نقش آسکوربیک اسید برای درمان بیماری شارکو‐ماری‐توث (Charcot‐Marie‐Tooth)
چکیده
پیشینه
بیماری شارکو‐ماری‐توث (Charcot‐Marie‐Tooth; CMT) شامل گروه بزرگی از اشکال مختلف نوروپاتیهای ارثی موتور (حرکتی) و حسی (سنسوری) میشود. اساس مولکولار زیر‐گروههای مختلف CMT در طول 20 سال گذشته شناسایی شدهاند. از آنجا که ضعف عضلانی و اختلال حسی که به تدریج و آهستگی پیشرفت میکند، مشخصه اصلی این سندرمها است، هدف درمان، بهبود اختلال در حرکت و اختلالات حسی برای بهبود تواناییهای بیماران است. کارآزماییهای مربوط به درمان فارماکولوژیک در CMT نادر هستند. این مرور از یک مرور کاکرین، درمان برای بیماری شارکو‐ماری‐توث، گرفته شده که از طریق همین مرور و یک عنوان جدید، درمانهایی به غیر از آسکوربیک اسید (ascorbic acid) برای بیماری شارکو‐ماری‐توث، بهروز خواهد شد.
اهداف
ارزیابی تاثیرات درمانی آسکوربیک اسید (ویتامین C) در CMT.
روشهای جستوجو
در تاریخ 21 سپتامبر 2015، ما پایگاه ثبت تخصصی گروه عصبیعضلانی در کاکرین، پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE؛ EMBASE و LILACS را برای یافتن کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) که به درمان برای CMT پرداخته باشند، جستوجو کردیم. همچنین پایگاههای ثبت کارآزماییهای بالینی را برای یافتن مطالعات در حال انجام بررسی کردیم.
معیارهای انتخاب
RCTها و شبه‐RCTهایی را وارد کردیم که به بررسی هر گونه درمان آسکوربیک اسید برای افراد مبتلا به CMT پرداخته بودند. هر جایی که هدف یک مطالعه ارزیابی درمان نشانههای عمومی عصبیعضلانی در افراد مبتلا به نوروپاتی محیطی، مانند CMT بوده، اگر توانستیم تاثیرات درمان را در گروه CMT شناسایی کنیم، آن مطالعه را وارد این مرور کردیم. مطالعات مشاهدهای یا گزارش موردی را که به درمان با آسکوربیک اسید در افراد مبتلا به CMT پرداخته بودند، وارد این مرور نکردیم.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور (BG و JB) بهطور مستقل از هم، دادهها را استخراج و کیفیت مطالعه را ارزیابی کردند.
نتایج اصلی
شش RCT به مقایسه تاثیرات درمان با اسید آسکوربیک خوراکی (1 تا 4 گرم) و دارونما (placebo) در CMT1A پرداخته بودند. در پنج کارآزمایی که دربرگیرنده بزرگسالان مبتلا به CMT1A بودند، در مجموع 622 شرکتکننده آسکوربیک اسید یا دارونما دریافت کردند. کارآزماییها عمدتا در معرض خطر پائین سوگیری (bias) قرار داشتند. شواهد با کیفیت بالا وجود دارد که نشان میدهد آسکوربیک اسید دوره CMT1A را در بزرگسالان، که به وسیله نمره نوروپاتی CMT (در مقیاس 0 تا 36) در 12 ماه (تفاوت میانگین (MD): 0.37‐؛ 95% فاصله اطمینان (CI): 0.83‐ تا 0.09؛ پنج مطالعه؛ N = 533)، یا در 24 ماه (MD: ‐0.21؛ 95% CI؛ 0.81‐ تا 0.39؛ سه مطالعه؛ N = 388) اندازهگیری شده، بهبود نمیدهد. درمان با آسکوربیک اسید تاثیر مثبتی را در تست nine‐hole peg در برابر دارونما نشان داد (MD: ‐1.16 ثانیه؛ 95% CI؛ 1.96‐ تا 0.37‐)، اما تفاوت بالینی این نتایج احتمالا اندک و کوچک است. متاآنالیزهای دیگر از پارامترهای پیامد ثانویه هیچ مزیتی مرتبط با آسکوربیک اسید نشان نداد. در یک کارآزمایی، 80 کودک مبتلا به CMT1A تحت درمان با آسکوربیک اسید یا دارونما قرار گرفتند. این کارآزمایی، هیچ مزیت بالینی از درمان با اسید اسکوربیک نشان نداد. عوارض جانبی در طبیعت یا فراوانی میان دو گروه درمانی آسکوربیک اسید یا دارونما تفاوتی نداشت.
نتیجهگیریهای نویسندگان
شواهد با کیفیت بالا نشان دادند که آسکوربیک اسید نمیتواند دوره CMT1A را در بزرگسالان، از نظر پارامترهای پیامد استفاده شده بهبود دهد. بر اساس شواهد با کیفیت پائین، آسکوربیک اسید دوره CMT1A را در کودکان بهبود نمیبخشد. به هر حال، CMT1A به آهستگی پیشرفت کرده و پارامترهای پیامدها فقط تغییر کوچکی را در طول زمان نشان میدهند. مطالعه با دوره زمانی طولانیتر باید مد نظر قرار گیرد و پارامترهای پیامدهایی با حساسیت بیشتر که در طول زمان تغییر کنند، باید طراحی شوند و برای مطالعات آینده اعتبارسنجی شوند.
PICO
خلاصه به زبان ساده
ویتامین C برای بیماری شارکو‐ماری‐توث (Charcot‐Marie‐Tooth; CMT) (نوروپاتی ارثی حرکتی و حسی)
سوال مطالعه مروری
مزایا یا مضرات ویتامین C (آسکوربیک اسید (ascorbic acid)) در درمان بیماری شارکو‐ماری‐توث (Charcot‐Marie‐Tooth; CMT) چه هستند؟
پیشینه
بیماری CMT طیف گستردهای از نوروپاتیهای محیطی ارثی (شرایطی که در آن، نورونهای خارج از مغز و طناب نخاعی صدمه میبینند) را نشان میدهد، که پیشرفت آن عموما آهسته بوده و باعث ضعف عضلانی و از دست رفتن حس میشود. ضعف عضلانی و از دست رفتن حس در اثر تخریب فیبرهای عصبی صورت میگیرد که به عضلات یا پوست میروند. اینطور فرض میشود که ویتامین C به عنوان درمانی برای CMT موثر است، زیرا این ویتامین برای میلیناسیون (myelination) (تکامل میلین یا عایق در اطراف الیاف عصبی) سلولهای عصبی در محیطهای کشت آزمایشگاهی و اعصاب محیطی موش ضروری بوده است.
ویژگیهای مطالعه
منابع علمی پزشکی را برای یافتن کارآزماییهایی جستوجو کردیم که در آنها ویتامین C در بیماری CMT به کار گرفته شده بود و شش کارآزمایی ـ پنج مورد در بزرگسالان و یک مورد در کودکان ـ را در زمینه درمان CMT تیپ 1A (یا CMT1A) با ویتامین C یافتیم. همه این مطالعات دوزهای 1 تا 4 گرمی از ویتامین C در روز را با دارونما (placebo) (یک قرص ساختگی یا قند که به عنوان ویتامین C به بیماران داده میشد) مقایسه کرده بودند، این مطالعات 12 یا 24 ماه به طول انجامیده بودند. کارآزماییهای بزرگسالان در مجموع، 622 فرد را در بر گرفته بودند. دیگر کارآزمایی شامل 80 کودک میشد. معیار اصلی تاثیرات ویتامین C در این مرور، تغییر در اختلالهای ایجاد شده بود. همچنین اطلاعاتی را در مورد ناتوانی، مطالعات هدایت عصبی، حس، قدرت عضلانی، کیفیت زندگی و تاثیرات مضر ویتامین C گردآوری کردیم.
نتایج کلیدی و کیفیت شواهد
ما دریافتیم که درمان آسکوربیک اسید اختلال ناشی از CMT1A را در بزرگسالان، که به وسیله نمره نوروپاتی CMT (یا CMTNS) اندازهگیری شده بود، ارتقا نمیبخشد. در کودکان، CMTNS گزارش نشده بود، چرا که معیاری است تکاملی برای بزرگسالان مبتلا به CMT. معیارهای مورد استفاده در این مطالعه برای کودکان مزیتی از ویتامین C را نشان نداد. مطالعات تا حد زیادی در معرض خطر پائین سوگیری (bias) قرار داشتند، به این معنی که آنها به خوبی طراحی شده بودند و نتایج به راحتی تحت تاثیر شانس قرار نگرفت. حوادث جانبی از نظر ماهیت و تعداد در دو گروه دارونما و ویتامین C مشابه هم بودند.
شواهد با کیفیت بالا برای بزرگسالان و شواهد با کیفیت پائین برای کودکان وجود دارد که نشان میدهد ویتامین C دوره CMT1A را بهبود نمیبخشد. با این حال، CMT به آهستگی پیشرفت میکند، و شاید دورههای 12 یا 24 ماهه مطالعه به اندازه کافی طولانی نباشند که تاثیرات درمان را نشان دهند. پژوهش بیشتر با دورههای مطالعاتی طولانیتر و پارامترهای پیامدهای حساستر باید انجام شوند، هر چند هر تاثیر بزرگ در بزرگسالان یا کودکان بعید به نظر میرسد.
Authors' conclusions
Summary of findings
| Ascorbic acid treatment compared with placebo for CMT1A in adults | ||||||
| Participants or population: adults with CMT1A Intervention: oral ascorbic acid (1 g to 4 g/day) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ascorbic acid | |||||
| Impairment ‐ change in CMTNS at 24 months (0 to 36 points) | The mean change in CMTNS ranged across control groups from ‐0.92 to 1 point | The mean change in CMTNS in the intervention groups was 0.21 lower (0.81 lower to 0.39 higher) | ‐0.21 (‐0.81 to 0.39) | 388 (3) | ⊕⊕⊕⊕ | |
| Impairment ‐ change in CMTES at 24 months (0 to 28 points) | The mean change in CMTES ranged across control groups from ‐0.64 to 0.5 point | The mean change in CMTES in the intervention groups was 0.12 lower (0.67 lower to 0.42 higher) | ‐0.12 (‐0.67 to 0.42) | 337 (2) | ⊕⊕⊕⊝ | |
| Change in timed 10‐m walk at 12 months (seconds (s)) | The mean change in timed 10‐m walk ranged across control groups from ‐0.15 to 0.56 s | The mean change in timed 10‐m walk in the intervention groups was 0.02 lower (0.32 lower to 0.29 lower) | ‐0.02 (‐0.32 to 0.29) | 401 (3) | ⊕⊕⊕⊕ | |
| Change in foot dorsiflexion at 12 months (N) | The mean change in foot dorsiflexion force ranged across control groups from | The mean change in foot dorsiflexion force in the intervention groups was 1.1 higher (3.47 lower to 5.67 higher) | 1.1 (‐3.47 to 5.67) | 423 (4) | ⊕⊕⊕⊕ | |
| Change in 9‐hole peg test (HPT) at 12 months (seconds) | The mean change in 9‐HPT ranged across control groups from | The mean change in 9‐HPT in the intervention groups was 0.39 lower (0.76 lower to 0.02 lower) | ‐0.39 (‐0.76 to ‐0.02) | 286 | ⊕⊕⊕⊝ | |
| Serious adverse events (SAE; %) | The relative abundance of SAE was 12% in the placebo group ((number of SAE/number of participants)*100) | The relative abundance of SAE was 11.7% in the intervention groups ((number of SAE/number of participants)*100) | Not estimable | 702 (6, including 1 study in children) | ⊕⊕⊕⊕ | |
| *The assumed risk is based on the mean change or the range of mean change) across control groups in included studies.. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence downgraded because of number of studies and participants, which was lower than for other outcomes. Further research may thus have an impact on our confidence in the estimate of effect and may change the estimate. | ||||||
Background
Description of the condition
Charcot‐Marie‐Tooth disease (CMT) comprises a large group of inherited neuropathies, which primarily affect either the myelin sheath or the axon of the peripheral nerve (Shy 2005b). Primary loss of the myelin sheath, called demyelination, leads to secondary axonal degeneration, resulting in muscular atrophy and paresis (Krajewski 2000; Martini 2001). The main clinical motor signs are atrophy and weakness, starting in the feet and calves and later affecting the hands (Shy 2005b). The prevalence of CMT ranges from 10 to 80/100,000, depending on the population studied (Braathen 2012; Kurihara 2002). Sensory deficits and positive sensory symptoms are less prominent, but may cause discomfort and add to the disability. Skeletal deformities like scoliosis or foot deformities can be additional features (Shy 2005b). Depending on the underlying molecular genetic defect, the clinical severity varies from mild weakness to severe paralysis and disability (Baets 2014), but in general they progress very slowly over decades rather than years. Histopathological findings in peripheral nerve biopsies reveal demyelination or axonal degeneration, depending on the specific type of CMT (Gabreels‐Festen 1993). Demyelination results in slowed nerve conduction velocities (NCVs). Types of CMT with primary axonal loss and preserved myelin are characterised by normal or nearly normal NCVs, but variably reduced compound muscle action potentials (CMAPs).
Genetics
A broad spectrum of gene mutations have been identified as causes of CMT (Baets 2014). We will give a short introduction to the most frequent forms of CMT: autosomal‐dominant demyelinating CMT (CMT1), X‐chromosome linked CMT (CMTX), autosomal‐dominant axonal CMT (CMT2), and autosomal‐recessive CMT (CMT4).
CMT1 is primarily a demyelinating condition, and is the most common form of CMT (Nelis 1996). The most frequent genetic defect in CMT1 is an intrachromosomal duplication on chromosome 17p11.2 (Lupski 1991; Raeymakers 1991). The gene encoding peripheral myelin protein 22 (PMP22) is located within the duplicated region (Timmerman 1992). This gene has been shown to be the culprit for the genetic subgroup CMT1A (duplication) and CMT1E (point mutations). The presence of mutations in the gene encoding myelin protein zero (MPZ) defines CMT1B (Hayasaka 1993a; Hayasaka 1993b; Hayasaka 1993c).
The cause of CMTX1 is mutations in the gene encoding gap junction protein beta1 (GJB1), which is localised on the X‐chromosome. Histopathological signs of axonal degeneration and demyelination characterise CMTX1 (Hahn 2001; Nicholson 1993). These cause the typical electrophysiological features of mildly reduced NCV and reduction of CMAPs. In addition to CMTX1, other, rarer forms of X‐chromosomal CMT have been recently discovered (Bird 2014).
In addition to CMTX1, other intermediate CMT subtypes exist, which are inherited in an autosomal dominant or recessive fashion. These are termed dominant‐intermediate or recessive‐intermediate CMT (DI‐CMT, RI‐CMT). Several genes have been implicated in DI‐ and RI‐CMT (Liu 2014).
The purely axonal form of CMT is called CMT2. Mutations in the gene encoding mitochondrial fusion protein mitofusin 2 (MFN2) were shown to be a cause of CMT2A (Verhoeven 2006; Zuchner 2004). Many other genes for CMT2 have been described; these often contribute only a small proportion of the genetic spectrum.
The autosomal‐recessive form of CMT is CMT4. The autosomal‐recessive forms of CMT are generally much rarer than the autosomal‐dominant forms. Autosomal‐recessive forms account for only 10% of people with CMT in outbred populations, rising to 40% in populations with a higher degree of consanguinity. Among the many genes implicated in CMT4, some of the more common are SH3TC2, GDAP1 and HINT1 (Baxter 2002; Saporta 2011; Senderek 2003; Zimon 2012).
Hereditary neuropathy with liability to pressure palsies (HNPP) represents the most common form of inherited neuropathy with recurrent focal nerve palsies, although some people also display signs of a generalised demyelinating neuropathy with a more chronic disease course. The underlying genetic defect is the reciprocal state of CMT1A, namely the loss of one allele of PMP22 due to the loss of a chromosomal fragment on chromosome 17p11.2 (Chance 1993), or less commonly, PMP22 missense mutations.
Furthermore, hereditary neuropathies exist with either predominant motor involvement, termed distal hereditary motor neuropathies (dHMN), or predominant sensory involvement, termed hereditary sensory and autonomic neuropathies (HSAN). In recent years, there has been major progress in the elucidation of the genetics of these neuropathies (Auer‐Grumbach 2013; Rossor 2012).
Many rare dominant and recessive forms of CMT have now been classified (see the Inherited Peripheral Neuropathies Mutation Database www.molgen.ua.ac.be/CMTMutations or recent review articles (Baets 2014; Rossor 2013).
Pathophysiology
The pathophysiological mechanisms that lead to the clinical phenotype are still unclear for many of the genes implicated in CMT. In general, mutations in CMT‐associated genes lead to disturbed functions of either Schwann cells or axons. Disturbed functions of Schwann cells cause secondary axonal degeneration in the course of the disease. The molecular mechanisms leading to axonal degeneration in CMT are still elusive. Evidence is accumulating for not only a convergence of several forms of CMT, but also hereditary spastic paraplegia (HSP), and hereditary sensory and autonomic neuropathies (HSAN), owing to common mechanisms such as disorganisation of the axonal cytoskeleton, axonal transport, and mitochondrial dysfunctions (d'Ydewalle 2012; Pareyson 2014).
Clinical features
Although molecular genetic methods for the identification of new genes in CMT have rapidly expanded, CMT1A remains the most common subtype (Gess 2013; Murphy 2012; Saporta 2011). The most prominent symptom in all forms of CMT1 is progressive distal wasting of peroneal and calf muscles resulting in distal lower limb weakness, beginning in the first or second decade of life (Vallat 2013). Many people with CMT have foot deformities, such as high‐arched feet and hammer toes. Distal sensory loss is typically found but positive sensory symptoms, such as neuropathic pain, are more rare. Autonomic nervous system involvement is rare and not prominent. The need for a wheelchair is unusual.
The pathobiological hallmarks of CMT1 are demyelination and axonal degeneration with consequent length‐dependent muscle atrophy (Krajewski 1999; Krajewski 2000). In nerve conduction studies, the reduction of motor and sensory NCV can identify demyelination. However, the degree of reduction in NCV does not reflect the clinical severity of CMT1 (Krajewski 1999; Krajewski 2000). The diagnosis of CMT1 requires a reduced motor NCV of less than 38 m/s in the upper limbs (Shy 2005b). Axonal CMT2 cannot be differentiated by clinical means from CMT1. Electrophysiologically, the motor NCV is normal or only slightly reduced (greater than 38 m/s) in CMT2, but the CMAP amplitudes are significantly decreased (Harding 1980; Shy 2005b). Intermediate slowing of NCV (25 to 45 m/s), with mildly reduced CMAPs, characterise intermediate CMT (Harding 1980).
Description of the intervention
Established strategies for the treatment of CMT and its symptoms are lacking (Young 2008). Some trials that aimed to improve muscle strength or sensory deficits have been undertaken. Trials have been performed with corticosteroids and antioxidants, and with physiotherapy for improving muscle function (Grandis 2005; Young 2001).
Ascorbic acid (vitamin C) has been shown to be necessary for correct myelination of peripheral nerves in vitro and in vivo (Eldridge 1987; Gess 2010; Gess 2011). Ascorbic acid improved muscle function and reduced demyelination in a mouse model of CMT1A (Passage 2004). Based on these experimental data, several randomised controlled trials (RCTs) were undertaken on the treatment of CMT1A with ascorbic acid.
The recommended daily allowance of vitamin C is between 75 and 120 mg (depending upon sex and physiological status). Usual daily doses of ascorbic acid purchased over the counter are 500 mg/day.
Why it is important to do this review
Six RCTs on the treatment of CMT1A with ascorbic acid have been performed. It is important to analyse these trials, compare their data and summarise the results in order to reach a conclusion on the treatment of CMT1A with ascorbic acid.
This review and a forthcoming Cochrane review, Treatments other than ascorbic acid for Charcot‐Marie‐Tooth disease, which is currently in preparation, will update the previous Cochrane review, 'Treatment for Charcot‐Marie‐Tooth disease' (Young 2008).
Objectives
To assess the effects of ascorbic acid (vitamin C) treatment for CMT.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs of ascorbic acid for the treatment of people with CMT. Where a study aimed to evaluate the treatment of general neuromuscular symptoms of people with peripheral neuropathy including CMT, we included the study if we were able to identify the effect of treatment in the CMT group. We did not include observational studies and case reports on the treatment of people with CMT.
Types of participants
Because of the heterogeneity of CMT, we included people with all types of CMT regardless of which clinical, electrophysiological, histopathological or molecular genetic criteria were used. Due to the fast progress in the elucidation of the genetic basis of CMT, we considered rare forms of CMT. Since all trials on ascorbic acid treatment for CMT were conducted in people with CMT1A, the focus of this review is effectively on CMT1A only. We did not include experimental data from animal models.
Types of interventions
We included all ascorbic acid (vitamin C) treatments, regardless of daily dosage, form of application or dosage regimen. Treatment had to have extended for at least 12 successive months. We chose 12 months of treatment as a minimum because the very slow progressive course of CMT makes it unlikely that a shorter period of treatment would be adequate to test the efficacy of the intervention.
Types of outcome measures
We rescaled changes and numbers of events occurring over time to 'change or numbers of events in a fixed period', for example, three months, to permit the pooling of studies with differing follow‐up periods.
Primary outcomes
Change in impairment after at least 12 months.
We accepted any measure of impairment made with a validated scale, such as the MRC sum score, Neuropathy Impairment Score (NIS) (Dyck 2005b), Total Neuropathy Score (Cornblath 1999), CMT Neuropathy score (Shy 2005a), or CMT neuropathy score version 2 (Murphy 2011).
Secondary outcomes
Secondary outcome measures included:
-
Change in disability after at least 12 months. We accepted any measure of disability made with a validated scale such as the Rankin Scale (van Swieten 1988) or the Inflammatory Neuropathy Cause and Treatment Overall Disability Status Scale (Merkies 2000).
-
Change of maximum compound muscle action potential (CMAP) amplitudes of the ulnar, median or peroneal nerve, after at least 12 months.
-
Change in sensory impairment measured with a validated scale, after at least 12 months.
-
Change in sensory nerve action potential (SNAP) amplitude of the sural or other sensory nerves, after at least 12 months.
-
Change in muscle force as measured with the aid of a dynamometer or vigorimeter, after at least 12 months.
-
Change in quality of life assessment using validated scores, such as SF‐36 Health Survey questionnaire (Jenkinson 1993) or EuroQol (Anderson 1996), after at least 12 months.
-
Adverse events, whether minor or severe, caused by any form of treatment. We distinguished between minor adverse events and severe adverse events (those which were fatal, life‐threatening, required or prolonged hospitalisation or caused discontinuation of the treatment).
Search methods for identification of studies
Electronic searches
On 21 September 2015, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8 in theCochrane Library), MEDLINE (January 1966 to September 2015), EMBASE (January 1980 to September 2015) and LILACS (January 1982 to September 2015) for randomised controlled trials. The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register Appendix 1, CENTRAL Appendix 2, MEDLINE (Appendix 3), EMBASE (Appendix 4), LILACS (Appendix 5). In August 2015, we searched the database ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO international Clinical Trials Registry (www.who.int/ictrp/en/).
Searching other resources
We contacted authors from clinical trials and experts from the field to identify unpublished trials. We also checked the bibliographies of identified trials.
Data collection and analysis
Selection of studies
Three review authors (BG, PY and DP) checked the titles and abstracts of the articles retrieved by the search. The review authors worked independently. The review authors obtained full‐text reports of potentially eligible studies and independently selected studies for inclusion. In case of disagreement, the three authors were to make a consensual decision. We did not apply any language limitations.
Data extraction and management
Two review authors (BG and JB) independently extracted data onto a specially designed data extraction form and checked the extracted data for disagreements. In case of disagreement, the two review authors came to a consensus. BG performed the data entry into the Cochrane software Review Manager (RevMan) 5.3 (Review Manager 2014) and JB checked the data. We contacted trial authors to obtain missing data or raw data if necessary.
Assessment of risk of bias in included studies
Two review authors (BG and JB) graded the risk of bias using the Cochrane approach: high, low or unclear risk of bias (Higgins 2011). In case of disagreement, the two review authors discussed this further and came to a unanimous decision. The 'Risk of bias' assessment took into account security of randomisation, allocation concealment, participant blinding, observer blinding, selective reporting, incomplete outcome reporting (completeness of follow‐up), and the extent to which the study took into account any imbalance in important prognostic variables present at time of randomisation.
Measures of treatment effect
We summarised continuous data with mean differences (MDs) and 95% confidence intervals (CIs). If we had found outcomes measured with different scales, we would have used a standardised mean difference (SMD) with 95% CI.
None of our outcomes were dichotomised; however, we would have reported these using a risk ratio (RR) and 95% CI.
We reported all the data in terms of change over time.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial unexplained heterogeneity, we would have reported it and explored possible causes by pre‐specified subgroup analysis (Higgins 2011).
Data synthesis
When pooling data was possible and appropriate, we used RevMan to perform a fixed‐effect model meta‐analysis.
We reported the results for adults and children separately.
We assessed treatment effects by meta‐analysis of data using forest plots. We determined the overall treatment effect by the Z‐score. We considered a significance level of P < 0.05 as the threshold for statistically significant treatment effects.
In the Discussion, we considered adverse events and cost‐effectiveness, taking into account information from the non‐randomised literature.
'Summary of findings' tables
We generated 'Summary of findings' tables using the GRADE approach. Our selected outcomes were: Impairment ‐ change in the CMTNS at 24 months; Impairment ‐ change in CMTES at 24 months, change in timed 10‐metre walk at 12 months, change in foot dorsiflexion at 12 months, change in 9‐hole peg test at 12 months, and serious adverse events. The GRADE Working Group established the following grades of evidence:
-
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low quality: We are very uncertain about the estimate.
We used the five GRADE considerations (namely, study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence (all studies that contribute data for the pre‐specified outcomes). We followed methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and used GRADEpro software (GRADEpro 2015).
Subgroup analysis and investigation of heterogeneity
We had planned to compare CMT1, CMT2, and where possible, other CMT subgroups separately. However, all the trials of ascorbic acid treatment of CMT only included people with CMT1A (the most common form of CMT).
We assessed heterogeneity using forest plots. We determined the overall level of heterogeneity using the Chi² statistic. We set a significance level of P < 0.05 to be the threshold for significant heterogeneity. If we had found heterogeneity, we would have explored and described possible reasons for differences between studies such as types of participants, intervention or quality.
Sensitivity analysis
We performed sensitivity analyses by omitting trials which were at high risk of bias in one or more domains. If necessary, we would have used the random‐effects model to obtain the pooled estimate.
Results
Description of studies
Results of the search
The number of papers found by the searches outlined in the appendices were: Cochrane Neuromuscular Specialised Register 23, CENTRAL 21, MEDLINE 29, EMBASE 31, LILACS 0, DARE 0, HTA 0 and NHSEED 0. We identified no ongoing trials of ascorbic acid treatment for CMT in trial registers. One study was found by personal communication. Of the 105 studies found in total, 94 remained after removal of duplicates. After review of abstracts and titles, we selected seven of the 94 for full‐text assessment. One of these was excluded with reasons. Six studies fitted the inclusion criteria and were included in the quantitative analysis (see flow chart in Figure 1).
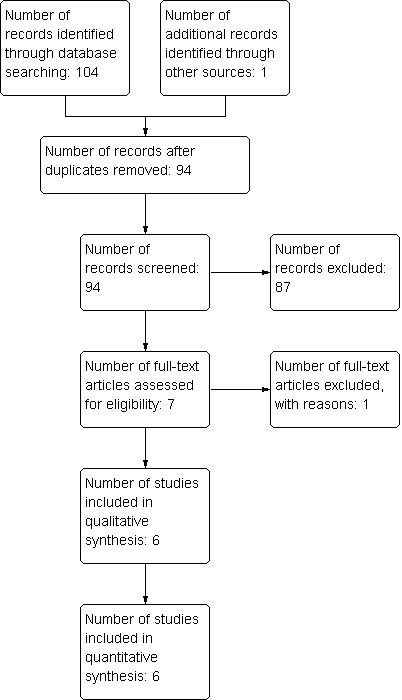
Study flow diagram.
Included studies
We identified seven controlled clinical trials on the treatment of CMT with ascorbic acid (see Characteristics of included studies). We excluded one study because it was not randomised (Toth 2009; Characteristics of excluded studies). The other studies fulfilled all inclusion parameters and we were thus able to include them in this review. One of these studies involved 80 children (Burns 2009), the other five included a total of 622 adults (Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a). Because the natural course of the disease differs between children and adults in terms of progression rates and outcome measures, we reported the study that was performed in children separately. None of the randomised studies defined a minimum level of impairment or disability at baseline. The study by Lewis et al used a futility design. Further, this study compared treated patients to historical controls, which were not randomised. Historical, natural history controls differ from placebo‐treated controls in their rate of progression. However, a placebo‐treated group was included too, so we included this study in this review (Lewis 2013).
All studies described interventions and the related procedures in detail, including the dosages, regimens and appearance of medication. The studies used different dosages (1 g to 4 g) and durations (12 to 24 months) of ascorbic acid treatment. All studies compared ascorbic acid to placebo treatment. Two studies continued treatment for 12 months (Micallef 2009; Verhamme 2009a), and three studies for 24 months (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011). To conduct a meta‐analysis of as many studies as possible, we took 12‐month results from the 24‐month trials, for a comparison of treatment at 12 months. In addition, we compared data at 24 months in a meta‐analysis of the three 24‐month trials. Five of the six included studies were published reports. One of the studies was not yet published, but we obtained data by personal communication with the principal investigator (Mazanec 2013 [pers comm]).
All the included studies provided definitions and descriptions of outcome criteria. All five trials in adults used the CMTNS as a composite disease‐specific score. Four studies used foot dorsiflexion force and SF‐36 physical functioning score. Three studies measured the CMTES (clinical component of the CMTNS (Shy 2005a)), nine‐hole peg test, 10‐metre walk test, hand grip force, three‐point pinch force, SF‐36 bodily pain and ascorbic acid concentration. Two studies used the ODSS, ONLS, SF‐36 energy, motor nerve conduction velocity (mean from ulnar and median nerves), and ulnar compound muscle action potential. Several other outcome parameters were used by only one study each.
Risk of bias in included studies
Figure 2 presents a summary of the 'Risk of bias' assessments of the included studies by the review authors. We considered only one of the selected studies to be at low risk of bias in all domains using the Cochrane approach (Pareyson 2011), but all studies were in general at low risk of bias.
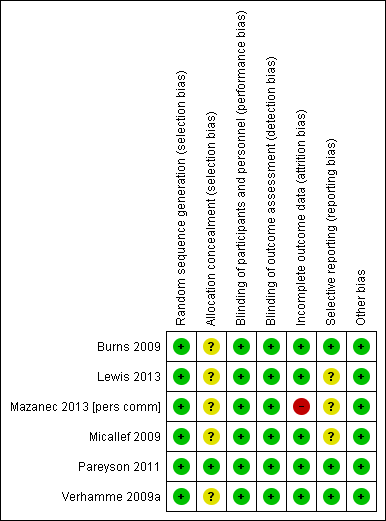
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.
Six of the studies described randomisation properly. Only one study supplied adequate information about allocation concealment.
Five of the studies explicitly considered differences in baseline characteristics. None of the study reports gave the age of onset of CMT. Two studies gave the duration of the disease. Five of the studies measured and stated disease severity. Four studies provided baseline CMTNS scores. In the trial involving children, for whom the CMTNS is not validated, trialists assessed other baseline parameters of severity, such as pain, pes cavus and regular trips and falls.
Five studies were at low risk of bias in terms of follow‐up. Five studies explicitly recorded the number and reasons for drop‐outs from the different experimental groups and used intention‐to‐treat analyses, while in one study, the number of drop‐outs was not given and data of dropped‐out patients were not included in the analysis (Mazanec 2013 [pers comm]). Three studies were at low risk for reporting bias (selective reporting of outcomes). Three studies were at unclear risk for reporting bias, as they showed minor differences between study protocol and reported outcomes.
All six included studies reported blinding of both the observer and the participant (Burns 2009; Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a).
Effects of interventions
See: Summary of findings for the main comparison
We performed meta‐analyses for outcome parameters used by two or more studies. We considered the trial of ascorbic acid treatment in children separately because of the different study population. We reported data from published results for four of the trials (Lewis 2013; Micallef 2009; Pareyson 2011; Verhamme 2009a) and from personal communication for one trial (Mazanec 2013 [pers comm]). For one trial, we obtained additional data by personal communication in order to make a comparison possible (Lewis 2013).
We have used 'no significant difference' when differences were neither clinically nor statistically significant.
Comparison: Oral ascorbic acid treatment versus placebo for adults with CMT1A (12 months)
Primary outcome ‐ impairment at 12 months on a validated scale
High‐quality evidence found no evidence of clinically significant differences between ascorbic acid and placebo at 12 months, on any of the validated measures for the primary outcome. Using the CMTNS (scale 0 to 36 points), the mean difference (MD) was ‐0.37; 95% CI ‐0.83 to 0.09; five studies; N = 533 (see Analysis 1.1; Figure 3). The CMTES (scale 0 to 28 points), which represents the CMTNS without the electrophysiological parameters, showed a significant benefit for ascorbic acid in one trial (Micallef 2009), but the meta‐analysis of all three trials that reported this outcome parameter found no significant difference (MD ‐0.15; 95% CI ‐0.57 to 0.27; three trials; N = 460; Analysis 1.2). For both outcome parameters, Pareyson 2011 dominated the meta‐analysis as it is the largest of the trials. The one study using the NIS reported no significant difference (MD 1.91; 95% CI ‐2.24 to 6.06; N = 110; Lewis 2013; Analysis 1.3). No study reported the MRC score, TNS, or CMTNS v2.

Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months, outcome: 1.1 CMTNS.
Secondary outcomes at 12 months
Change in disability on a validated scale
Treatment with ascorbic acid was not associated with any significant difference compared to placebo in the disability outcomes measured, including ODSS and ONLS, which were each measured in two trials (totals in analyses N = 134 and N = 289, respectively; Analysis 1.4; Analysis 1.5). These comparisons were dominated by single studies because of unequal study sizes. A single study (N = 11) reported the ALDS, with no significant change versus placebo after ascorbic acid (Verhamme 2009a; Analysis 1.6).
Change in CMAP amplitudes
No significant differences were found between ascorbic acid and placebo in the electrophysiological parameters of CMAP amplitude in the ulnar nerve (two studies, N = 161) or median nerve (one study, N = 11), or in a summated amplitude measure (one study, N = 217; Analysis 1.7 to Analysis 1.9).
Change in sensory impairment measured with a validated scale
When measured semi‐quantitatively using the INCAT Sensory Sum (ISS) score in one study (N = 10), sensory function demonstrated no significant change with ascorbic acid versus placebo (Verhamme 2009a; Analysis 1.10).
Change in SNAP amplitude
One study (N = 123) reported data on SNAP amplitudes (Micallef 2009). SNAP amplitudes changed only mildly over the study period of 12 months. Micallef et al. reported a statistically significant effect favouring ascorbic acid when comparing all treatment groups (placebo, 1 g and 3 g ascorbic acid) by ANCOVA. However, when comparing placebo to the higher dosage of 3 g, there was no significant difference (Analysis 1.11).
Change in grip force and other measures of force
Objective measures of force including hand grip force (N = 412; Mazanec 2013; Micallef 2009; Pareyson 2011), three‐point pinch (N = 300; Mazanec 2013; Pareyson 2011; Verhamme 2009a), and foot dorsiflexion force (N = 423; Mazanec 2013; Micallef 2009; Pareyson 2011; Verhamme 2009a) were not significantly changed after treatment with ascorbic acid versus placebo (Analysis 1.12; Analysis 1.13; Analysis 1.14).
Change in quality of life
No significant changes were recorded in any of the SF‐36 measures (Analysis 1.15 to Analysis 1.20) when compared to placebo.
Other non pre‐specified secondary outcomes at 12 months
Change in the nine‐hole peg test
Three studies, with a total of 286 participants, reported data on the nine‐hole peg test as a secondary outcome parameter. Data were available for all 286 participants (Mazanec 2013 [pers comm]; Pareyson 2011; Verhamme 2009a). Ascorbic acid treatment showed a positive effect on the nine‐hole peg test over placebo; the effect was small and probably clinically insignificant (mean difference (MD) ‐0.39 seconds 95% CI ‐0.76 to ‐0.02). The studies were very unequal in size, so that the largest of the three trials (Pareyson 2011) achieved a weight of 82.2% (Analysis 1.21; Figure 4). Furthermore, the nine‐hole peg test is not a CMT‐specific outcome parameter and has not been a primary endpoint in any CMT treatment trial, although recently it was found to have good discrimination power between people with CMT who were mildly and severely affected (Mannil 2014).
![Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months (adults), outcome: 1.21 9‐hole peg test [s].](/cdsr/doi/10.1002/14651858.CD011952/media/CDSR/CD011952/image_n/nCD011952-AFig-FIG04.png)
Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months (adults), outcome: 1.21 9‐hole peg test [s].
Change in walking tests
There was no change with ascorbic acid compared to placebo in the 10‐metre walk in the three studies (N = 401) that measured this outcome (Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Analysis 1.22). A single study (N = 11) assessed 50‐metre walk and found no significant difference between ascorbic acid and placebo on this measure (Verhamme 2009a; Analysis 1.23).
Comparison: Oral ascorbic acid treatment versus placebo for adults with CMT1A (24 months)
Primary outcome ‐ impairment at 24 months on a validated scale
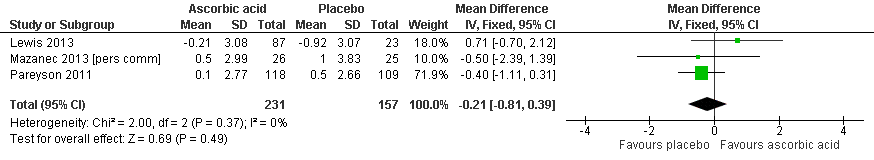
There was moderate‐quality evidence for the primary outcome that ascorbic acid treatment had no clinically meaningful effect over placebo using any of the validated outcome parameters (CMTNS in three studies, N = 388; CMTES in two studies, N = 337; Analysis 2.1; Analysis 2.2; Figure 5). It should be noted that these comparisons are strongly dominated by Pareyson 2011 because of its larger number of participants.

Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.1 Impairment ‐ CMTNS.
Secondary outcomes at 24 months
Change in disability on a validated scale
Treatment with ascorbic acid was not associated with any significant difference in the disability outcome ONLS (two studies, N = 276; Mazanec 2013 [pers comm]; Pareyson 2011;
Change in CMAP amplitudes
There was no evidence of significant differences in ulnar nerve (abductor digiti minimi) CMAP (two studies, N = 161; Lewis 2013; Mazanec 2013 [pers comm]; Analysis 2.4) when compared to placebo.
Change in sensory impairment measured with a validated scale
Sensory impairment was not separately measured by validated scales in the 24‐month trials.
Change in SNAP amplitude
SNAP amplitudes were not studied in the 24‐month trials.
Change in grip force and other measures of force
Objective measures of force including hand grip (N = 186), three‐point pinch (N = 276), and foot dorsiflexion (N = 278) showed no significant difference after treatment with ascorbic acid versus placebo (Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.5 to Analysis 2.7).
Change in quality of life
No significant changes were recorded in any of the SF‐36 measures when compared to placebo (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.8 to Analysis 2.10).
Other non pre‐specified secondary outcome measures at 24 months
Change in the nine‐hole peg test
Two studies with a total of 275 participants used the nine‐hole peg test as a secondary outcome parameter (Mazanec 2013 [pers comm]; Pareyson 2011). As in the 12‐month data, ascorbic acid treatment did show a positive effect on the nine‐hole peg test versus placebo (P = 0.004). The MD was ‐1.16 seconds, 95% CI ‐1.96 to ‐0.37. The clinical significance of this result is probably small. The studies were very unequal in size, so that Pareyson 2011, the larger of the two trials, achieved a weight of 82.8% (Analysis 2.11, Figure 6).
![Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.11 Impairment ‐ 9‐hole peg test [s].](/cdsr/doi/10.1002/14651858.CD011952/media/CDSR/CD011952/image_n/nCD011952-AFig-FIG06.png)
Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.11 Impairment ‐ 9‐hole peg test [s].
Change in walking test
The 10‐metre walk test was used by two studies with a total of 278 participants. The results showed no evidence of significant difference between ascorbic acid and placebo (Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.12).
Change in PMP22 mRNA
Skin biopsies of study participants did not show a clinically relevant difference in PMP22 mRNA (two studies, N = 114; Lewis 2013; Pareyson 2011; Nobbio 2014; Analysis 2.13).
Comparison: Oral ascorbic acid treatment versus placebo for children with CMT1A
Children were studied in Burns 2009, N = 80.
Primary outcome ‐ impairment at 12 months on a validated scale
The trial did not report the CMTNS, CMTES or CMTNS v2 because they are not validated for children. A paediatric CMT scale has been recently developed (Burns 2012), but was not available at the time of the study.
Secondary outcomes at 12 months
Change in disability on a validated scale
No validated disability scales were used.
Change in CMAP amplitudes
The mean change in CMAP (abductor pollicis brevis) was not significantly different between ascorbic acid and placebo groups (Analysis 3.1).
Change in sensory impairment measured with a validated scale
Sensory impairment was not measured on a specific scale.
Change in SNAP amplitude
SNAP amplitudes were not reported.
Change of grip force and other measures of force
Muscle force measurements by dynamometry of hand grip, finger pinch, foot dorsiflexion and foot plantar flexion, did not show significant differences when compared to placebo (Analysis 3.2 to Analysis 3.5).
Change in quality of life
Change in health‐related quality of life outcomes were reported in the web appendix of the study (Burns 2009). However, quality of life changes did not show any discernible differences between the treatment groups.
Other, non pre‐specified secondary outcome parameters
Change in median nerve conduction velocity
Change in median nerve conduction velocity was the primary outcome parameter of this study. However, there was no significant differences between the treatment groups (Analysis 3.6).
Change in the nine‐hole peg test
The nine‐hole peg test did not show a significant difference in ascorbic acid‐treated versus placebo‐treated children (Analysis 3.7, N = 78).
Change in walking tests
The six‐minute walk test did not show significant differences in ascorbic acid‐treated versus placebo‐treated children (Analysis 3.8, N = 65).
A number of further outcome parameters, particularly functional parameters assessing different aspects of motor function, such as balance, agility, long jump, speed, step time and length, were measured. None of these parameters showed evidence of differences between ascorbic acid and placebo (Analysis 3.9 to Analysis 3.16).
Adverse events, whether minor or severe, caused by ascorbic acid
We distinguished between minor and severe adverse events (those which were fatal, life‐threatening, required or prolonged hospitalisation or caused discontinuation of the treatment). Overall, ascorbic acid treatment was well tolerated. Most adverse events concerned nausea, gastro‐intestinal discomfort and constipation, but these complaints were not more common in the ascorbic acid groups compared to the placebo groups. Overall, 77 serious adverse events with ascorbic acid and placebo occurred in all studies combined (N = 702). The relative abundance of serious adverse events was 11.7% in the ascorbic acid and 12% in the placebo groups. (Participants can have more than one serious adverse event, so the percentages were calculated in the following way: (number of serious adverse events/number of participants) * 100.) (Burns 2009; Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a).
Discussion
Summary of main results
We identified a total of six different trials describing medical treatments of Charcot‐Marie‐Tooth disease (CMT) in a randomised controlled study design that fulfilled the inclusion criteria defined in the protocol. All of the six trials studied the effects of oral ascorbic acid versus placebo treatment in participants with CMT1A. The total number of participants in all trials amounted to 702 (Burns 2009; Micallef 2009; Verhamme 2009a; Pareyson 2011; Lewis 2013; Mazanec 2013 [pers comm]). One of these trial was conducted in children with CMT1A (N = 80, Burns 2009); the other five in adults (N = 622;Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a). Overall, 414 participants were treated with ascorbic acid and 288 with placebo.
The six included RCTs all used a medical intervention in the form of an oral medication with ascorbic acid or placebo. Dosages of ascorbic acid were different, ranging from 1 g to 4 g per day. However, there was no significant dose‐response relationship in a regression analysis (data not shown).
These trials provide high‐quality evidence that ascorbic acid does not change the course of CMT1A over 12 or 24 months in adults (summary of findings Table for the main comparison). The observed pooled effect size of ‐0.37 (95% CI ‐0.83 to 0.09) on the CMT neuropathy score (CMTNS) is probably not clinically relevant. Further research including a large number of participants and a trial period longer than 24 months may improve confidence in this estimate. There is low‐quality evidence that ascorbic acid does not improve the course of CMT1A in children over a treatment period of 12 months.
Overall completeness and applicability of evidence
The small number of trials that fulfilled the selection criteria for this review reflects the difficulties of undertaking clinical trials in CMT. Nonetheless, we did identify six trials conforming to the inclusion criteria, and it is very likely that we identified all available trials on ascorbic acid treatment of CMT. In terms of ascorbic acid treatment, this review comprises sufficient data to reach a meaningful conclusion. The consideration of possible effects of ascorbic acid on CMT1A is limited to a certain extent by the treatment period of the clinical trials (12 to 24 months). Due to the slow progression of the disease, the treatment period might have to be even longer than 24 months in order to detect changes in the disease course, but any large effect at longer time periods is unlikely.
Since CMT is biologically and genetically heterogenous and shows great variability in severity, analysis of different subgroups would have been appropriate, but the lack of data made this impossible. The ascorbic acid trials showed that the rate of change in CMTNS and other outcome parameters is very low, which makes a longer study duration, a higher number of participants or a more sensitive outcome measure necessary. The ascorbic acid trials also showed that a power analysis could account for slow rates of change of most outcome parameters. On the other hand, novel outcome parameters are being developed that are potentially more sensitive to change over time and therapeutic effects (Burns 2012; Komyathy 2013; Mannil 2014).
Although the trial in children appeared to provide good quality evidence in terms of trial design and reporting, we graded it as low quality according to GRADE criteria because it was a single trial with a relatively small number of participants, and the primary outcome parameter (motor nerve conduction velocity) was not ideal.
Quality of the evidence
The studies that were identified as RCTs used appropriate randomisation methods. Blinding and follow‐up were appropriate in all studies. All studies were placebo controlled. The treatment trial duration was two years in three studies (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011), and one year in three studies (Burns 2009; Micallef 2009; Verhamme 2009a). Follow‐up was generally good, with low numbers of drop‐outs. Most studies provided reasons for drop‐outs and all performed intention‐to‐treat analyses. One study did not include data of participants who dropped out and did not report the number of drop‐outs, therefore, it was not clear how many data were actually missing (Mazanec 2013 [pers comm]). The chosen outcome parameters were different between trials. Some trials used electrophysiologic outcome parameters (Burns 2009; Verhamme 2009a). The limitation of this approach is that electrophysiologic parameters change very little over time in CMT1A, especially in adults (Verhamme 2009b). However, most studies used the CMTNS as the primary outcome parameter. The CMTNS has been validated for people with CMT and was considered the most appropriate outcome parameter at the time the studies were done. Overall, the choice of outcome parameters was reasonable, being partly based on a workshop by the European Neuromuscular Centre (ENMC) on the conduct of trials in CMT (Reilly 2006). Importantly, there was good overlap of outcome measures between the different studies, so that we were able to perform meta‐analyses of results for several outcome measures. Taken together, we identified six RCTs with proper methodology and design, all studying the effects of ascorbic acid versus placebo on CMT1A.
Potential biases in the review process
BG and PY have been involved in basic research on ascorbic acid function in the peripheral nervous system (Gess 2010; Gess 2011). DP and MMR were investigators in one of the trials on ascorbic acid treatment in adults with CMT1A (Pareyson 2011). These involvements could present an intellectual bias, which we cannot altogether exclude. However, DP and MMR did not assess risk of bias in Pareyson 2011.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other studies or reviews, particularly no other meta‐analyses, on ascorbic acid treatment in Charcot‐Marie‐Tooth disease.
Outlook
Further studies of ascorbic acid in CMT disease with longer study duration and more sensitive outcome parameters may be sensible in the future. However, it is likely that ascorbic acid does not provide any significant benefit for CMT1A, and even with adaptation of study design or longer time periods, no significant effect is likely to be forthcoming. Further research into the mechanism of action of vitamin C in the peripheral nervous system may lead to novel therapeutic approaches. Active research on other medical and physical therapies of CMT is currently undergoing and will be reviewed in a separate Cochrane review on treatments of CMT other than ascorbic acid.
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.

Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months, outcome: 1.1 CMTNS.
![Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months (adults), outcome: 1.21 9‐hole peg test [s].](/es/cdsr/doi/10.1002/14651858.CD011952/media/CDSR/CD011952/image_n/nCD011952-AFig-FIG04.png)
Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months (adults), outcome: 1.21 9‐hole peg test [s].
Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.1 Impairment ‐ CMTNS.
![Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.11 Impairment ‐ 9‐hole peg test [s].](/es/cdsr/doi/10.1002/14651858.CD011952/media/CDSR/CD011952/image_n/nCD011952-AFig-FIG06.png)
Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.11 Impairment ‐ 9‐hole peg test [s].
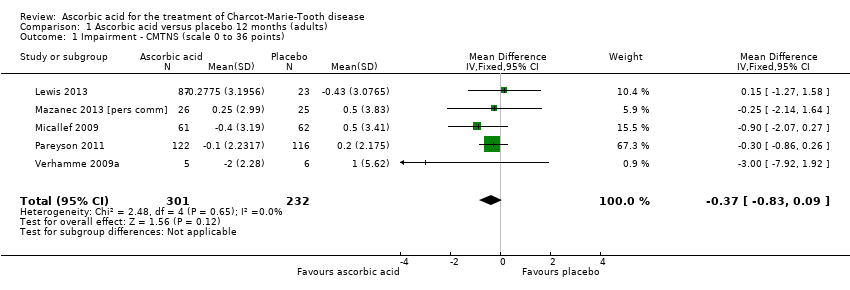
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 1 Impairment ‐ CMTNS (scale 0 to 36 points).
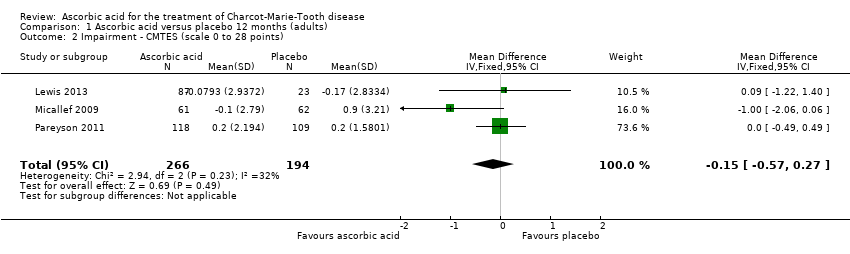
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 2 Impairment ‐ CMTES (scale 0 to 28 points).

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 3 Impairment ‐ NIS (scale ‐26 to 26 points).
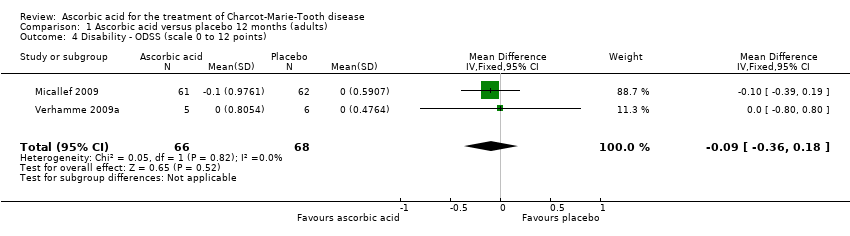
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 4 Disability ‐ ODSS (scale 0 to 12 points).

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 5 Disability ‐ ONLS (scale 0 to 12 points).
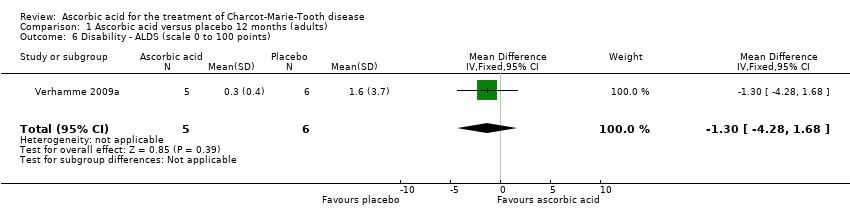
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 6 Disability ‐ ALDS (scale 0 to 100 points).

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 7 CMAP, ulnar nerve (abductor digiti minimi).

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 8 CMAP, median nerve (abductor pollicis brevis).

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 9 CMAP summatory from ulnar, median and peroneal nerves.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 10 Sensation ‐ INCAT Sensory Sum score (scale 0 to 20 points).
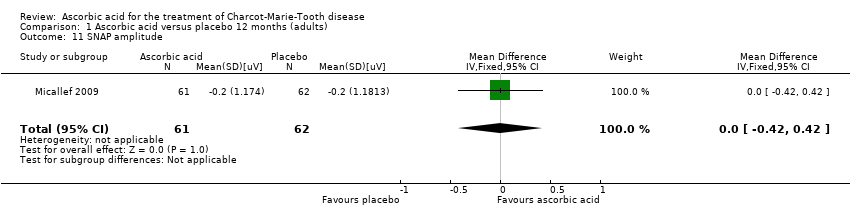
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 11 SNAP amplitude.
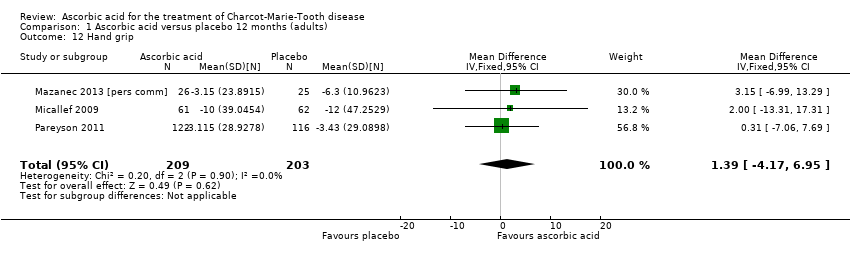
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 12 Hand grip.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 13 Three‐point pinch.
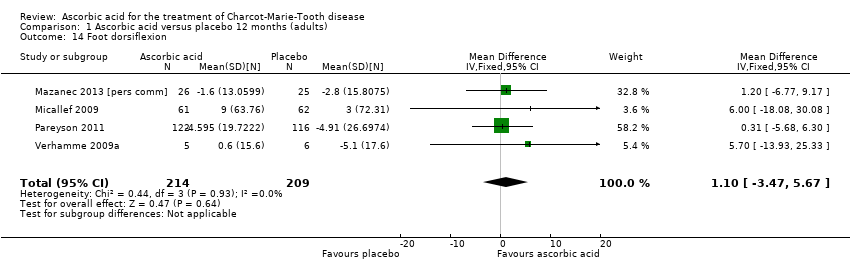
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 14 Foot dorsiflexion.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 15 Quality of life ‐ SF36 ‐ physical functioning.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 16 Quality of life ‐ SF36 ‐ bodily pain.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 17 Quality of life ‐ SF36 ‐ energy.

Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 18 Quality of life ‐ SF36 ‐ psychological.
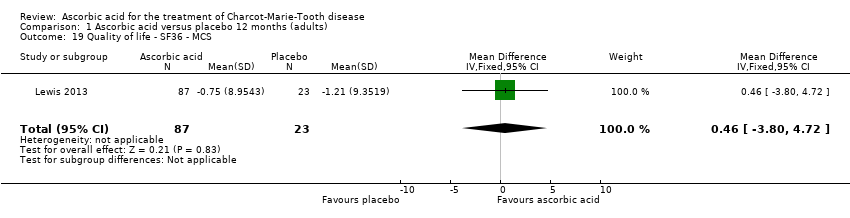
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 19 Quality of life ‐ SF36 ‐ MCS.
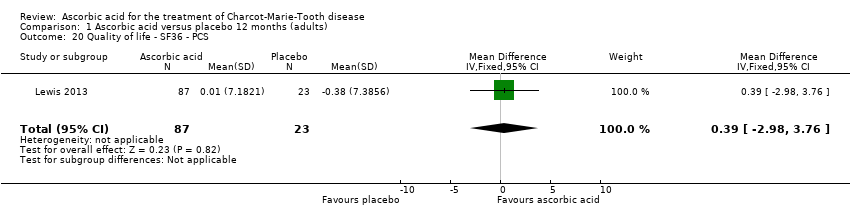
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 20 Quality of life ‐ SF36 ‐ PCS.
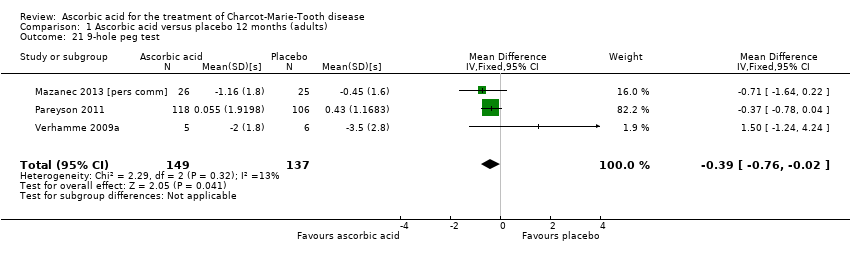
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 21 9‐hole peg test.
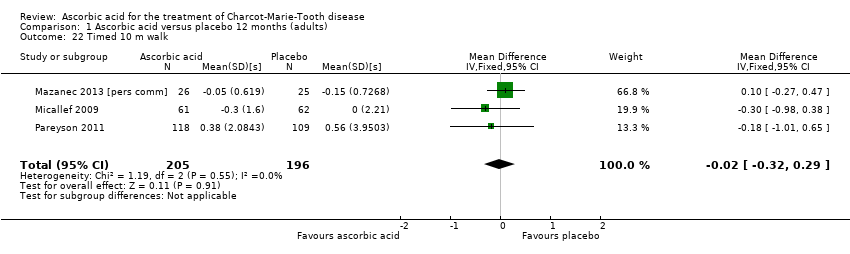
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 22 Timed 10 m walk.
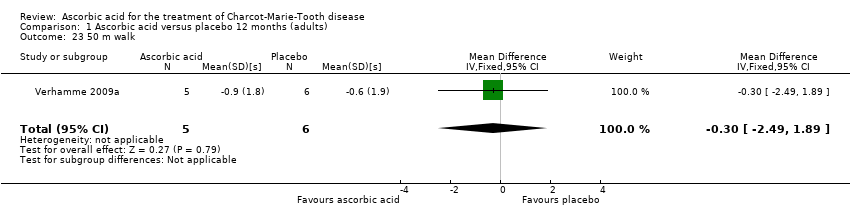
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 23 50 m walk.

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 1 Impairment ‐ CMTNS (scale 0 to 36 points).

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 2 Impairment ‐ CMTES (scale 0 to 28 points).

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 3 Disability ‐ ONLS (scale 0 to 12 points).
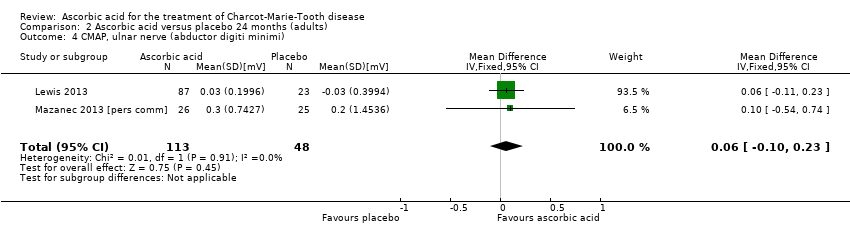
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 4 CMAP, ulnar nerve (abductor digiti minimi).

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 5 Hand grip.
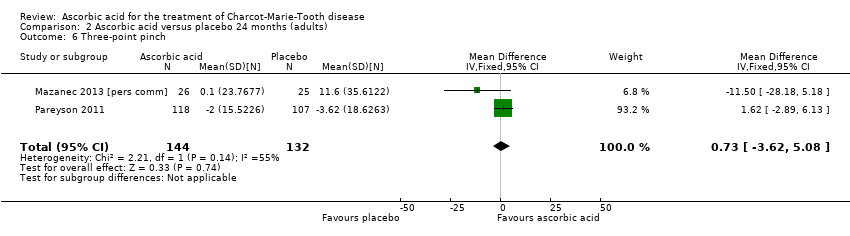
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 6 Three‐point pinch.

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 7 Foot dorsiflexion.

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 8 Quality of life ‐ SF36 ‐ physical functioning.
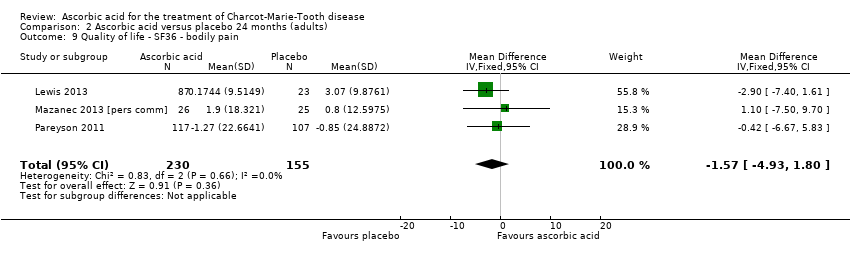
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 9 Quality of life ‐ SF36 ‐ bodily pain.

Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 10 Quality of life ‐ SF36 ‐ energy.
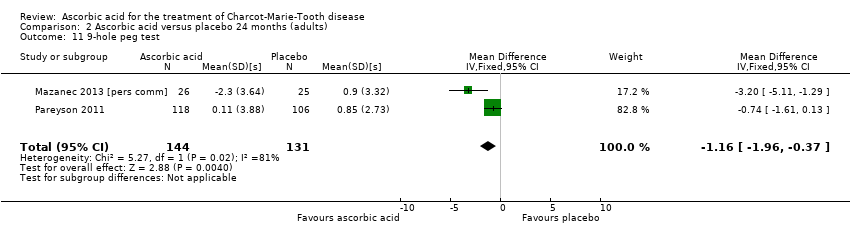
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 11 9‐hole peg test.
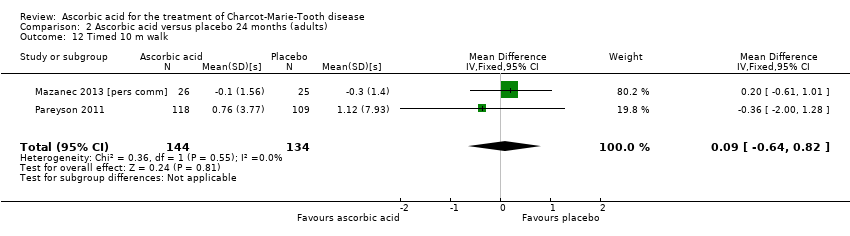
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 12 Timed 10 m walk.
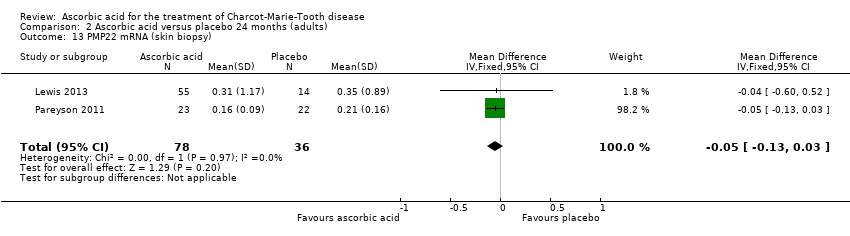
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 13 PMP22 mRNA (skin biopsy).
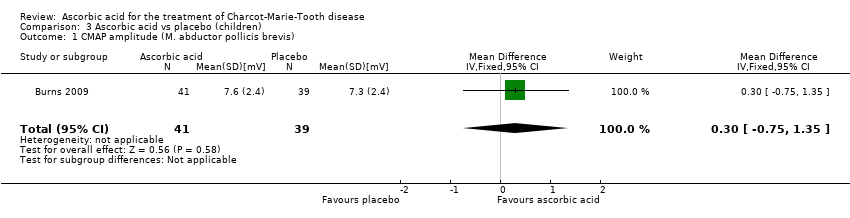
Comparison 3 Ascorbic acid vs placebo (children), Outcome 1 CMAP amplitude (M. abductor pollicis brevis).
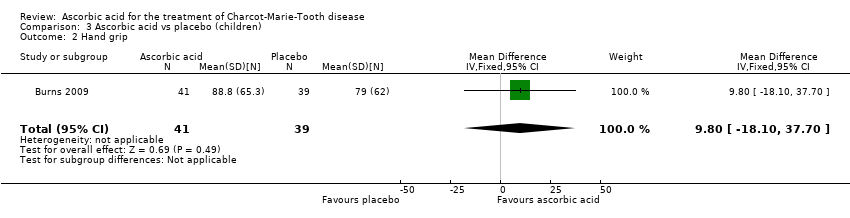
Comparison 3 Ascorbic acid vs placebo (children), Outcome 2 Hand grip.
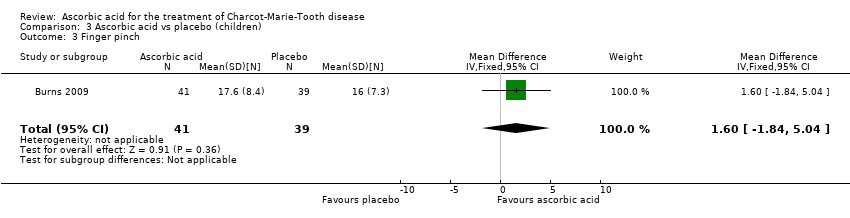
Comparison 3 Ascorbic acid vs placebo (children), Outcome 3 Finger pinch.
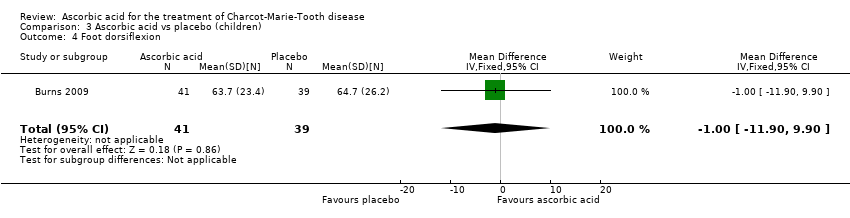
Comparison 3 Ascorbic acid vs placebo (children), Outcome 4 Foot dorsiflexion.
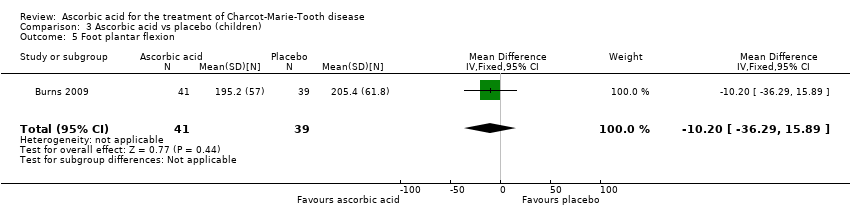
Comparison 3 Ascorbic acid vs placebo (children), Outcome 5 Foot plantar flexion.

Comparison 3 Ascorbic acid vs placebo (children), Outcome 6 Median nerve motor conduction velocity.
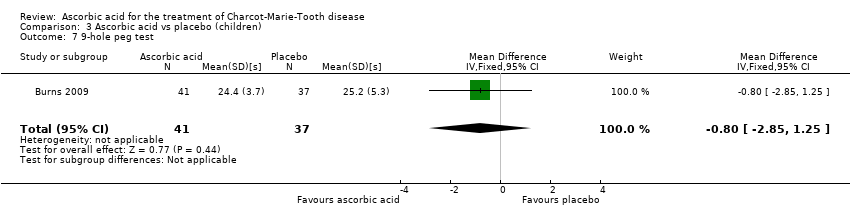
Comparison 3 Ascorbic acid vs placebo (children), Outcome 7 9‐hole peg test.
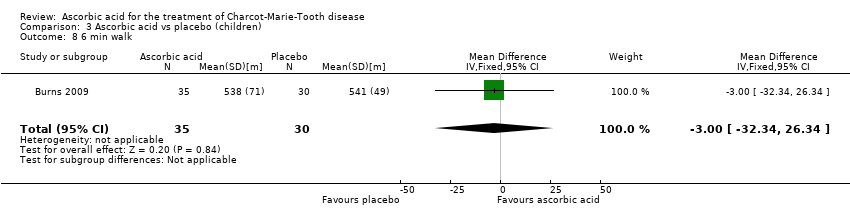
Comparison 3 Ascorbic acid vs placebo (children), Outcome 8 6 min walk.
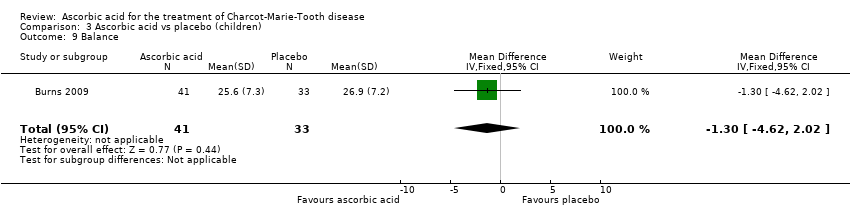
Comparison 3 Ascorbic acid vs placebo (children), Outcome 9 Balance.

Comparison 3 Ascorbic acid vs placebo (children), Outcome 10 Agility.
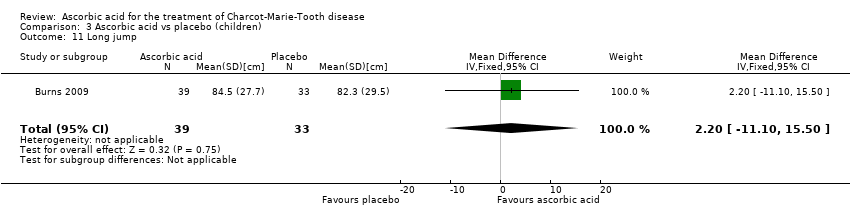
Comparison 3 Ascorbic acid vs placebo (children), Outcome 11 Long jump.

Comparison 3 Ascorbic acid vs placebo (children), Outcome 12 Speed.

Comparison 3 Ascorbic acid vs placebo (children), Outcome 13 Cadence.
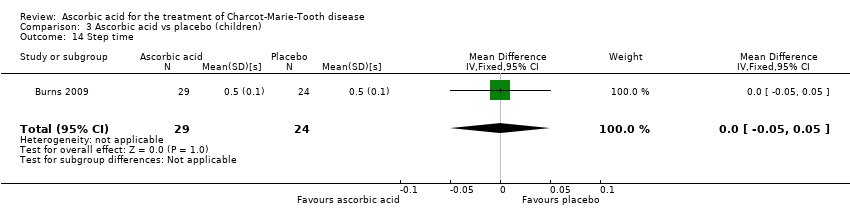
Comparison 3 Ascorbic acid vs placebo (children), Outcome 14 Step time.
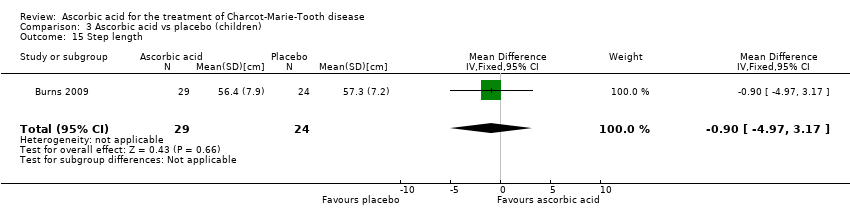
Comparison 3 Ascorbic acid vs placebo (children), Outcome 15 Step length.

Comparison 3 Ascorbic acid vs placebo (children), Outcome 16 Stride length.
| Ascorbic acid treatment compared with placebo for CMT1A in adults | ||||||
| Participants or population: adults with CMT1A Intervention: oral ascorbic acid (1 g to 4 g/day) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ascorbic acid | |||||
| Impairment ‐ change in CMTNS at 24 months (0 to 36 points) | The mean change in CMTNS ranged across control groups from ‐0.92 to 1 point | The mean change in CMTNS in the intervention groups was 0.21 lower (0.81 lower to 0.39 higher) | ‐0.21 (‐0.81 to 0.39) | 388 (3) | ⊕⊕⊕⊕ | |
| Impairment ‐ change in CMTES at 24 months (0 to 28 points) | The mean change in CMTES ranged across control groups from ‐0.64 to 0.5 point | The mean change in CMTES in the intervention groups was 0.12 lower (0.67 lower to 0.42 higher) | ‐0.12 (‐0.67 to 0.42) | 337 (2) | ⊕⊕⊕⊝ | |
| Change in timed 10‐m walk at 12 months (seconds (s)) | The mean change in timed 10‐m walk ranged across control groups from ‐0.15 to 0.56 s | The mean change in timed 10‐m walk in the intervention groups was 0.02 lower (0.32 lower to 0.29 lower) | ‐0.02 (‐0.32 to 0.29) | 401 (3) | ⊕⊕⊕⊕ | |
| Change in foot dorsiflexion at 12 months (N) | The mean change in foot dorsiflexion force ranged across control groups from | The mean change in foot dorsiflexion force in the intervention groups was 1.1 higher (3.47 lower to 5.67 higher) | 1.1 (‐3.47 to 5.67) | 423 (4) | ⊕⊕⊕⊕ | |
| Change in 9‐hole peg test (HPT) at 12 months (seconds) | The mean change in 9‐HPT ranged across control groups from | The mean change in 9‐HPT in the intervention groups was 0.39 lower (0.76 lower to 0.02 lower) | ‐0.39 (‐0.76 to ‐0.02) | 286 | ⊕⊕⊕⊝ | |
| Serious adverse events (SAE; %) | The relative abundance of SAE was 12% in the placebo group ((number of SAE/number of participants)*100) | The relative abundance of SAE was 11.7% in the intervention groups ((number of SAE/number of participants)*100) | Not estimable | 702 (6, including 1 study in children) | ⊕⊕⊕⊕ | |
| *The assumed risk is based on the mean change or the range of mean change) across control groups in included studies.. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence downgraded because of number of studies and participants, which was lower than for other outcomes. Further research may thus have an impact on our confidence in the estimate of effect and may change the estimate. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Impairment ‐ CMTNS (scale 0 to 36 points) Show forest plot | 5 | 533 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.83, 0.09] |
| 2 Impairment ‐ CMTES (scale 0 to 28 points) Show forest plot | 3 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.57, 0.27] |
| 3 Impairment ‐ NIS (scale ‐26 to 26 points) Show forest plot | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [‐2.24, 6.06] |
| 4 Disability ‐ ODSS (scale 0 to 12 points) Show forest plot | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.36, 0.18] |
| 5 Disability ‐ ONLS (scale 0 to 12 points) Show forest plot | 2 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.02, 0.17] |
| 6 Disability ‐ ALDS (scale 0 to 100 points) Show forest plot | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐4.28, 1.68] |
| 7 CMAP, ulnar nerve (abductor digiti minimi) Show forest plot | 2 | 161 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.34, 0.59] |
| 8 CMAP, median nerve (abductor pollicis brevis) Show forest plot | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.19, 0.83] |
| 9 CMAP summatory from ulnar, median and peroneal nerves Show forest plot | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.48, 0.94] |
| 10 Sensation ‐ INCAT Sensory Sum score (scale 0 to 20 points) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.53, 1.53] |
| 11 SNAP amplitude Show forest plot | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.42, 0.42] |
| 12 Hand grip Show forest plot | 3 | 412 | Mean Difference (IV, Fixed, 95% CI) | 1.39 [‐4.17, 6.95] |
| 13 Three‐point pinch Show forest plot | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.53, 2.73] |
| 14 Foot dorsiflexion Show forest plot | 4 | 423 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐3.47, 5.67] |
| 15 Quality of life ‐ SF36 ‐ physical functioning Show forest plot | 4 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐1.32, 1.22] |
| 16 Quality of life ‐ SF36 ‐ bodily pain Show forest plot | 3 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐2.69, 1.62] |
| 17 Quality of life ‐ SF36 ‐ energy Show forest plot | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.84, 3.74] |
| 18 Quality of life ‐ SF36 ‐ psychological Show forest plot | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.65, 4.45] |
| 19 Quality of life ‐ SF36 ‐ MCS Show forest plot | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐3.80, 4.72] |
| 20 Quality of life ‐ SF36 ‐ PCS Show forest plot | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐2.98, 3.76] |
| 21 9‐hole peg test Show forest plot | 3 | 286 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.76, ‐0.02] |
| 22 Timed 10 m walk Show forest plot | 3 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.32, 0.29] |
| 23 50 m walk Show forest plot | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.49, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Impairment ‐ CMTNS (scale 0 to 36 points) Show forest plot | 3 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.81, 0.39] |
| 2 Impairment ‐ CMTES (scale 0 to 28 points) Show forest plot | 2 | 337 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.67, 0.42] |
| 3 Disability ‐ ONLS (scale 0 to 12 points) Show forest plot | 2 | 276 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.05, 0.34] |
| 4 CMAP, ulnar nerve (abductor digiti minimi) Show forest plot | 2 | 161 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.10, 0.23] |
| 5 Hand grip Show forest plot | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐3.86, 6.37] |
| 6 Three‐point pinch Show forest plot | 2 | 276 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐3.62, 5.08] |
| 7 Foot dorsiflexion Show forest plot | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐7.21, 8.76] |
| 8 Quality of life ‐ SF36 ‐ physical functioning Show forest plot | 3 | 382 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐2.41, 2.26] |
| 9 Quality of life ‐ SF36 ‐ bodily pain Show forest plot | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐4.93, 1.80] |
| 10 Quality of life ‐ SF36 ‐ energy Show forest plot | 2 | 267 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐3.35, 4.77] |
| 11 9‐hole peg test Show forest plot | 2 | 275 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.96, ‐0.37] |
| 12 Timed 10 m walk Show forest plot | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.64, 0.82] |
| 13 PMP22 mRNA (skin biopsy) Show forest plot | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CMAP amplitude (M. abductor pollicis brevis) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.75, 1.35] |
| 2 Hand grip Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 9.80 [‐18.10, 37.70] |
| 3 Finger pinch Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐1.84, 5.04] |
| 4 Foot dorsiflexion Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐11.90, 9.90] |
| 5 Foot plantar flexion Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐36.29, 15.89] |
| 6 Median nerve motor conduction velocity Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.89, 4.89] |
| 7 9‐hole peg test Show forest plot | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.85, 1.25] |
| 8 6 min walk Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐32.34, 26.34] |
| 9 Balance Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.62, 2.02] |
| 10 Agility Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐5.43, 2.03] |
| 11 Long jump Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐11.10, 15.50] |
| 12 Speed Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐9.33, 8.93] |
| 13 Cadence Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐6.54, 8.14] |
| 14 Step time Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 15 Step length Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐4.97, 3.17] |
| 16 Stride length Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐9.58, 5.98] |

