laryngitis, pharyngitis, bronchitis and pneumonia. ARTI could be either clinically confirmed or self‐reported by parents in studies.
Secondary outcomes
-
Fever clearance time. This refers to the time from commencing treatment until body temperature returns to normal; self‐reported by parents or reported by clinicians based on formal thermometer readings. We did not include causes of fever other than ARTI in the fever clearance time.
-
Respiratory symptom clearance time. This refers to the time from commencing treatment until respiratory symptoms resolve, such as cough and sputum reported by parents or clinicians. We planned to evaluate clearance time for each respiratory symptom.
-
Immune system indicator changes referred to changes in:
-
serum immunoglobulins such as IgG, IgA, or IgM; or
-
peripheral blood counts; or
-
percentage of immunocompetent cells such as haematopoietic progenitor cell, or antigen three T lymphocytes.
-
-
Adverse events. We planned to report all adverse events during medication administration and frequency of severe adverse events during the study period, including hospitalisations and deaths, and additional medications used in both groups.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 12 2015) (Appendix 1), MEDLINE (Ovid) (1946 to 31 December 2015) (Appendix 2), Embase (Elsevier) (Appendix 3) (1974 to 31 December 2015), AMED (Ovid) (1985 to 31 December 2015) (Appendix 4), Chinese National Knowledge Infrastructure (CNKI) (1979 to 31 December 2015) (Appendix 5) and Chinese Scientific Journals full text database (CQVIP) (1989 to 31 December 2015) (Appendix 6), China Biology Medicine disc (CBM 1976 to 31 December 2015) (Appendix 7) and Wanfang Data Knowledge Service Platform (WanFang) (1998 to 31 December 2015) (Appendix 8).
We applied the strategy described in Appendix 2 to search MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). We adapted the search strategy to search the other databases. We did not impose language, date or publication limits.
Searching other resources
We searched the trials registries: Current Controlled Trials (www.controlled‐trials.com), National Research Register (www.nihr.ac.uk), Chinese Clinical Trial Register (www.chictr.org), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (WHO ICTRP) (www.who.int/ictrp/en), and ClinicalTrials.gov for completed and ongoing trials (www.clinicaltrials.gov). We planned to search the references of included studies. We attempted to identify the grey literature by searching reviews, conference proceedings and academic degree dissertations. Our search identified relevant studies published up to 31 December 2015.
Data collection and analysis
Selection of studies
Two review authors (Zhang L, Liu ZZ) scanned all titles and abstracts found in the searches. We retrieved potentially eligible studies as full‐text articles and the same review authors independently assessed eligibility for inclusion. We resolved disagreements by reaching consensus after rechecking source papers and following discussion with a third review author (Liu XS). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1; Moher 2009), and Characteristics of excluded studies table.
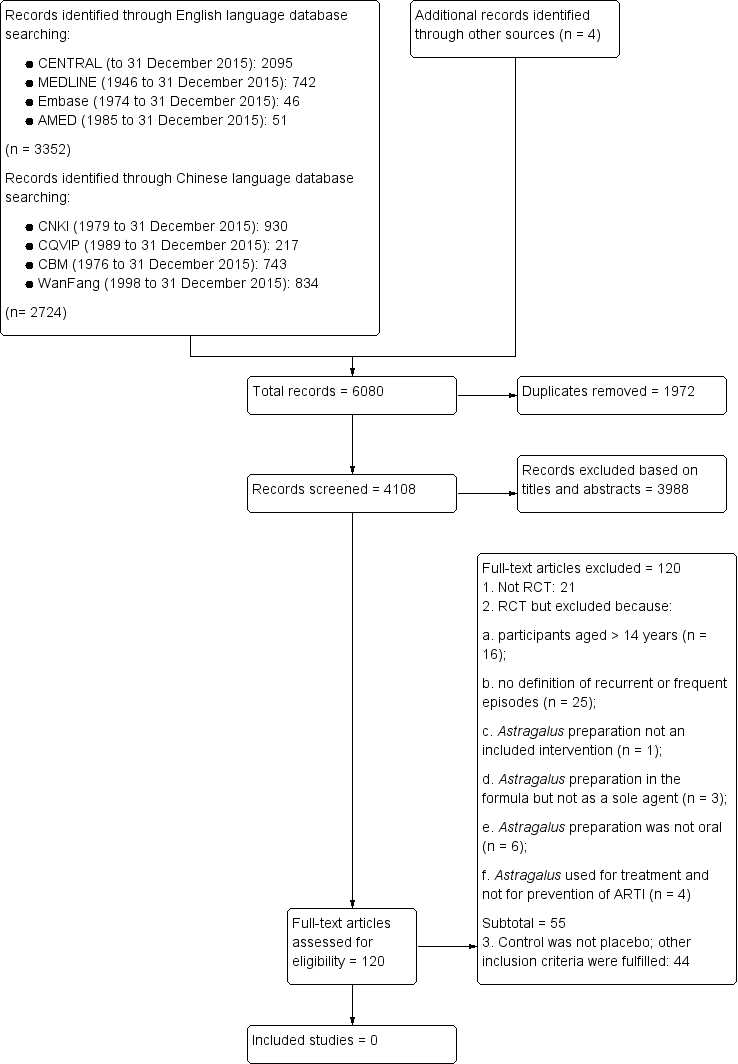
Study flow diagram
Data extraction and management
We designed a data extraction form based on the checklist of items for data collection in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and as proposed by Cochrane Acute Respiratory Infections. We included information on source, eligibility, study methods, participants, intervention details, outcomes and results. All review authors reviewed the form, which was piloted using a sample of studies, and revised to produce the final version. Two review authors independently extracted data from the included trials. When more than one publication of a study existed, we planned to group reports together and to only use the publication with the most complete data in analyses. We planned to contact trial authors for study details and additional information for incomplete results in the available reports, if needed. We resolved disagreements by discussion, and when necessary, by consulting a third review author.
Assessment of risk of bias in included studies
We planned that two review authors would independently assess risk of bias of each included trial using the Cochrane 'Risk of bias' tool (Higgins 2011). Risk of bias tool domains are:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We planned to assign a quality rating for each domain for all included studies as high, low or unclear risk of bias.
Measures of treatment effect
We were to analyse data using Review Manager 5 (RevMan 2014). We planned to use mean difference (MD) or standardised mean difference (SMD) for continuous data, and risk ratio (RR) or odds ratio (OR) to analyse dichotomous data, both with 95% confidence intervals (CIs).
Unit of analysis issues
The individual participant was to be the unit of analysis. If the unit of analysis was not the same as the unit of randomisation, we were to adjust the outcomes for clustering based on the intra‐cluster correlation as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For cross‐over trials, we were only to include data from the first period. For multiple treatment groups, we were to combine all relevant experimental intervention groups of the study into a single test group and combine all relevant control intervention groups into a single control group. We were to subject different units of analysis to a sensitivity analysis.
Dealing with missing data
We planned to obtain additional information as required by contacting study authors and to include information obtained in the review. We were to investigate attrition rates. Analyses were to be based on an intention‐to‐treat approach. We were to impute missing data. For missing dichotomous outcomes, we were to assume that they were ineffective in both treatment and control groups. For missing continuous outcomes, we were to try to obtain participant data by contacting authors and to impute missing data using the last observation carried forward approach (Higgins 2011). If we failed to obtain additional data, we were to perform an available‐case analysis.
Assessment of heterogeneity
We planned to assess heterogeneity among trials in two steps. First, we were to assess face value heterogeneity by comparing trial populations, settings and methods. Secondly, we were to assess statistical heterogeneity using a Chi² test and the I² statistic (Higgins 2003). An I² statistic value of 25%, 50% and 75% refers to low, medium and high levels of heterogeneity, respectively. If heterogeneity were to be present, we would have examined the methodological and clinical characteristics of the included trials to explore the possible causes. We were to then conduct subgroup analyses and summarise our findings. If we had failed to identify the possible causes of heterogeneity, we were not to perform a meta‐analysis. Instead, we were to use a qualitative approach to present the results.
Assessment of reporting biases
We planned to construct funnel plots if more than 10 studies were included to assess risk of publication bias. Because factors other than publication bias (such as study quality) might lead to an asymmetrical funnel plot, we were to perform Egger's test using STATA 11.0 software (StataCorp, USA) (Stata 2009), to detect funnel plot asymmetry.
Data synthesis
We planned to use a random‐effects model to synthesise all data, regardless of heterogeneity among the studies. We planned to test for publication bias by constructing a funnel plot or other corrective analytical methods, depending on numbers of included studies.
GRADE and 'Summary of findings' table
We planned to create a 'Summary of findings' table using the following outcomes: ARTI frequency, fever clearance time, respiratory symptom clearance time, effectiveness rate and adverse events. We were to use the GRADE approach to rate the quality of the body of evidence based on five considerations: study limitations, consistency of effect, imprecision, indirectness and publication bias (Atkins 2004). We planned to use methods and recommendations introduced in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the GRADE Handbook (www.guidelinedevelopment.org/handbook), using GRADEpro GDT software (GRADEpro GDT 2014). We planned to justify all decisions to downgrade or upgrade the quality of studies in footnotes, and to make comments to aid readers' understanding of the review, where necessary. We were to use Review Manager 5 for all calculations (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses based on interventions and treatment duration. Clinical heterogeneity among interventions could be related to the different doses and types of Astragalus preparations, such as decoction of dried Astragalus, powder capsule of dried Astragalus, capsule of Astragalus extracts, etc. If the onset of clinical effect and how long the effect would last were still unclear, we were to perform a subgroup analysis by stratifying by treatment duration and time of follow‐up to investigate the appropriate duration of treatment.
Sensitivity analysis
We planned to perform a sensitivity analysis by stratifying studies at low, high and unclear risk of bias to explore the impact of risk of bias on the pooled estimate, and to investigate potential methodological heterogeneity. We were also to perform a sensitivity analysis with the available data to assess the impact of imputation. Analyses were not possible because no studies were found to be eligible for inclusion.
Results
Description of studies
Results of the search
Searches identified a total of 6080 records: 3352 from English language databases, 2724 from Chinese databases, and four from other sources (Figure 1). After removing 1972 duplicate records, we assessed the remaining 4108, and excluded 3988 records based on titles and abstracts. We retrieved 120 full‐text records and assessed them for inclusion.
Included studies
We did not identify any studies that met our inclusion criteria for this review.
Excluded studies
We excluded 120 studies, of these, 21 studies were not RCTs. We excluded a further 55 records because: participants were aged over 14 years (n = 16); no definition was included for recurrent or frequent episodes (n = 25); Astragalus preparation was not an intervention (n = 1); Astragalus preparation was in the formula, but not a sole agent (n = 3); Astragalus preparation was not oral (n = 6); and Astragalus was used for treatment rather than ARTI prevention (n = 4). We excluded 44 records because the control was not a placebo, although other inclusion criteria were fulfilled.
We identified no ongoing studies.
Risk of bias in included studies
We were unable to conduct a 'Risk of bias' assessment because no studies were identified that met our inclusion criteria.
Effects of interventions
We did not identify any studies that met our inclusion criteria for this review.
Discussion
Frequent acute respiratory tract infection (ARTI) episodes are common during childhood. Caring for children with ARTIs consumes considerable medical, parental and carer resources. In China, Astragalus (Huang qi) has traditionally been used to prevent ARTI. Although Astragalus is widely used and widely available in China, its effectiveness and safety have not been evaluated systematically in the context of ARTI prevention.
We aimed to assess the effectiveness and safety of oralAstragalus for preventing frequent ARTI episodes among children in community settings. However, we did not identify any studies that met our inclusion criteria. We found a gap in the evidence: well‐designed and well‐conducted randomised controlled trials (RCTs) investigating oral Astragalus to prevent frequent ARTI in children are needed to inform assessment of safety and efficacy.
A limitation of this review is that definitions of ARTI and episode frequency were not well defined. We did not find a definition for 'acute', nor whether there were differences in how episodes of upper and lower airway infections were counted, nor length of intervals between episodes. This is problematic because it can result in over‐ or underestimation of numbers of ARTI episodes reported.
Another limitation was that ARTI frequency in children was the only primary outcome; ARTI severity and duration are also very important to evaluate the effect of prevention. Instruments such as the Wisconsin Upper Respiratory Symptom Survey for Kids (WURSS‐K) and the Canadian Acute Respiratory Infection and Flu Scale (CARFIS) have been developed to enable evaluation of severity and duration of RTIs among children (Fischer 2014; Jacobs 2000; Shepperd 2004; www.fammed.wisc.edu/wurss). WURSS‐K is a self‐report quality of life instrument for children, designed to assess the negative impact of acute URTI. WURSS‐K is completed by children and focuses on the impact of quality of life; CARFIS is used by parents and focuses on ARTI severity. Both instruments could be used in future studies looking at the preventive effect of Astragalus for children with frequent episodes of ARTI.
We did not capture the exact ingredients of the intervention in the inclusion criteria. As a natural product, the method of plant extraction and whether the product is standardised will determine the proportion of constituents and dose, thus influencing effectiveness and safety of oral Astragalus preparations. The aspects of standardisation of oral Astragalus preparation should be considered, such as which part of the plant was used, species, age, size, growing conditions, medicinal preparations/extract methods, proportion of constituents, and storage methods of Astragalus (Natural Medicines 2016), all of which have implication for future research.
Summary of main results
We did not identify any RCTs that investigated the effectiveness and safety of oralAstragalus compared with placebo to prevent frequent episodes of ARTIs in children.
Overall completeness and applicability of evidence
We did not identify any RCTs for inclusion.
Quality of the evidence
We did not identify any RCTs for inclusion.
Potential biases in the review process
We found no studies that met our inclusion criteria; our search strategies were comprehensive, and It is unlikely that we missed potentially eligible studies. We included two additional Chinese language databases (CBM and Wanfang) than planned in the protocol, to extend our search and capture all potential studies.
We planned to include only placebo‐controlled studies and ARTI was to be clinically confirmed or self‐reported by patients. Since patient‐reported occurrence of ARTI is subjective, placebo was nominated as the only control that would avoid expectation and measurement bias. We did not find any studies that were placebo‐controlled, and surmised that difficulties in obtaining ethics approval, especially in trials for children, limited this objective. Obtaining informed consent could be challenging because of reluctance by parents and carers to consent to children's participation in the knowledge that they may be allocated to the placebo arm. Of the 44 studies that fulfilled all inclusion criteria, except being placebo‐controlled, most were add‐on design studies (Huang qi + concurrent treatment versus concurrent treatment). Further evaluation of the add‐on effect might be possible by reviewing these studies.
Agreements and disagreements with other studies or reviews
We found no studies or systematic reviews that investigated the effectiveness and safety of oral Astragalus in preventing frequent ARTIs in children. Current evidence is diverse in study design but all support oral Astragalus in preventing ARTIs in children, such as the 44 non‐placebo‐controlled RCTs excluded from this review, and a mini systematic review embedded in a case report of Huang qi (Astragalus) granules in upper respiratory tract infection (URTI) prevention in children with nephrotic syndrome (Zou 2013).

Study flow diagram
| Age (years) | Recurrent URTI (episodes per year) | Recurrent LRTI (episodes per year) | |
| Recurrent tracheitis or bronchitis | Recurrent pneumonia | ||
| Birth to 2 years of age | 7 | 3 | 2 |
| 2 to 5 years of age (> 2 years and < 5 years) | 6 | 2 | 2 |
| 5 to 14 years of age (> 5 years and < 14 years) | 5 | 2 | 2 |
| Abbreviations: LRTI ‐ lower respiratory tract infection; URTI ‐ upper respiratory tract infection | |||

